Amphiesma parallelum ( Boulenger, 1890 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3919.2.9 |
|
publication LSID |
lsid:zoobank.org:pub:B9AA0822-2908-4C4C-A14A-30007F40F9BE |
|
DOI |
https://doi.org/10.5281/zenodo.5681723 |
|
persistent identifier |
https://treatment.plazi.org/id/03A187E7-FFA9-D316-FF3B-3DA1FD80D776 |
|
treatment provided by |
Plazi |
|
scientific name |
Amphiesma parallelum ( Boulenger, 1890 ) |
| status |
|
Amphiesma parallelum ( Boulenger, 1890)
( Fig. 8–9 View FIGURE 8 View FIGURE 9 )
Tropidonotus parallelus Boulenger, 1890: 345 View in CoL . Type locality. By virtue of lectotype designation: “Sikkim”, currently the State of Sikkim, India. Lectotype. By designation of Kramer (1977: 728): BMNH 1946.1.13.53, adult male; collected and deposited by Sir Joseph Dalton Hooker.
Material (n = 18 + 1 unpreserved specimen). India. Sikkim (? See below). BMNH 1946.1.13.53 (lectotype of Tropidonotus parallelus Boulenger, 1890 ), “Sikkim”.—Meghalaya. BMNH 1946.1.12.83–84, BMNH 1946.1.13.48 (3 former syntypes of Tropidonotus parallelus Boulenger, 1890 ), “Khasi Hills”; ZSI 3852, “Shillong”; ZSI/ERS 112, ZSI/ERS 205, ZSI/ERS 2785, ZSI/ERS 3076–3077, Risa Colony, Shillong; ZSI/ERS 272, ZSI/ERS 9059, Tripura Castle Road, Shillong; ZSI/ERS 450, ZSI/ERS 970, Fruit Garden, Shillong; ZSI/ERS 3253, Mawlai, East Khasi Hills District; ZSI/ERS 8262, Mawphlang, East Khasi Hills; ZSI/ERS 9060, Selbelgiri, Garo Hills.—Nagaland. Unpreserved specimen ( Fig. 9 View FIGURE 9 ), near the Tragopan Sanctuary (25.63549N- 094.01261E), Khonoma.—No locality. ZSI 4397, “Madras Hills”, obviously in error.
Systematics. Boulenger (1890) did not specify the number of syntypes upon which he based his description but he mentioned the type locality as “Sikhim [sic], Khási Hills, hills of Upper Burma and Yunnan”. Subsequently, Boulenger (1893: 223) considered six specimens to be syntypes, i.e. one from Sikkim ( BMNH 1946.1.13.53), three from the Khasi Hills ( BMNH 1946.1.13.48 and BMNH 1946.1.12.83 – 84), one from Sanda, in northern Myanmar ( BMNH 1946.1.21.87) and the last one from Hotha Valley, Yunnan ( BMNH 1946.1.13.58). These two latter specimens were collected by J. Anderson. We examined them and they are referable to Amphiesma bitaeniatum ( Wall, 1925) . The four other original type specimens, all are referable to the same species that should now be known as A. parallelum . Kramer (1977: 728) considered specimen BMNH 60.3.19.1359, from Sikkim but not collected by Sir J. Hooker, to be also a syntype of Tropidonotus parallelus . This assertion is erroneous, as this specimen did not belong to the original type material. However, quite interestingly, Kramer noted that this specimen disagreed in several points with the other syntypes, which possibly represented two forms. This author was correct, as specimen BMNH 60.3.19.1359 is referable to Amphiesma clerki (see above). In order to fix the status of the taxon Tropidonotus parallelus Boulenger, 1890 , Kramer (1977) designated the specimen BMNH 1946.1.13.53, also from Sikkim, as its lectotype.
However, in our opinion, there remains an ambiguity on the exact collection locality of the lectotype. To our best knowledge, no other specimen of A. parallelum has ever been collected in Sikkim ( Sanyal et al. 2006) or in the adjacent State of West Bengal. Specimen BMNH 1946.1.13.53 was collected and deposited by Sir Joseph Dalton Hooker (1817–1911), one of the greatest botanists and explorers in Asia of his time. In 1847, he undertook an expedition to the Himalaya Range which lasted four years. Hooker travelled extensively in the region of Darjeeling and, during nearly the whole of 1849, in Sikkim where he gathered a large collection, mostly of plants. However, Sikkim was not his sole area of interest in India. He spent much of 1850 in Assam, especially in the Khasi Hills ( Hooker 1854; Turrill 1963; Desmond 2006). Unfortunately, his journal does not contain much information on the snakes collected during his trips. In Vol. 2 (p. 25–26), Hooker stated to have collected “about 12 species” in Sikkim, of which seven were colubrid snakes, without additional details. Moreover, in the same volume ( Hooker 1854: 305) wrote “ Reptiles , and especially Colubridae , are very common in the Khasia mountains, and I procured sixteen species and many specimens. The natives repeatedly assured us that these were all harmless, and Dr. Gray, who has kindly examined all my snakes, informs me of the remarkable fact (…) that whereas none are poisonous, (…).” Obviously, Hooker also collected numerous colubrid snakes in the Khasi Hills. Although proof is lacking, we cannot exclude a confusion in specimen labels and localities. Instead of Sikkim, the lectotype specimen might well come from the Khasi Hills, where numerous specimens of Amphiesma parallelum have been subsequently collected.
Amphiesma parallelum has been extensively confused in the literature with A. clerki but also with A. bitaeniatum and A. octolineatum , as well as with A. platyceps and A. sieboldii . A chresonymy of A. parallelum , however, is out of the scope of the present paper. Such confusion appeared in major authors such as Bourret (1936) and Smith (1943). As currently conceived, Amphiesma parallelum is a monotypic species.
As far as we know, Amphiesma parallelum had never been depicted alive in the literature before the present paper. Only a colour painting appeared in Das (2010: Pl. 67: Fig. 13).
Diagnosis. A species of the genus Amphiesma characterized by (1) a dark dorsal background in life, dark reddish-brown or probably dark chestnut-brown in life, turning to cream, grey, pinkish-grey, tan, more or less dark greyish-brown or dark yellowish-grey in preservative, (2) on each side, a conspicuous brown or reddish-brown (cream, pale greyish-brown or pale yellowish-brown in preservative) dorsolateral stripe, narrowly edged on its both sides with dark brown or black, extending on the 5th–7th dorsal scale rows from the occipital region along the whole of the body, (3) many dorsal scales longitudinally edged with dark brown, especially on the lower part of the sides, producing a faint reticulation or irregular streaks, (4) upper edge of scales of the 1st dorsal scale row and lower edge of those of 2nd row blackish-brown, producing a very irregular ventrolateral stripe, (5) a rather narrow, irregular, blackish-brown postocular streak extending from behind the eye to the corner of the mouth, usually separated by a wide gap from the dark line edging the lower side of the dorsolateral stripe, (6) no chevron on the upper part of the neck, (7) supralabials mostly uniform, not (or barely) spotted, (8) venter uniformly cream, beige or yellowishbrown, with the outer edge of ventral tips blackish-brown, (9) 21–22 maxillary teeth, the last two moderately enlarged and separated from anterior teeth by a distinct diastema, (10) 19–19–17 dorsal scale rows, (11) dorsal scales moderately to strongly keeled on 2nd–9th dorsal and vertebral scale rows, smooth or at best feebly keeled 1st dorsal row, (12) tail moderate, proportion tail length / total length 0.221–0.252 (0.239–0.252 in males, 0.221–0.249 in females), (13) 160–173 ventrals, (14) 63–77 subcaudals, (15) anal plate divided, (16) usually 2 preoculars, and (17) 1 or 2 anterior temporals.
Variation (based on 18 preserved and 1 living specimens). Habitus. Body elongate but rather plump in females, cylindrical; head ovoid, elongate, distinct from the neck; snout length average, accounting for 19.7–23.1 % of HL or 1.1–1.2 times as long as eye diameter, flattened, narrowing anteriorly, blunt when seen from above and in profile, with no defined canthus rostralis; nostril laterally positioned; eye size average, its diameter 1.6–2.0 times the distance between its inferior margin and upper lip edge; pupil round; tail length average, cylindrical, and progressively tapering.
The maximal total length known is 633 mm (SVL: 483 mm, TaL: 150 mm; female; specimen ZSI/ERS 9070). The longest known male is 601 mm long (SVL 450 mm, TaL 151 mm; ZSI/ERS 272). Proportion TaL / TL: 0.221–0.252, without clear sexual dimorphism.
Dentition. Maxillary teeth: 21–22, with 19–20 subequal teeth + 2 moderately enlarged posterior teeth, not twice as long as an anterior teeth, separated from the anterior teeth by a distinct diastema, at least as wide as the length of a posterior tooth.
Body scalation. Dorsal scales in 19-19-17 rows, scales of the 2nd–9th rows strongly keeled and deeply notched at their apical part, especially on the posterior part of the body, more strongly keeled on the upper part of the dorsum; scales of the 1st dorsal row enlarged, smooth or feebly keeled; no apical pit. VEN: 160–173 (plus 1 or 2 preventrals); SC: 63–77, usually all paired but some single scales has been recorded in a few specimens; anal plate divided.
Head scalation. Rostral about 1.5–1.6 times as wide as high; nasal rectangular, longer than high, nostril lateral and piercing in the middle of the scale; internasals subtriangular, 0.9–1.1 times as long as wide, anteriorly narrowed but truncated, with the fore width about 0.45–0.55 times as great as the hind width; prefrontals about 1.0–1.3 times as long as internasals; 1 large, longer than wide undivided supraocular on each side; frontal bell-shaped, rather small, 1.3–1.5 times as long as wide, 2.2–2.7 times longer than prefrontals; parietals large, in contact along a suture about 0.8–1.2 times as long as frontal; one subrectangular loreal on each side, as long as high or barely longer than high; 8 supralabials in all examined specimens, 1st and 2nd SL in contact with nasal, 2nd or 2nd and 3rd SL in contact with loreal, 3rd–5th SL entering orbit, 7th SL largest; no subocular; on each side 2 (exceptionally 1) preoculars, small and narrow; 3 (rarely 2) small postoculars; 1 (in 17/34 occurrences) or 2 (in 17/34) anterior temporals with the formulas 1+1, 1+2, 2+2 or rarely 2+3 temporals; 9 or 10 infralabials, 1st pair in contact, 1st–4th or 1st–5th infralabials in contact with anterior chin shields, 4th and 5th infralabials largest.
Coloration and pattern. In preservative ( Fig. 8 View FIGURE 8 ), the body is cream, pinkish-grey, tan, pale to dark greyishbrown, dark yellowish-grey or even dark brown, not or barely darker on the upper part of the body than on the sides; many scales of the sides and upper dorsum surface either narrowly and irregularly edged with blackishbrown, especially on the sides, or paler centred, i.e., pale yellowish-grey or yellowish-brown, or both, producing an irregular, diffuse reticulate pattern or dark elongate streaks; scales more broadly edged or mottled with dark brown or blackish-brown a short distance just behind the head, where sides can be irregularly but distinctly darker than other part of the sides; scales of the 1st dorsal scale row more largely pale centred; upper dorsal surface sometimes with scattered black spots; on each side, a broad, conspicuous dorsolateral stripe, cream or pale greyish-brown, anteriorly, slightly larger, i.e. yellowish-grey or pale yellowish-brown after the anterior quarter of the body, and narrowly edged with a dark brown or black line on both its lower and upper sides, extends on the upper part of 5th, the whole of 6th and much of 7th dorsal scale rows from the nuchal region behind temporals scales, along the whole of the body; this dorsolateral stripe is narrowly but distinctly edged with irregular, discontinuous lines, more conspicuous on the forepart of the body, the lower one composed of dark brown or blackish-brown streaks on the upper half of 5th DSR, the upper one by similar streaks on the upper part of scales of the 7th DSR; upper halves of scales of the 1st DSR and often lower part of those of the 2nd DSR row marked with short, irregular blackish-brown streaks, producing a very discontinuous stripe, sometimes present only in the hind part of the body or barely noticeable; lower part of scales of 1st with irregular blackish-brown streaks, producing a very discontinuous, zigzag-like ventrolateral stripe. Upper tail surface as the body but, anteriorly, somewhat paler on its upper dorsolateral half due to the continuation of the pale dorsolateral stripe at least to the fore part of the tail, stripes separated by dorsal background colour on a narrow vertebral area; scales of the lower part of the sides rather pale, distinctly edged with dark brown, strongly edged for the scales of the 1st row, producing a strongly reticulate pattern; hind half of tail uniformly pale as the dorsolateral stripe with scales strongly edged with brown.
The head is beige, pale greyish-brown, yellowish-brown or yellowish-grey above, distinctly paler than body in dark specimens; sides of the snout, supralabials and temporal areas ivory, creamish-yellow or pale creamishbrown; upper head scales irregularly and incompletely edged with dark brown; a short, narrow, dark streak in front of the eye on the upper edge of lower preocular and loreal, not reaching nasal and often reduced to an elongate blotch between the two preoculars; supralabials entirely uniform or with, at most, a few dark dots, not edged with dark brown; a narrow, oblique blackish-brown or black postocular streak extends from behind the eye to the corner of the mouth, covering lower postocular, lower half of anterior temporal, upper edge of 6th SL, the upper half of 7th SL and lower half of 8th SL; the postocular streak does not extend beyond 8th SL or at most only a short distance, and is usually widely separated from both the dark line edging the lower side of the dorsolateral stripe and, if present, the darker colour of the body side, by a pale coloured gap of same colour as the supralabials and chin; sometimes this gap is much reduced, with postocular streak and dark body colour nearly in contact; the pale nuchal gap above and behind the end of the postocular streak is edged with blackish-brown, more broadly on its lower edge, and merges into the dorsolateral stripe, the dark edges of the gap extending as edges of the dorsolateral stripe; a cream or pale yellow occipital streak, narrowly black-edged, extends from the tip of parietals to the upper part of the nuchal pale-coloured gap. The chin and throat are uniformly ivory, creamish-yellow or pale yellow; some infralabials with faint, darker edges.
The venter is uniformly ivory, creamish-yellow or pale yellowish-brown, with outer edges of tips of ventral scales dark brown or blackish-brown, in contact with the short streaks of the lower edges of scales of the 1st DSR. The subcaudal surface is uniformly coloured as the venter.
In life ( Fig. 9 View FIGURE 9 ), the colouration is much more vivid than in preserved specimens. According to the sole available specimen, the dorsum is dark reddish-brown with many dorsal scales irregularly black-edged at random locations, not forming any definite pattern; on each side, a pale brown dorsolateral stripe extending on the 6th and 7th dorsal scale rows from the nape to the end of the body; these two stripes appear to be truly parallel; on each side of the pale dorsolateral stripe, dorsal scales are regularly black-edged, producing a thin, regular black lines above and below the stripe; these black lines extend to the end of the tail dorsolaterally; tail as the body, with some of the lateral scales with a red centre.
The head is dark brown above, brighter reddish-brown on the nape; an irregular, thin, black preocular streak starts form the anterior nasal scale to the eye; supralabials, lower preocular and loreal white marked with irregular red and black spots; the last supralabial is reddish-brown; a thin postocular streak from the eye to the gap behind the corner of the mouth, extending on penultimate supralabial; last supralabial brown; chin and throat white with black and red markings.
The venter is pale yellow; outer tips of ventral scales with red spots, plus other black spots at the intersection of the pale yellow background colour and red spots, producing an irregular ventral red stripe dotted with black, that extends venterolateraly from the throat to the vent. Subcaudal surface pale yellow, progressively merging with the lateral red spots that extends to the tip of the tail.
Sexual dimorphism. It is weakly expressed only in the number of subcaudals: males: 72–77 (n = 8; x = 74.3; s = 1.6) vs. females: 63–73 (n = 8; x = 70.0; s = 3.8).
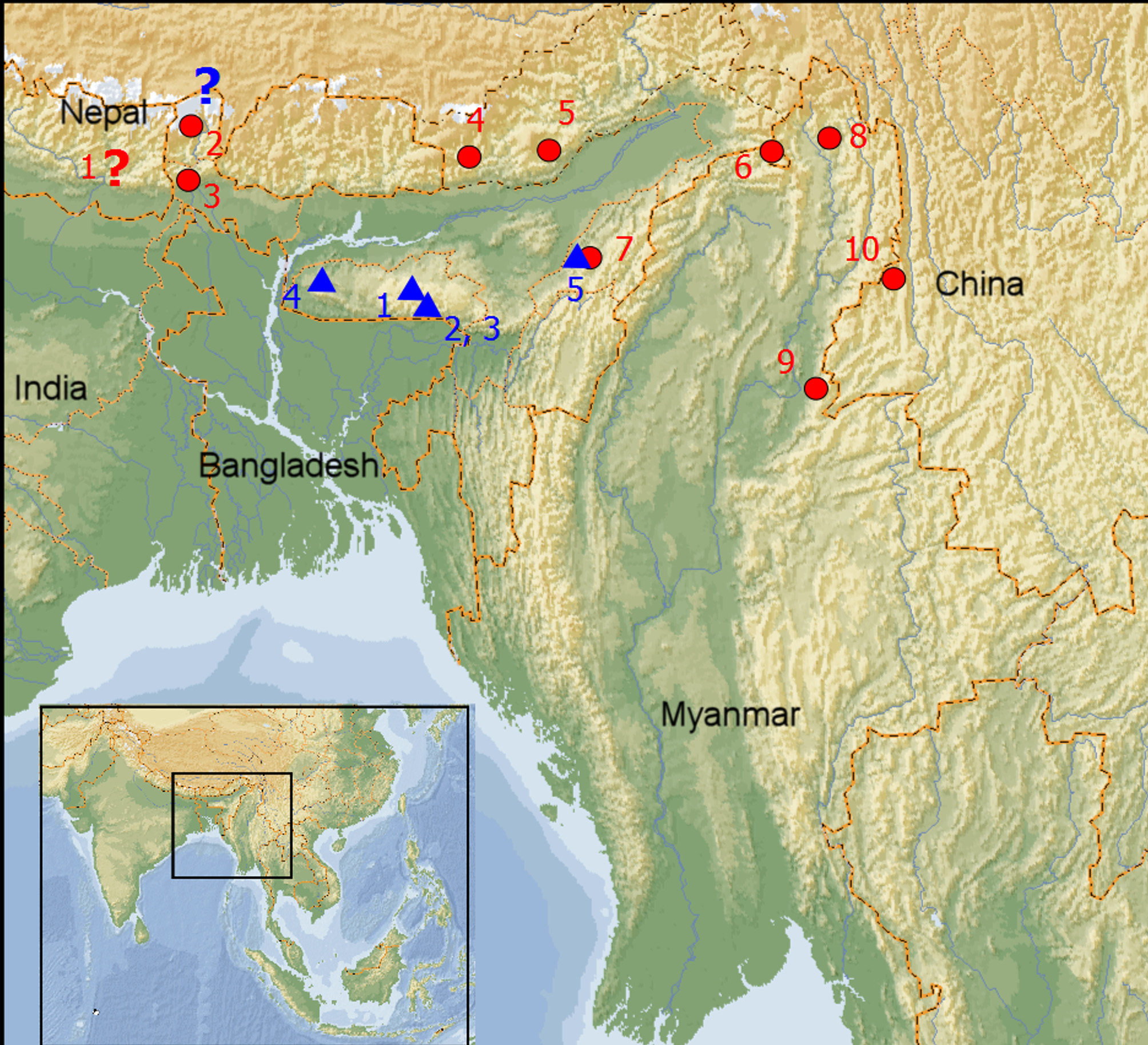
Distribution ( Fig. 7 View FIGURE 7 ; numbers refer to the triangles on the map). As currently conceived, Amphiesma parallelum is definitely known only from mountain ranges (Khasi Hills, Garo Hills and Mts. Naga) south of the Himalaya Range. India. Sikkim. Presence unconfirmed, perhaps in error (1). Meghalaya. Shillong and its vicinity (2); Mawphlang, East Khasi Hills (3); Mawlai, East Khasi Hills District (4); Selbalgiri, Garo Hills (5). Nagaland. Tragopan Sanctuary (25.63549N- 094.01261E), Khonoma (6).
Lastly, we examined specimen BMNH 58.6.24.5, alledged to come from “ Nepal ”. According to its pattern, it is indeed an Amphiesma clerki (see above).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Amphiesma parallelum ( Boulenger, 1890 )
| David, Patrick, Agarwal, Ishan, Athreya, Ramana, Mathew, Rosamma, Vogel, Gernot & Mistry, Viral K. 2015 |
Tropidonotus parallelus
| Kramer 1977: 728 |
| Boulenger 1890: 345 |