Tupirinna Bonaldo, 2000
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5004.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:60817167-2232-43BB-825D-B2DA67BD54D0 |
|
DOI |
https://doi.org/10.5281/zenodo.5123408 |
|
persistent identifier |
https://treatment.plazi.org/id/03A487D6-BA31-7043-8CEE-6862FEB1CB4A |
|
treatment provided by |
Plazi |
|
scientific name |
Tupirinna Bonaldo, 2000 |
| status |
|
Tupirinna Bonaldo, 2000 View in CoL
Tupirinna Bonaldo, 2000: 133 View in CoL (type species by original designation, Tupirinna rosae Bonaldo, 2000 View in CoL ); WSC, 2021.
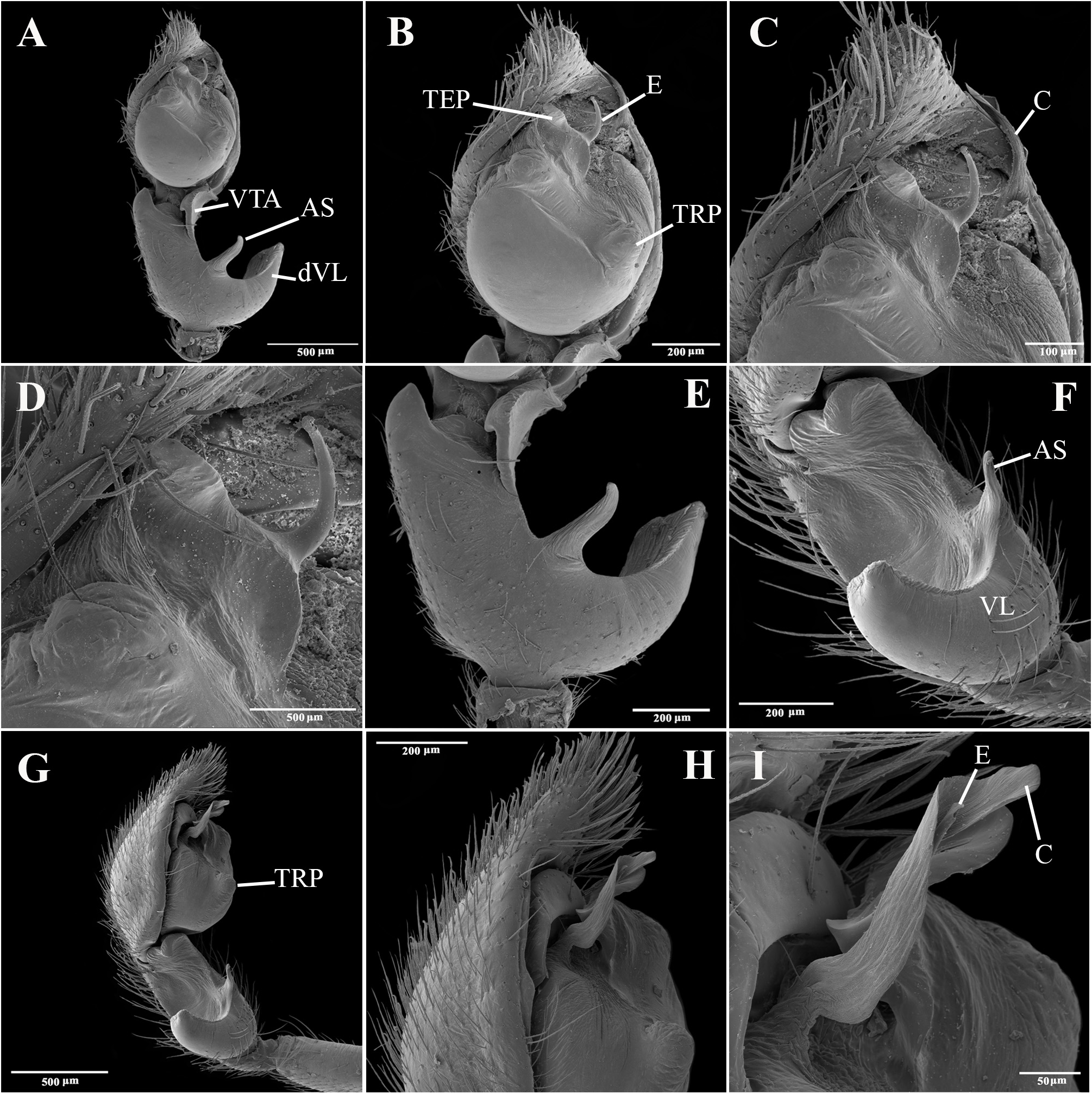
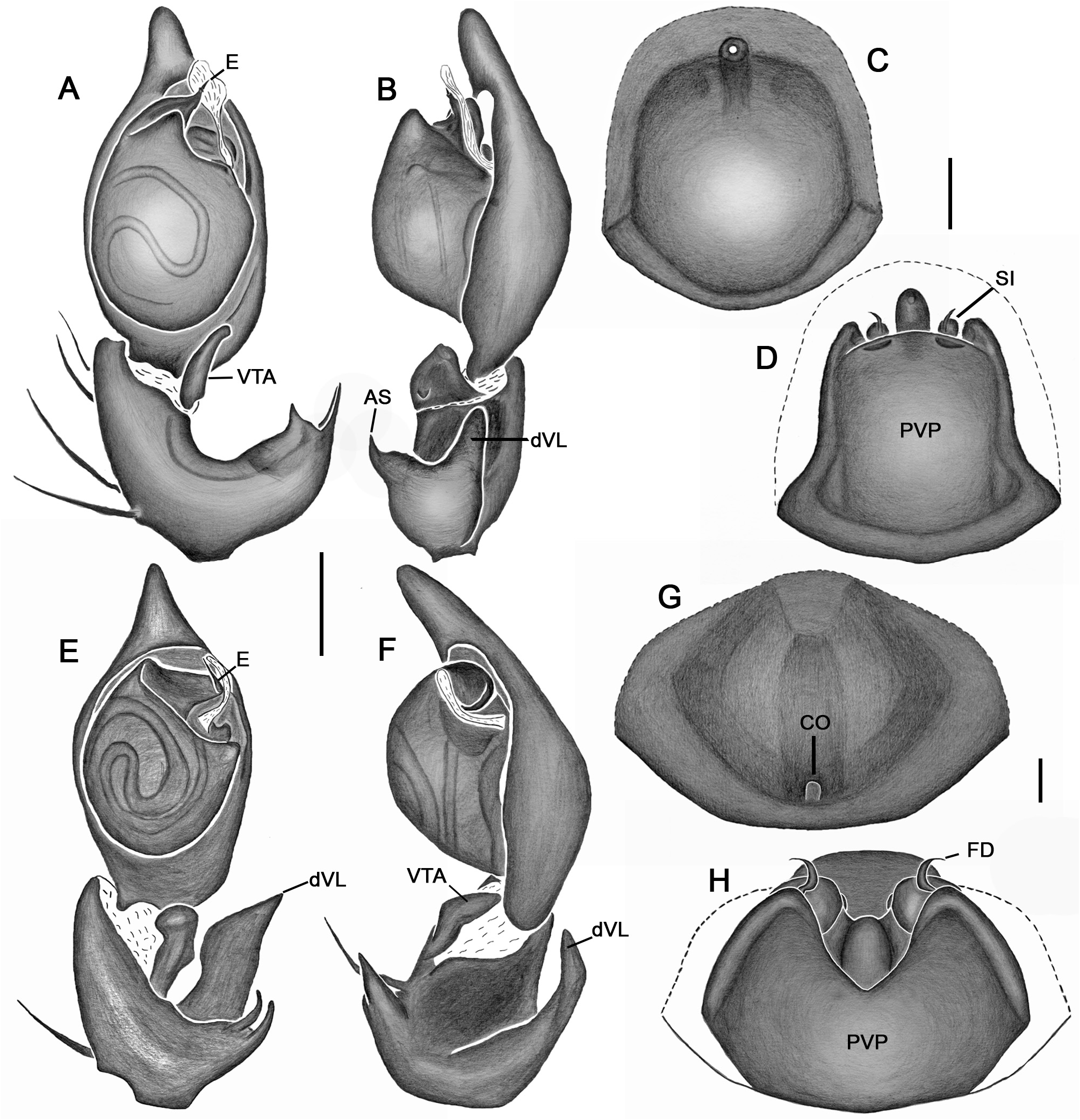
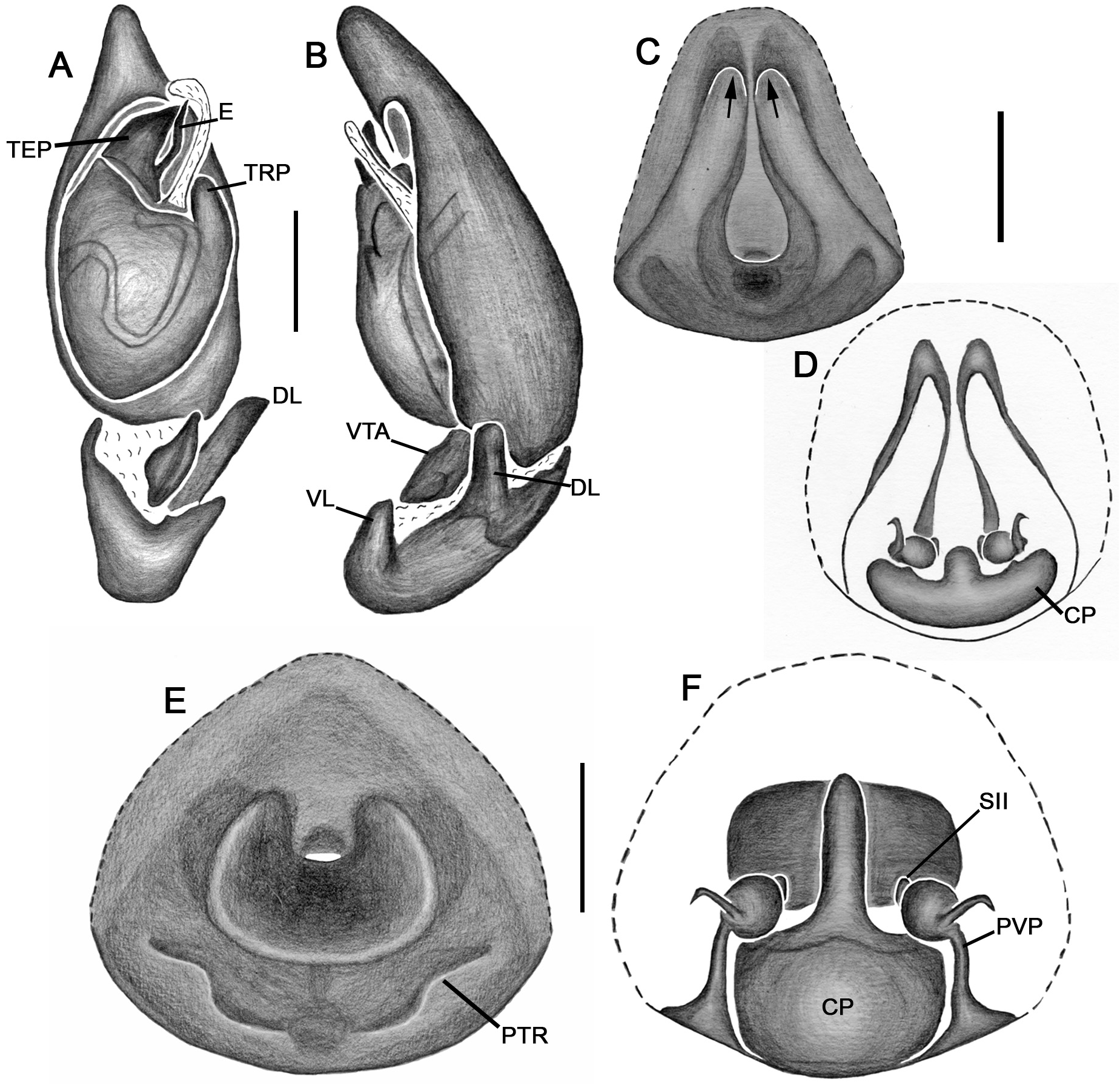
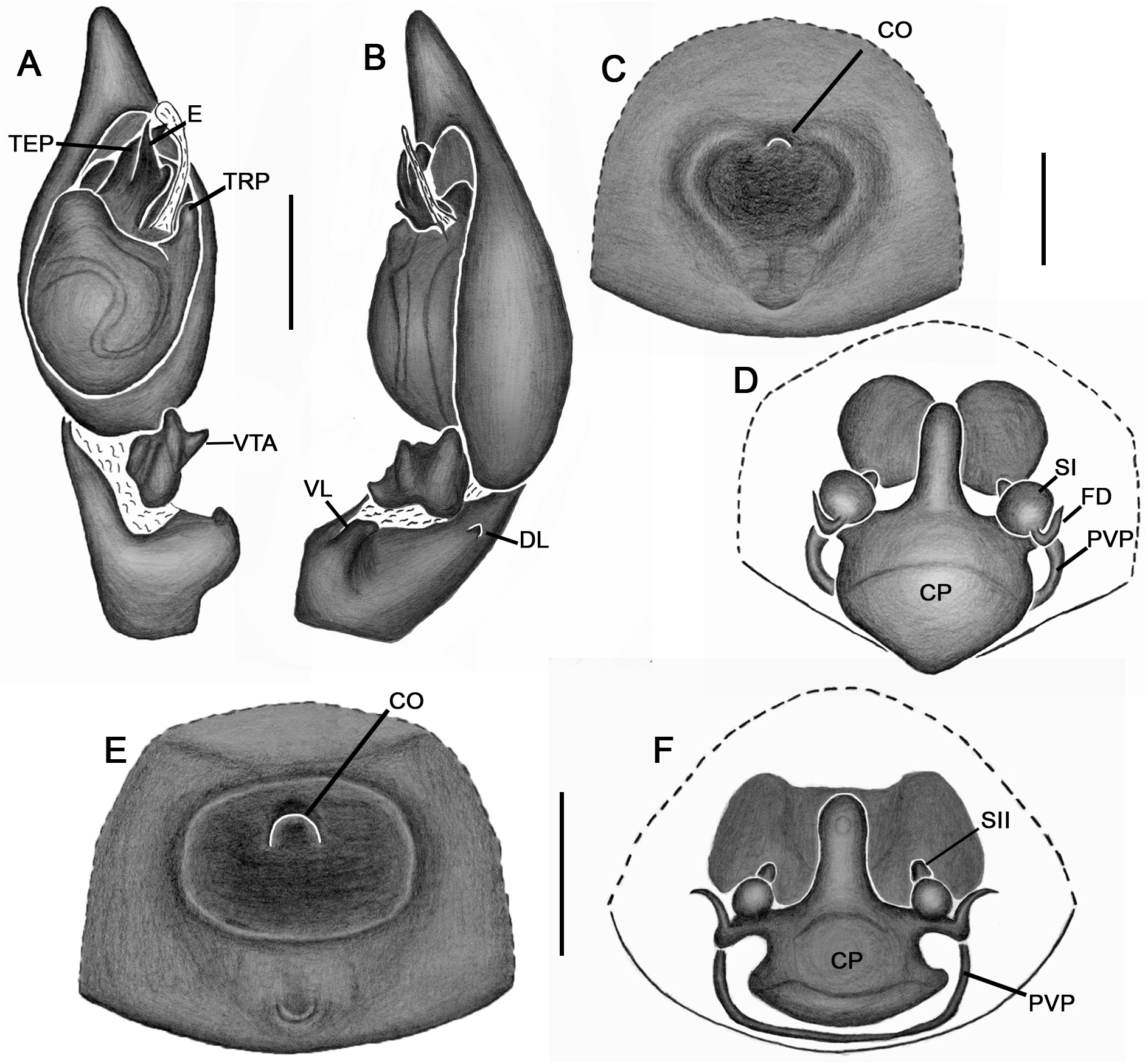
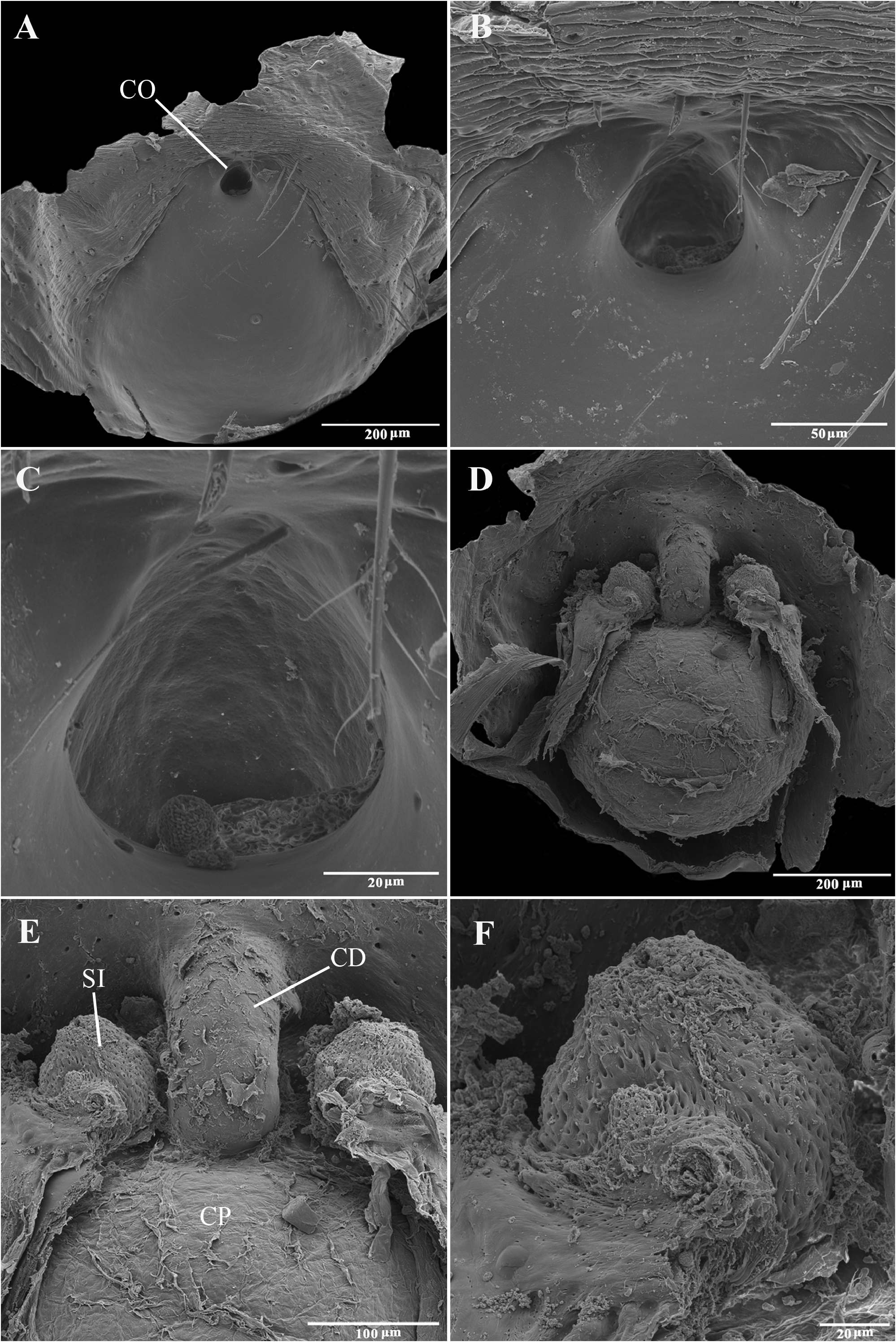
Emended diagnosis. Species of Tupirinna share with those of Stethorrhagus and Parachemmis the presence of anterior external excavations on the sternum in both sexes ( Figs 21A View FIGURE 21 , 22G View FIGURE 22 ) and an exclusive ventral tibial apophysis in the male palp (VTA in Figs 19C, 19F View FIGURE 19 , 20D View FIGURE 20 ). Tupirinna differs from both these genera by the following combination of characters: carapace with three longitudinal stripes of contrasting coloration, two marginal and one median ( Figs 1 View FIGURE 1 , 2A–B View FIGURE 2 ); chelicerae sexually dimorphic, longer in males, reaching about 2/3 the length of the carapace, with fangs nearly 2/3 the length of the paturon ( Fig. 22D View FIGURE 22 ); male palpal tegulum weakly sclerotized, spermophore slightly spiraled; embolus short, apex simple, generally with a curved elongated basal process (TEP) ( Figs 8B View FIGURE 8 , 16A View FIGURE 16 , 17A View FIGURE 17 , 20C View FIGURE 20 ); epigynum with one copulatory opening, vulva with enlarged copulatory duct forming a copulatory pouch ( Figs 9G, H View FIGURE 9 , 10E, F View FIGURE 10 , 11G, H View FIGURE 11 ).
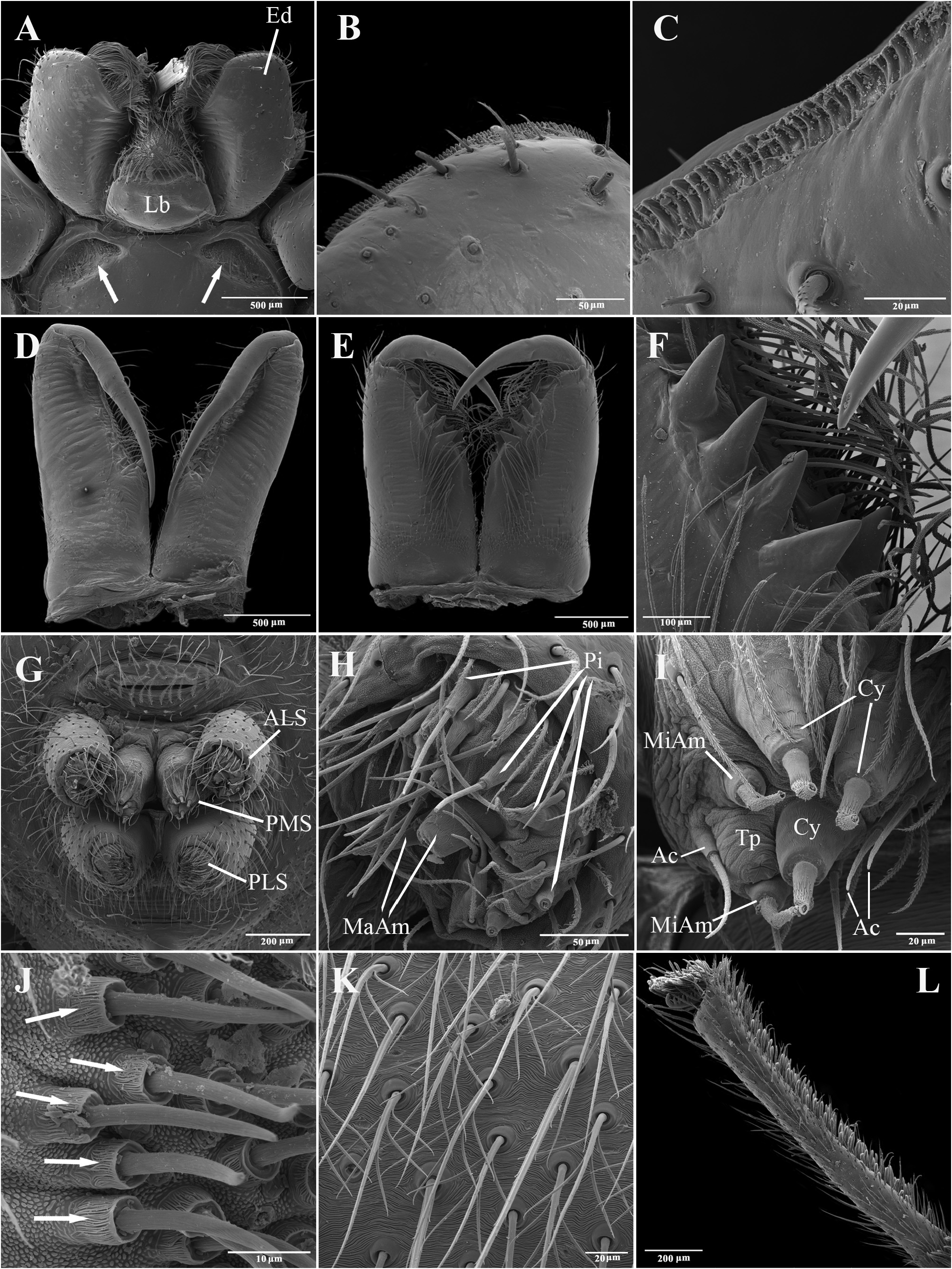
Description. Total lengths (males and females) 3.75–6.90. Carapace with three stripes of contrasting coloration, two marginal, edge-curved ones and an additional median, longitudinal one; suboval, longer than wide in dorsal view ( Fig. 1 View FIGURE 1 ); covered by short black hairs and few erect setae, more abundant in ocular region; with fine granulations, visible only through SEM ( Fig. 22A, B View FIGURE 22 ); largest width between coxae II and III, maximum height at palpal insertion. Cephalic region low, poorly delimited, cephalic constriction slightly pronounced; anterior margin nearly straight, median anterior interocular tubercle undeveloped; thoracic region abruptly recessed in lateral view. Short, deep thoracic groove, smaller in length than MOQ; posterior margin nearly straight. Clypeus high, about two times AME diameter; clypeal groove present. Posterior eye row strongly procurved in dorsal view; MOQ as wide as long in dorsal view, anterior width approximately equal to posterior width ( Fig. 21A, B View FIGURE 21 ). Median eyes rounded, lateral eyes suboval; all eyes subequal in size. Interdistances: AME-AME separated by half their diameter; AME-ALE by about one third of an AME diameter; PME-PME by about one and a half diameters; PME-PLE by slightly more than one diameter; ALE-PLE almost contiguous. Chilum entire, smooth, glabrous. Chelicerae with accentuated sexual dimorphism; in females, short, less than one-third of carapace length, slightly geniculate; in males, elongated, about two-thirds of carapace length, strongly geniculate ( Fig. 21D, E View FIGURE 21 ); basal condile triangular, conspicuous; cheliceral frontal surface smooth, without granules; retrolateral surface with inconspicuous transverse ridges ( Fig. 22D, E View FIGURE 22 ); fangs longer in males than in females, except in T. zebra sp. nov.. Endites convergent, promargin substraight, retromargin deeply notched ( Fig. 21A View FIGURE 21 ). Labium wider than long, less than half endite lengths ( Fig. 21A View FIGURE 21 ). Sternum approximately as long as wide, with long hairs not inserted in tubercles; sides of anterior margin projected; anterior sternal excavations present, deep ( Figs 21A View FIGURE 21 , 22G View FIGURE 22 ). Legs long, thin, covered by simple and feathery hairs; leg IV longer than others, leg I, II and III of sub-equal lengths ( Fig. 1 View FIGURE 1 ). All coxae with hairs not inserted in tubercles; tibia I with three to four pairs of ventral macrosetae; metatarsus I with two pairs of ventral macrosetae. Scopular setae of tarsi I and II sparse ( Fig. 21L View FIGURE 21 ); III and IV replaced by spiniform setae; metatarsus with no scopular setae, with inconspicuous ventro-distal setal tuft; tarsal claws with four to five large teeth; subungueal hairs dense; tarsal trichobothria in two dorsal rows; female palpal tarsal claw smooth; trochanters I and II slightly notched; III and IV notched. Abdomen with abundant simple and feathery hairs ( Fig. 21K View FIGURE 21 ), without erectile setae; dorsal and ventral scutum absent in both sexes; tracheal tubercle present. Colulus represented by a sub-triangular plate with few simple hairs. Spinnerets (surveyed only in female of T. caraca sp. nov. ( Fig. 21G–J View FIGURE 21 ). ALS ( Fig. 21G, H View FIGURE 21 ) with nearly 15 piriform spigots and two major ampullate spigots; PMS ( Fig. 21G, I View FIGURE 21 ) with two or three aciniform spigots, two minor ampullate spigots plus large, protruded tartipore and three large cylindrical spigots; PLS ( Fig. 21G, J View FIGURE 21 ) with multiple aciniform spigots and two cylindrical spigots.
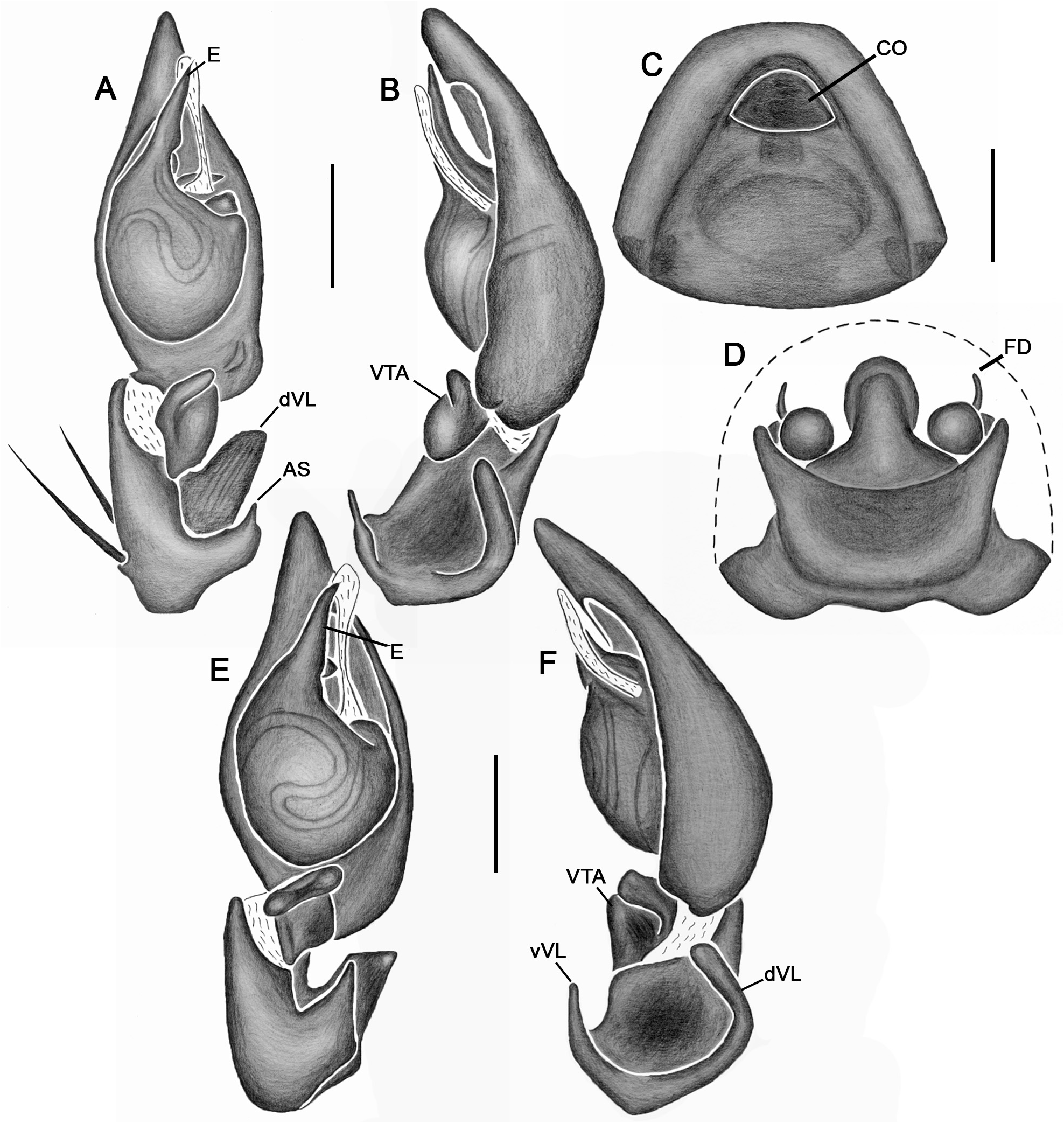
Male palp. Tibia long, longer than half of cymbial length or as long as cymbium; ventral lobe of retrolateral tibial apophysis (VL) always present, with or without apical spur; dorsal lobe absent in species of the rosae group. Ventral tibial apophysis (VTA) present, not fused retrolaterally to tibial margin. Cymbial retrolateral basal process weakly developed. Cymbial prolateral basal process absent. Tegulum wide, weakly sclerotized, spermophore weakly spiralled, with two to three wide, ventral S-shaped folds; conductor hyaline; embolus fused to tegulum, spiniform, short or relatively long, generally with basal process (absent in T. platnicki sp. nov., T. regiae sp. nov., T. mutum sp. nov., T. gigantea sp. nov. and T. araguaia sp. nov.).
Epigynum not projected over epigastric furrow, with one copulatory opening, generally anteriorly positioned (posteriorly positioned only in T. una sp. nov. and T. gigantea sp. nov.); posterior vulval plate well developed in rosae group ( Figs 9D, H View FIGURE 9 , 10D, F View FIGURE 10 , 11D, H View FIGURE 11 , 12D, F View FIGURE 12 , 13D View FIGURE 13 , 14D, H View FIGURE 14 ), weakly developed in trilineata group ( Figs 15D View FIGURE 15 , 16D, H View FIGURE 16 , 17H View FIGURE 17 , 18F View FIGURE 18 ). Copulatory duct expanded medially, forming copulatory pouch. In species with developed posterior vulval plate, copulatory pouch membranous; in species with undeveloped posterior vulval plate, copulatory pouch sclerotized; primary spermathecae globular; secondary spermathecae generally absent, but present and small in most species of trilineata group ( T. albofasciata , T. caraca sp. nov., T. lata sp. nov., T. cruzes sp. nov., T. palmares sp. nov., T. una sp. nov. and T. oba sp. nov.; Figs 14G, H View FIGURE 14 , 15C, D, 15G, H View FIGURE 15 , 16C, D, G, H View FIGURE 16 , 17C, D, G, H View FIGURE 17 , 20I View FIGURE 20 ).
Natural history. Most of the examined specimens were collected with pitfall traps, while a few were collected by beating tray, which suggests that species of Tupirinna may inhabit both the leaf litter and vegetation ( Figs 2A–B View FIGURE 2 ). The typical color pattern of Tupirinna species may be an example of disruptive coloration (Cott 1957), which leads to conspicuous convergences between phylogenetically unrelated animals. For example, the color pattern presented by Tupirinna species is similar to that presented by species of the Castianeirinae genera Copa Simon, 1886 (see Haddad 2013a: figs 1–6), Ticopa Raven, 2015 (see Raven 2015: fig. 127d) and Copuetta Haddad, 2013 (see Haddad 2013b: figs 7–13), and even to that present in the genus Cambridgea (Desidae) , endemic to the New Zealand, especially Cambridgea plagiata Foster & Wilton, 1973 and C. quadromaculata Blest & Taylor, 1995 (see Blest & Taylor 1995: fig. 1).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Tupirinna Bonaldo, 2000
| Xavier, Cláudia & Bonaldo, Alexandre B. 2021 |
Tupirinna
| Bonaldo, A. B. 2000: 133 |