Stenopelmatus sartorianus Saussure
|
publication ID |
https://doi.org/10.11646/zootaxa.4917.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:D89148CE-EE8A-46B8-8D8B-8F5790063FC4 |
|
DOI |
https://doi.org/10.5281/zenodo.4475925 |
|
persistent identifier |
https://treatment.plazi.org/id/03A4C420-8A24-FB11-9B84-22991E97F834 |
|
treatment provided by |
Plazi |
|
scientific name |
Stenopelmatus sartorianus Saussure |
| status |
|
Stenopelmatus sartorianus Saussure View in CoL
Winged Jerusalem Cricket
Figs 143–157 View FIGURE 143 View FIGURE 144 View FIGURE 145 View FIGURE 146 View FIGURE 147 View FIGURE 148 View FIGURE 149 View FIGURE 150 View FIGURE 151 View FIGURE 152 View FIGURE 153 View FIGURE 154 View FIGURE 155 View FIGURE 156 View FIGURE 157 , Table 1 View TABLE , 2 View TABLE 2
1859. Stenopelmatus sartorianus . Revue et Magasin de Zoologie 2(11): 211.
1932. Stenopelmatus sumichrasti . Hebard, Trans. Amer. Entomol. Soc. 58(3): 343
1945. Stenopelmatus sartorianus . Strohecker, Ann. Ent. Soc. Amer. 38(2): 207
1988. Stenopelmatopterus sartorianus Gorochov, Zoologicheskii zhurnal 67(4): 521
Distribution. The most widespread species of Stenopelmatus , known from Veracruz state, Mexico, south into Panama.
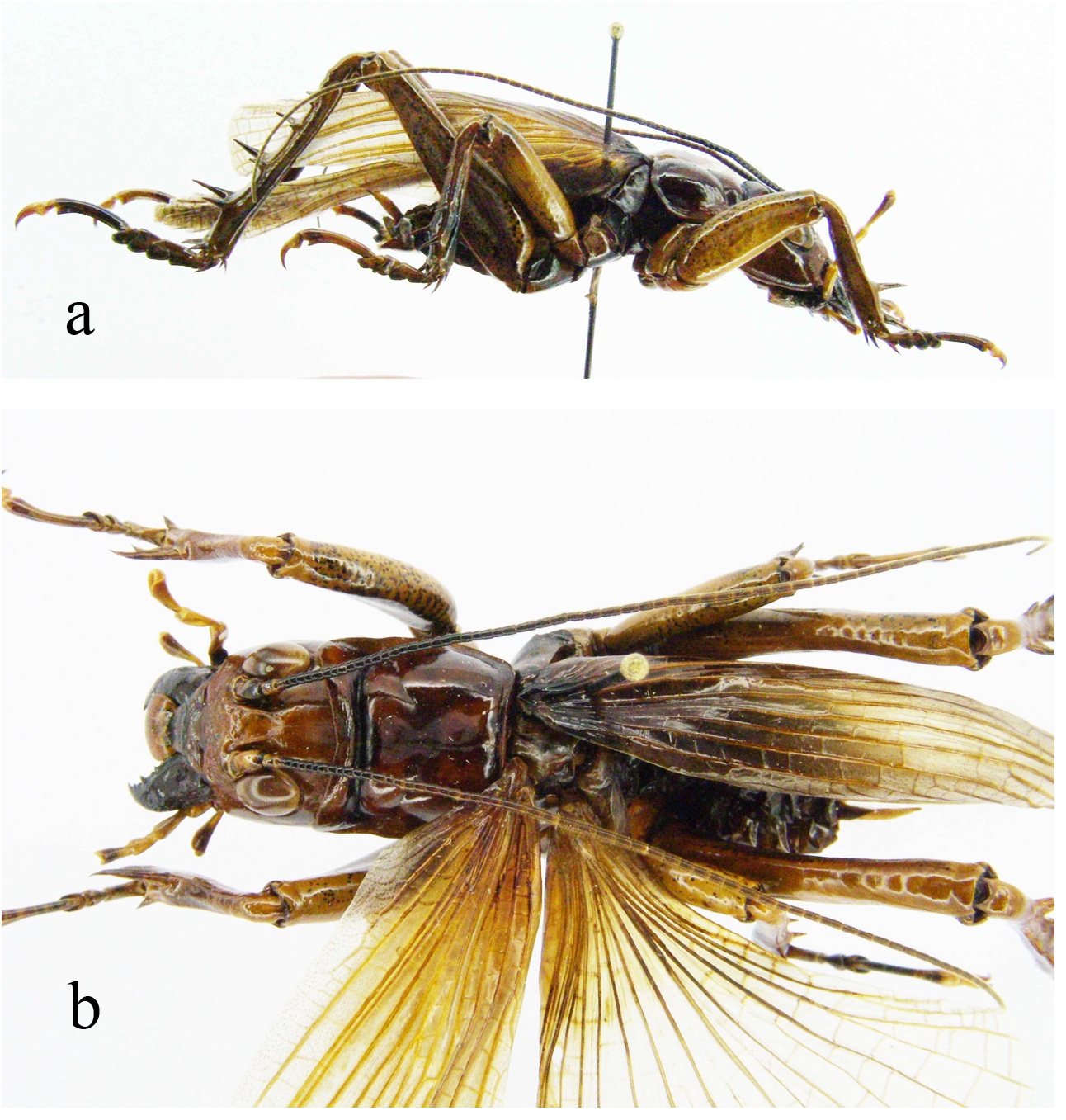
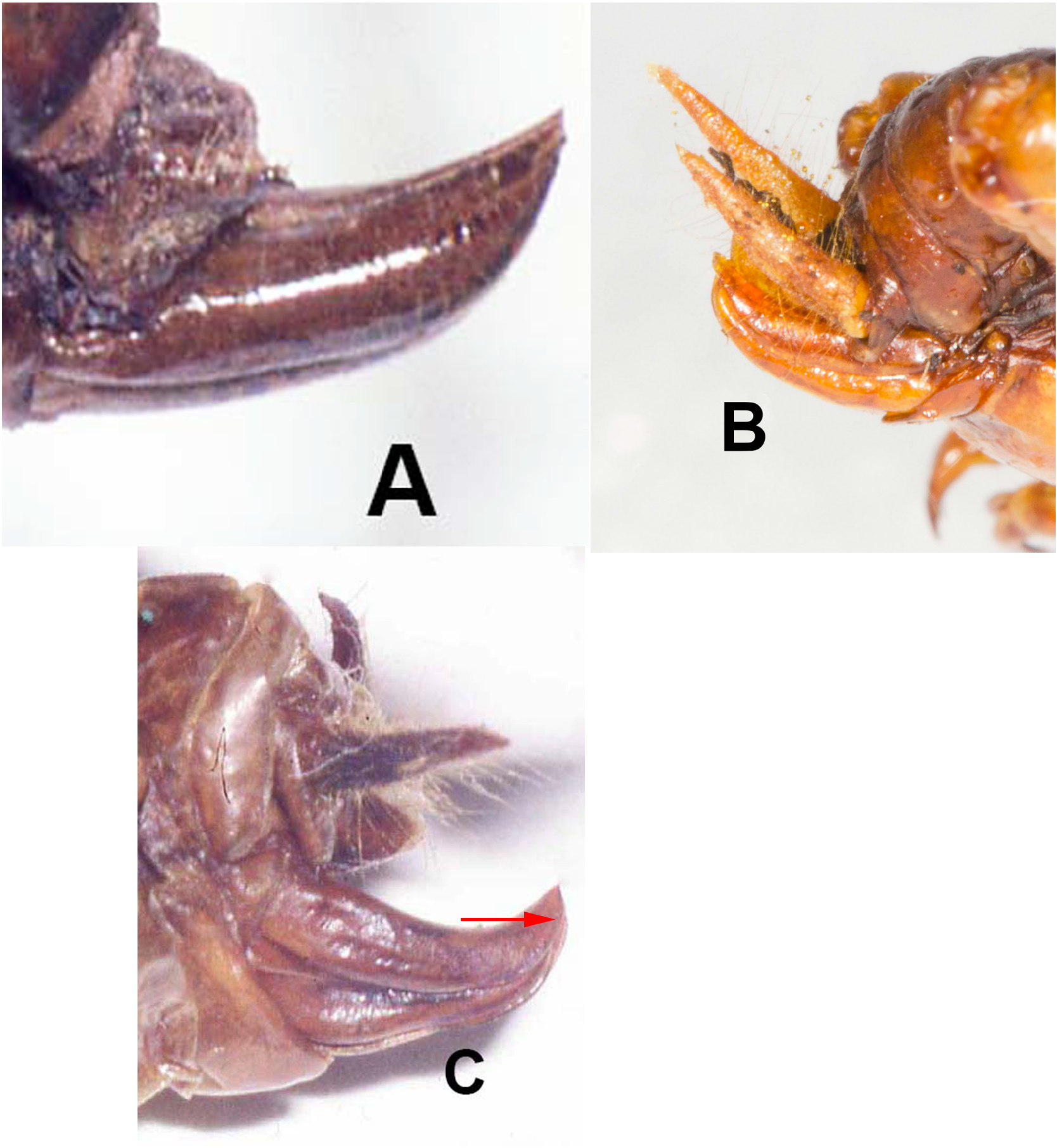
Recognition characters. Stenopelmatus sartorianus is the only species of New World Jerusalem cricket whose adults have fully developed front and hind wings. In all 15 adults examined, hind wings longer ( Fig. 144 View FIGURE 144 ) than tegmina in situ. Three other species, S. chiapas , S. piceiventris , and S. sanfelipe all have micropterous fore wings as adults, and, at least in the case of the first two, in apparently all early instars. There are no concealed, hind wings, in any instar, under the tegmina of these 3 latter taxa. The dense area of short hairs (see, for example, Fig. 134 View FIGURE 134 , p. 82 View FIGURE 82 ), as seen under the wings of S. chiapas , S. piceiventris and S. sanfelipe , is missing in S. sartorianus .
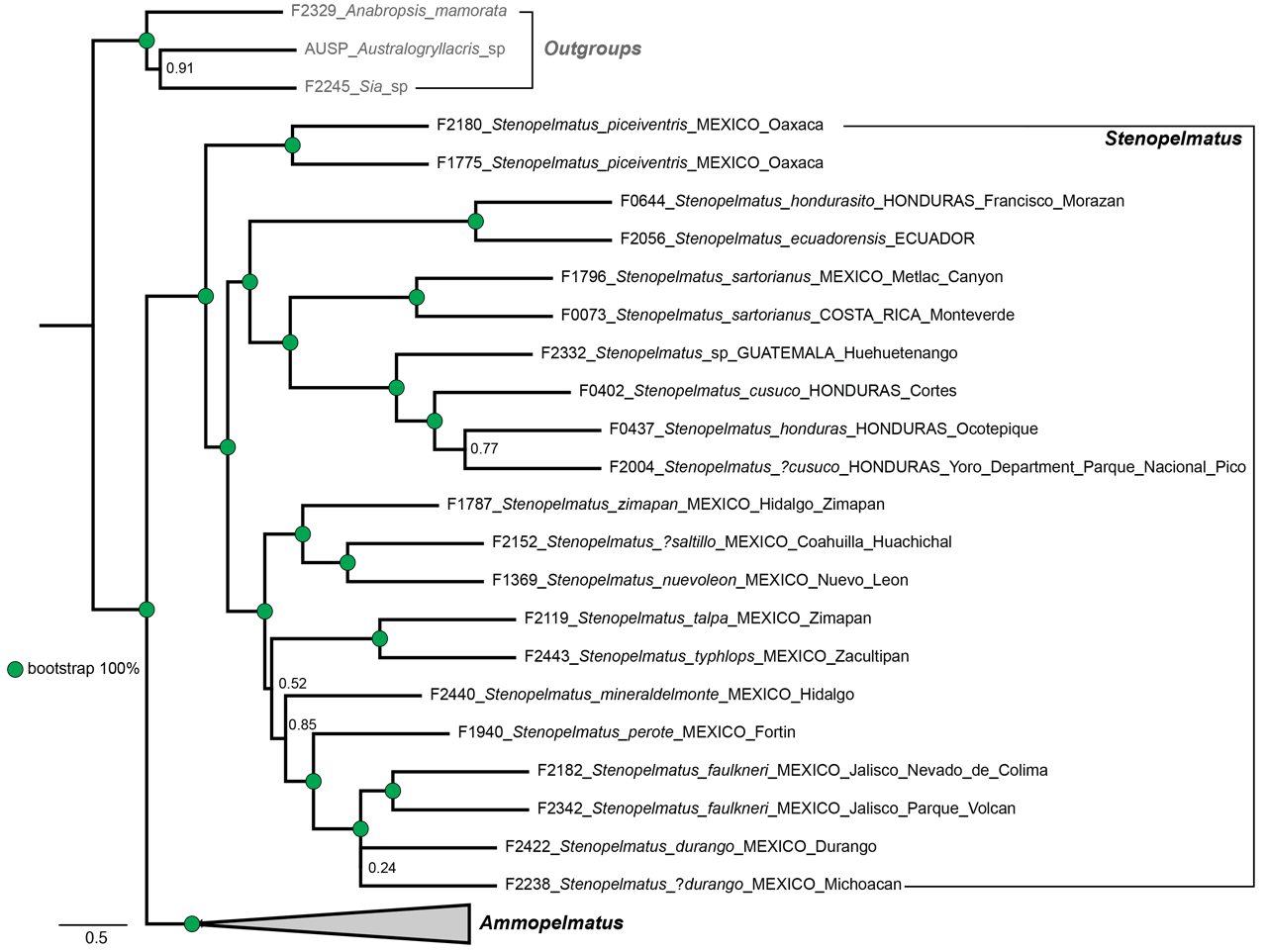
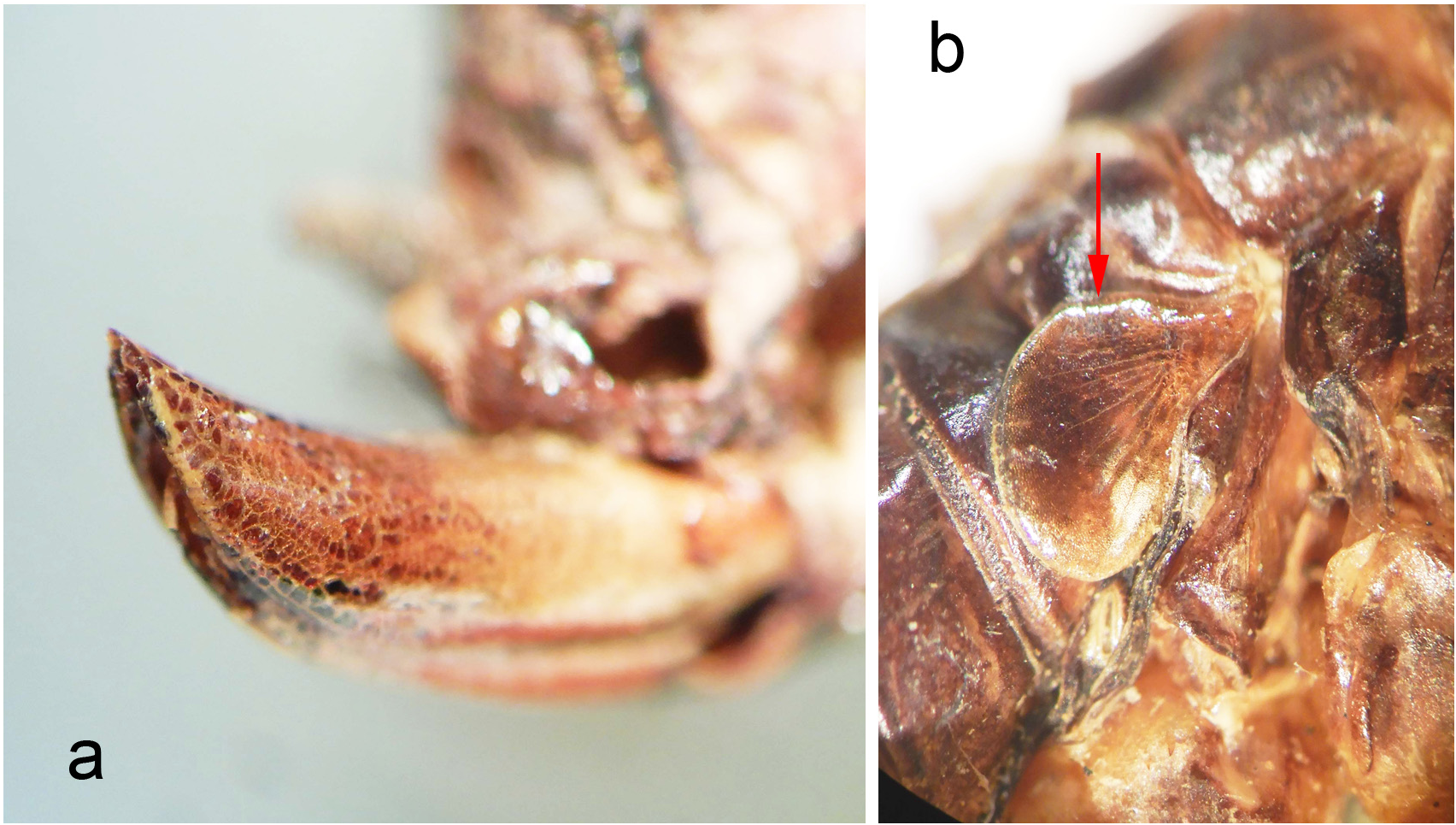
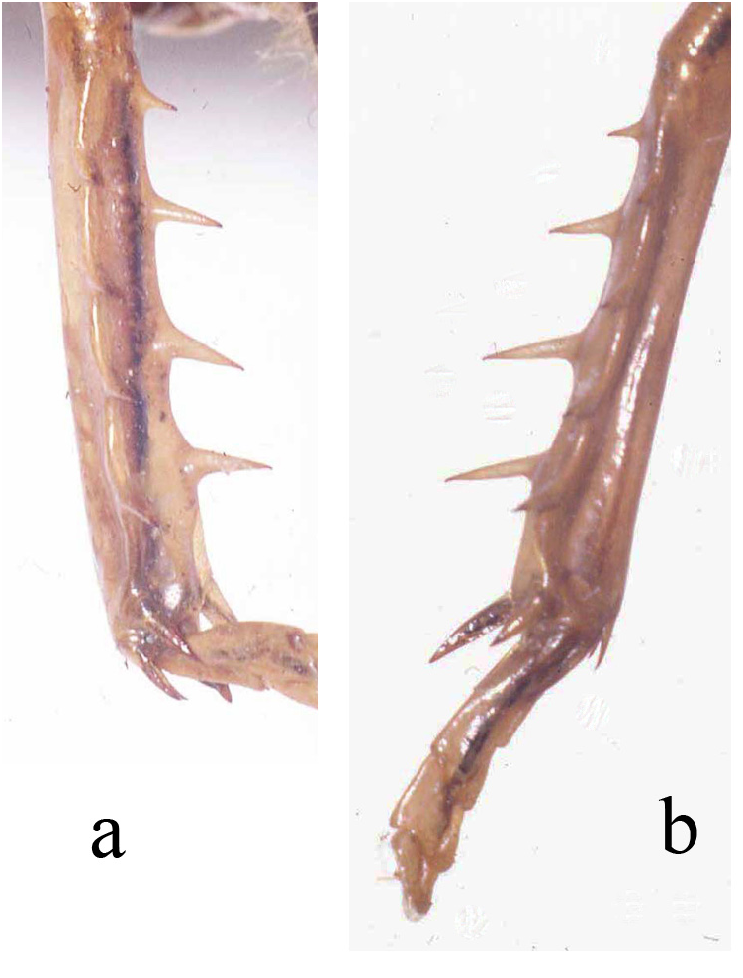
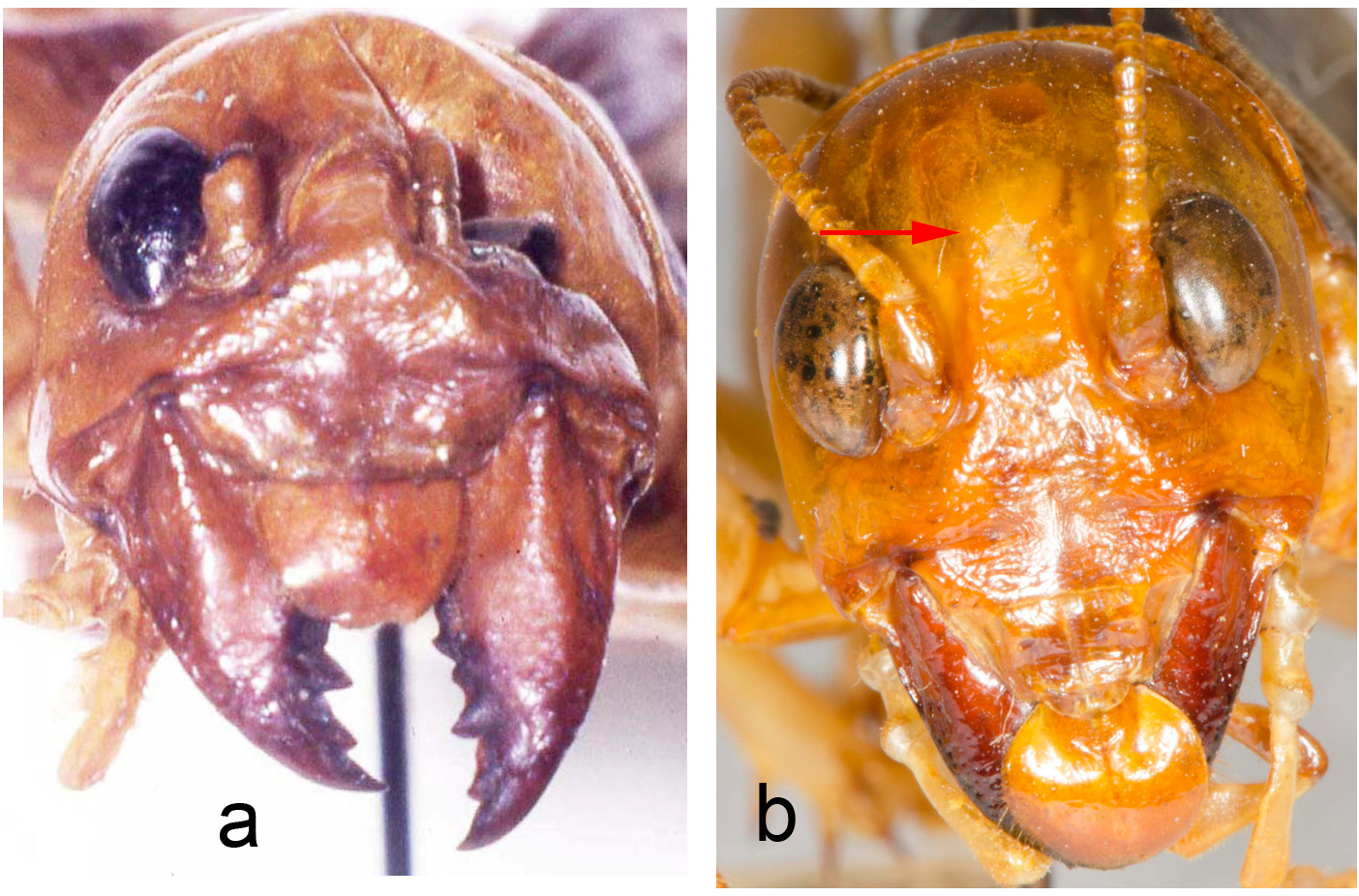
Holotype. Adult female ( Fig. 145 View FIGURE 145 , with fully formed front and hind wings, right side spread). (1) Anostostoma sartorii Sauss ♀ Tuxtla [Gutierrez, state of Chiapas, Mexico], M. H. S. (2) green label Stenopelmatus sartorianus Sauss. (3) green label 71 (4) red label Holotypus Stenopelmatus sartorianus Saussure. Measurement in mm: Body length 35.9, including ovipositor, hind femur length 14, hind femur width 5.1, tegmina 17.8 and almost reaching tip of abdomen. Left hind leg tibia ( Fig. 146 View FIGURE 146 ) with 4 inner and 4 outer spines. Face with apparent furrow although distorted by damage ( Fig. 147 View FIGURE 147 ). Ovipositor upturned ( Fig. 153 View FIGURE 153 ). Deposited MHNG Geneva. New status: transferred back to Stenopelmatus .
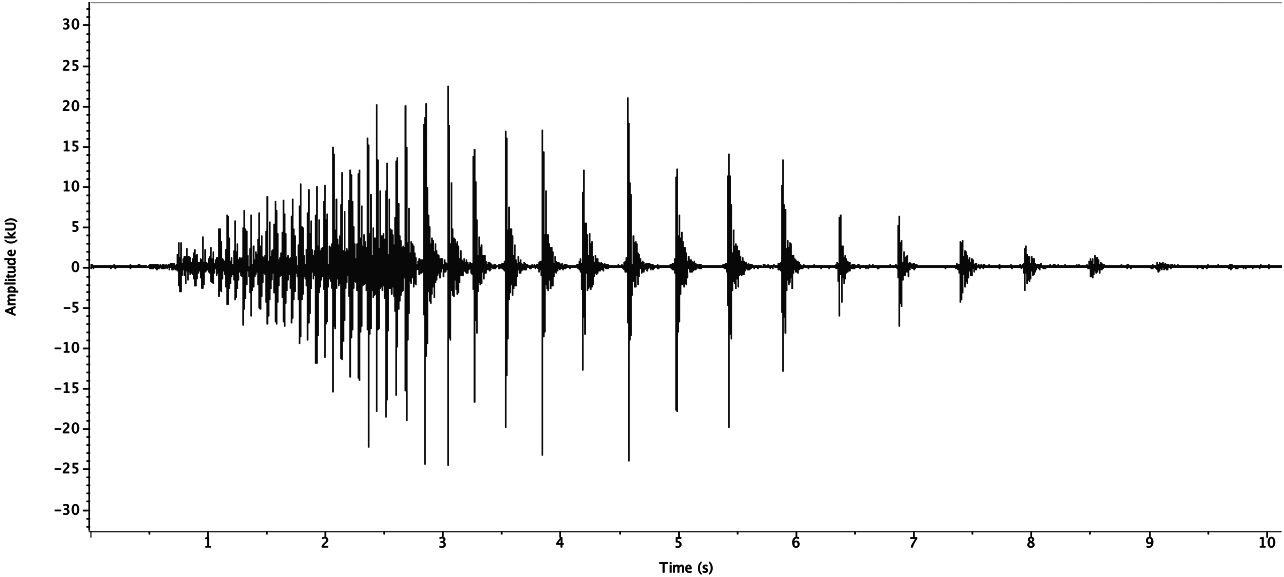
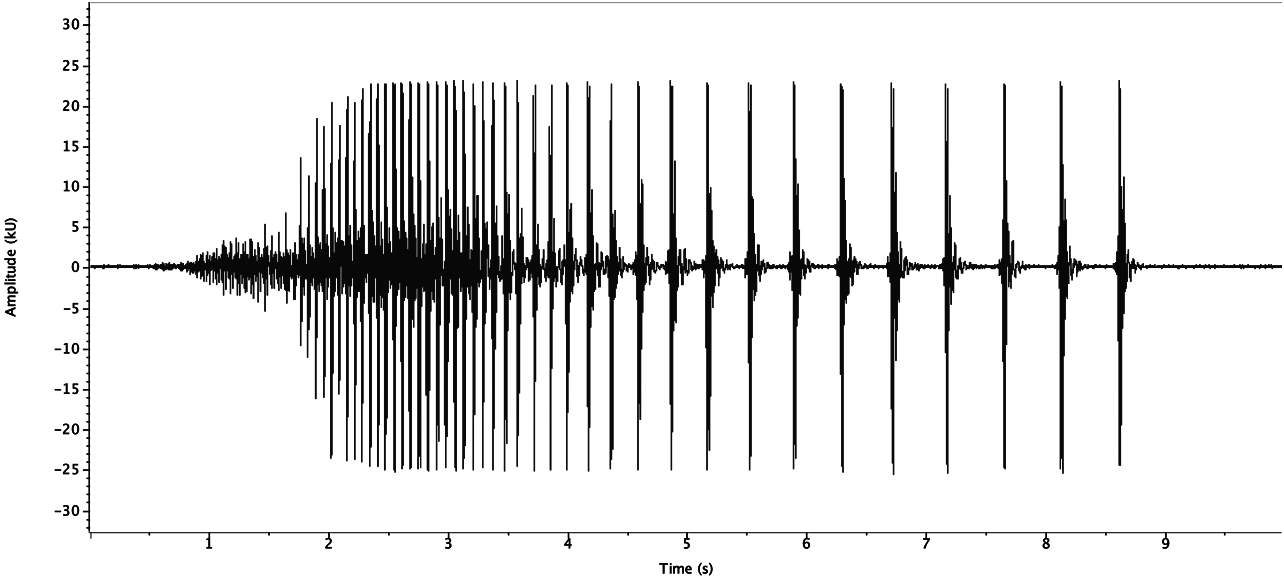
Drum. Both sexes from Mexico, Veracruz state, Metlac Canyon (S06-39) drummed similarly. Adult male (R06- 12) had one series ( Fig. 148 View FIGURE 148 ) recorded at 20.5°C, in 2:40 of recording time: 26 fast drums over 1.9s (13.7 d/s) followed by 15 slow drums over 5.8s (2.6 d/s). Adult female (R06-13) had one series ( Fig. 149 View FIGURE 149 ), similar to male R06-12, recorded at 23°C, in 4:15 hours: 39 fast drums over 2.8s (13.9 d/s) followed by 14 slow drums over 4.7s (3.0 d/s).
Derivation of name. “sartor” is Latin for tailor, patcher, maybe in reference to the holotype’s long wings and being “well dressed?”
Habitat. Riparian forest ( Fig. 150 View FIGURE 150 ) along the Rio Metlac (S06-39). Cloud forests at Monteverde, Costa Rica.
Behavior. All instars and adults jumped well (see Discussion below). Heard one mid instar female from Monteverde, Costa Rica (S00-21) femoral-abdominal stridulate. During mating trials (see Discussion below), an adult female femoral-abdominal stridulated while the male tremulated. All instars fed well on wax worm larvae.
Is this species an obligate predator? At Metlac Canyon, we put out a nighttime 16 kg long oatmeal trail, especially in the area where we collected the 4 late instars during the daytime. While the oatmeal attracted many other Orthoptera , such as Anabropsis, Glaphyrosoma, Anurogryllus, and Gryllus , no JCs were attracted, including no specimens of a second, sympatric, medium-large, jumping black JC that was also in a log during the daytime and that we discuss on p. 117.
Variation. Table 1 View TABLE . Adult female Monteverde (S00-21) with 8 left rear leg inner tibial spines ( Fig. 152 View FIGURE 152 ). This same female had the normal 4 inner rear leg tibial spines on her right side. This left rear tibia was almost 3.5 mm shorter than the right side and indicates, we believe, physical damage of some kind during an earlier instar, with subsequent regeneration (discussed in Weissman 2001a) during succeeding molts.
Specimens examined. Costa Rica: Puntarenas, Monteverde, 10° 18’ 01” -84° 47’ 47”, 16-vi-1995 (S95-48) ♀ 2, CAS ; 26-vi-1996 (S96-71) Ƌ2, CAS ; 14-vii-2000 (S00-21) ♀ 1, CAS . Lo. Palma, P Biolley, no date, 1 adult female. Cangrejal de Asseri , 800m, iv-1906, P. Biolley, 1 last instar female . Guatemala: Huehuetenango Paraiso Municipio La Libertad (~ 5 km E. Hojas Blancas ) 1650-1850m (forest, under logs) 29-vi-1966. LC Stuart, 3 late (last?) females with minimal lateral carinae on furrows, UMMZ . Las Mercedes , 3000 ft., Champion, Brit. Mus. 1899-235, BMNH. Other Guatemala nymphs in BMNH from Capetillo and El Tumbador . Mexico: Veracruz, Cualcuapan, between Orizaba & Cordoba, 3800’, 11-vii-1938, E.H. Taylor, adult ♀ 3, adult Ƌ1, ANSP ; same data, 18- vii-1938, E.H. Taylor, adult Ƌ1, ANSP . Metlac Canyon, 18° 54’ 30.78” -97° 00’ 44”, 3200’, 20-vi-2006 (S06-39), Ƌ1, ♀ 3, all late instars, DBW, DC Lightfoot, CAS ( See Ball & Whitehead (1967) for more information on Metlac Canyon ) . Portrero Viejo , 13-viii-1941, Mrs. Forbes + E.H. Taylor, adult Ƌ1, ANSP ; Chiapas, 8.0 m SE Las Casas ( San Cristobal ), 11-iv-1941, I. Cantrall, adult ♀ 1 (very small: body length 22.9, hind femur length 8.03, hind femur width 2.53. furrow only with distinct lateral carinae- not connected at top), UMMZ . Two additional Mexico records are cited in Gorochov & Cadena-Castañeda (2016).
DNA. Nuclear F0073 (S95-48, Monteverde, Costa Rica) and F1796 (S06-39, Metlac Canyon, Mexico) both recovered together ( Fig. 10 View FIGURE 10 ), and well nested, within Stenopelmatus . The same 2 individuals, along with F1769, also from Metlac Canyon, all recovered together ( Fig. 9 View FIGURE 9 ) for mtDNA. To have left this species in Stenopelmatopterus would have resulted in Stenopelmatus being paraphyletic.
Karyotype. Unknown.
Discussion.
Systematics. Saussure (1859) described 3 Mexican taxa, in one publication, that apparently represent the same species: S. sallei on p. 210, S. sumichastri also on p. 210, and S. sartorianus on p. 211. Elsewhere in this paper, we designate the names of S. sallei (p. 76, a juvenile female) and S. sumichrasti (p. 95, a juvenile male) as nomen dubium because both types are subadults without precise locality data, and such action is consistent with our attempts to modernize the prior taxonomy of this genus. While S. sallei has line priority if we chose to retain that ambiguous name, the type of S. sartorianus is an undisputed adult female with a good type locality, so we follow the suggested action of Strohecker (1945) and retain only that name. Junior synonym S. politus was described from an adult female by Walker, in 1869, from Orizaba, which, like Metlac Canyon (S06-39), is also in the Mexican state of Veracruz. Thus, from Metlac Canyon individuals, we have drums in both sexes, DNA, a small series of adults, mating observations, and habitat information which will now form the basis for further taxonomic decisions and the possibility of new species descriptions in this most widely distributed taxon in Stenopelmatus .
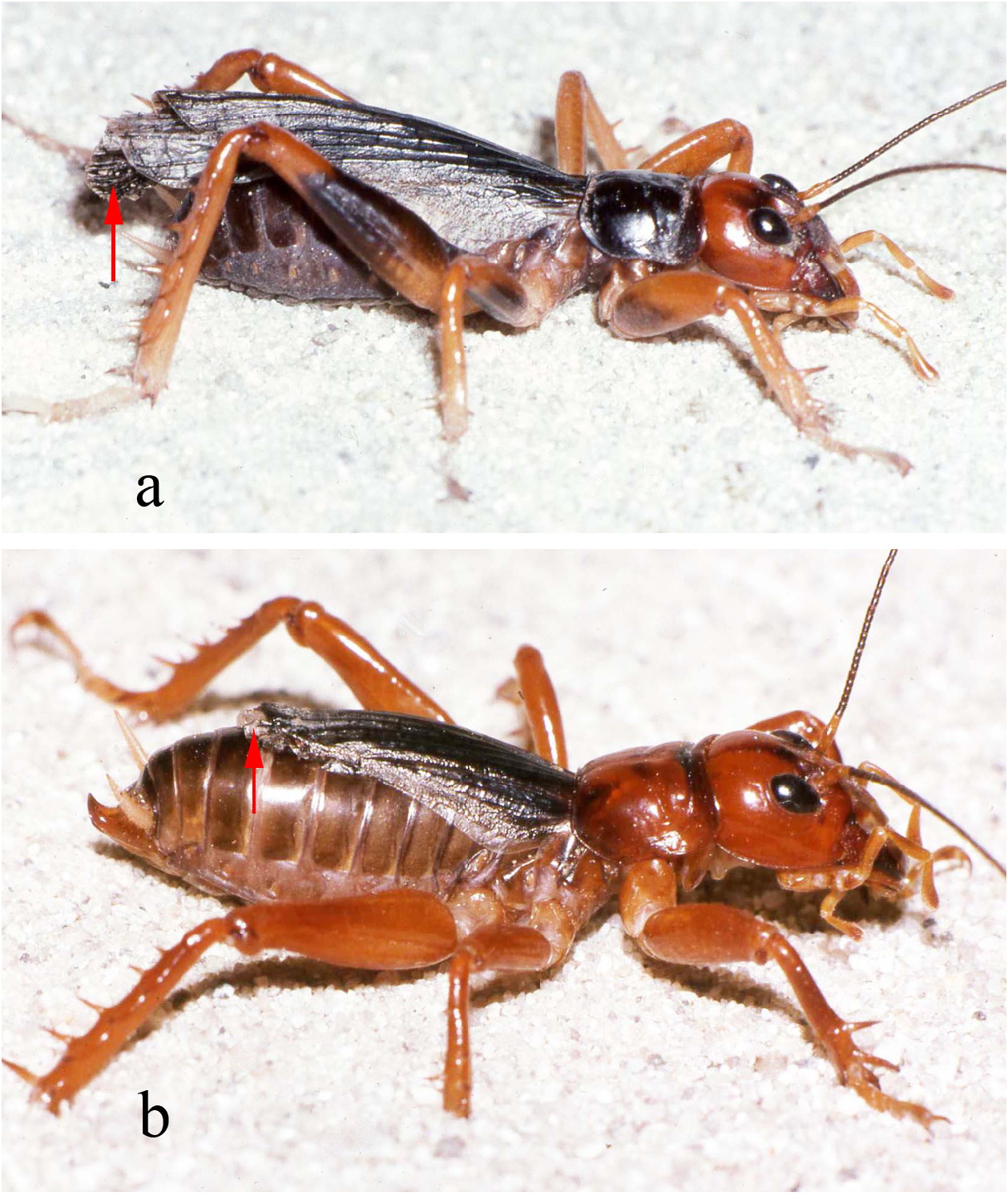
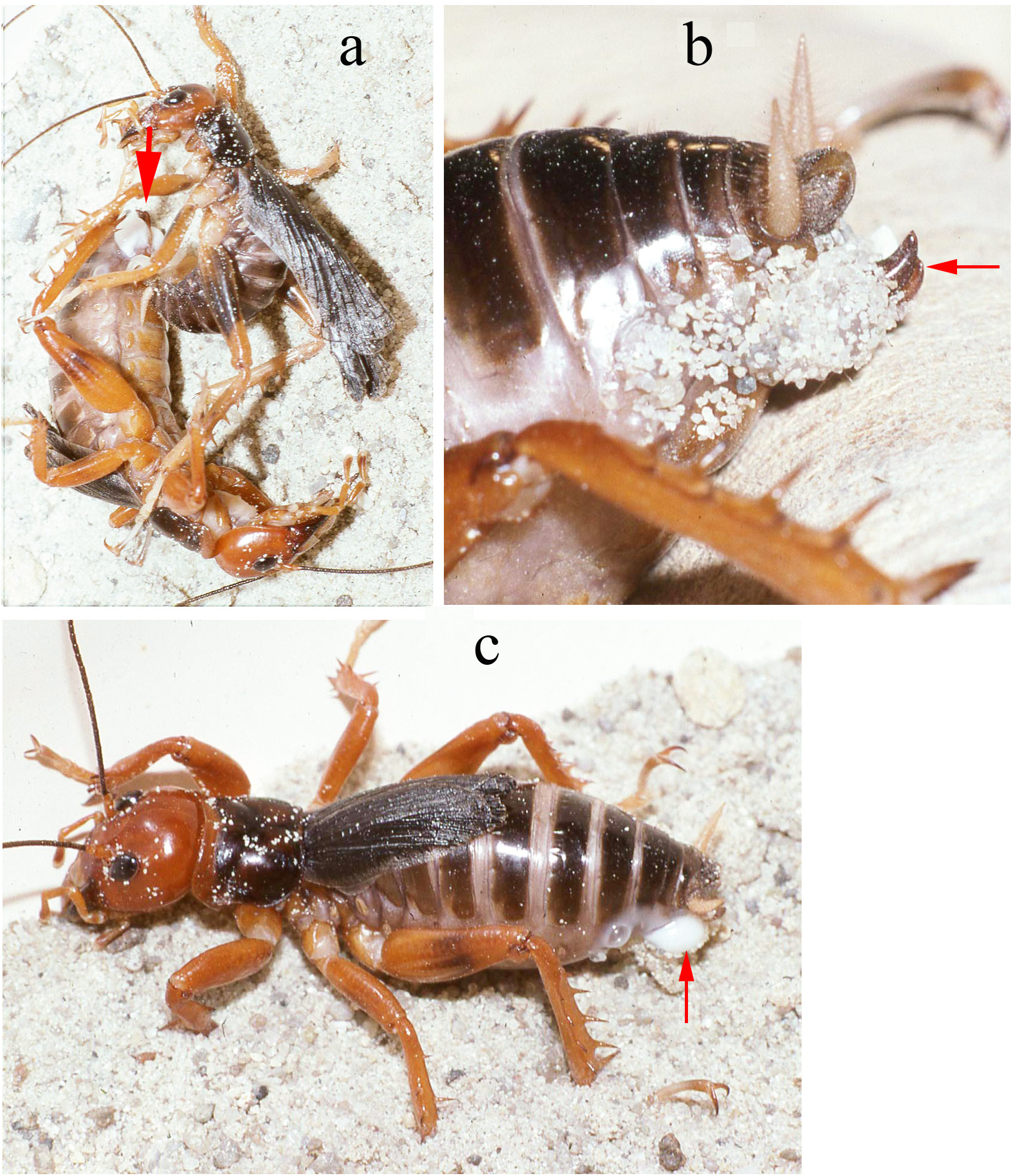
Past taxonomy in this species “complex” has been complicated by the surprising difficulty of discerning when females (and males in the case of S. sumichrasti ) are adult and for choosing females as holotypes. For instance, in 1859, Saussure illustrated a supposed adult female of S. sallei , an error repeated in 1897, by Saussure & Pictet. Hebard (1932) and Strohecker (1945) both recognized that this female of S. sallei was an immature that would have fully developed hind wings as an adult. When Hubbell (1960) examined this same female of S. sallei , he thought it an adult. In 1988 Gorochov erected his new genus Stenopelmatopterus to include 3 winged species: S. sallei , S. sumichrasti , and S. sartorianus . When DBW examined the holotype of S. sallei in 1999, he also thought that she was an adult female, based on her ovipositor (see Fig. 125 View FIGURE 125 , p. 78 View FIGURE ), and placed an appropriate label. Fortunately, after examination of a good series of S. sartorianus , of many different instars in the collections of ANSP, UMMZ, NHMUK, and MHNG Geneva, we can finally clarify this situation. First, one must distinguish between the 3 taxa ( S. piceiventris , S. sanfelipe , and S. chiapas ) with micropterous fore wings (and no hind wing) versus subadult specimens of S. sartorianus that have pad-like tegmina ( Figs. 124 View FIGURE 124 & 125 View FIGURE 125 ) in earlier instars, but which also have hind wing pads under those pad-like tegmina (confirmed for S. sallei , see p. 76). Second, once S. sartorianus reaches penultimate and last instar, then these hind wing pads are clearly visible ( Fig. 154 View FIGURE 154 ) without the need to lift the tegmina.
Flight attempts of winged adults. Winged adult male (S06-39). Observations made at 14 and 44 days after molt to adult, to ensure not teneral. Made under red and incandescent light. Male moved extremely fast, antennae always in motion, perhaps a wasp mimic? He was able to jump almost 45 cm laterally, without using wings. DBW threw him into air several times and although he clearly spread both his tegmina and hind wings, and maybe fluttered them a little, he still fell straight down onto padded surface. But outstretched wings might have slowed the rate of his descent. Blowing onto his face had no effect nor did he raise his wings when provoked. If he was up in a tree (postulated by Weissman & Lightfoot [2007] and confirmed by Gutiérrez-Rodríguez & Riverón [2018]), jumping would be a good escape mechanism. Winged adult female (S06-39): Jumped readily when disturbed or reached edge of desk. Could see her “cock” her rear legs and then spring. On flat surface, jumps exceeded 15 cm. When jumped off edge of desk, she extended wings as “parachutes” but still fell straight down. No flapping seen. Similar behavior if thrown into air. Another time, jumped from 30 cm above surface and landed a good 30 cm away. We conclude that while neither sex can fly, their wings could slow descent if they jump from a high elevation to escape a predator. Wings apparently not used to startle a predator. In any case, there must be a good reason to have such wings when residing deep inside logs during the daytime. We also never saw any fanning during courtship (see below) as might occur should the adults be releasing pheromones.
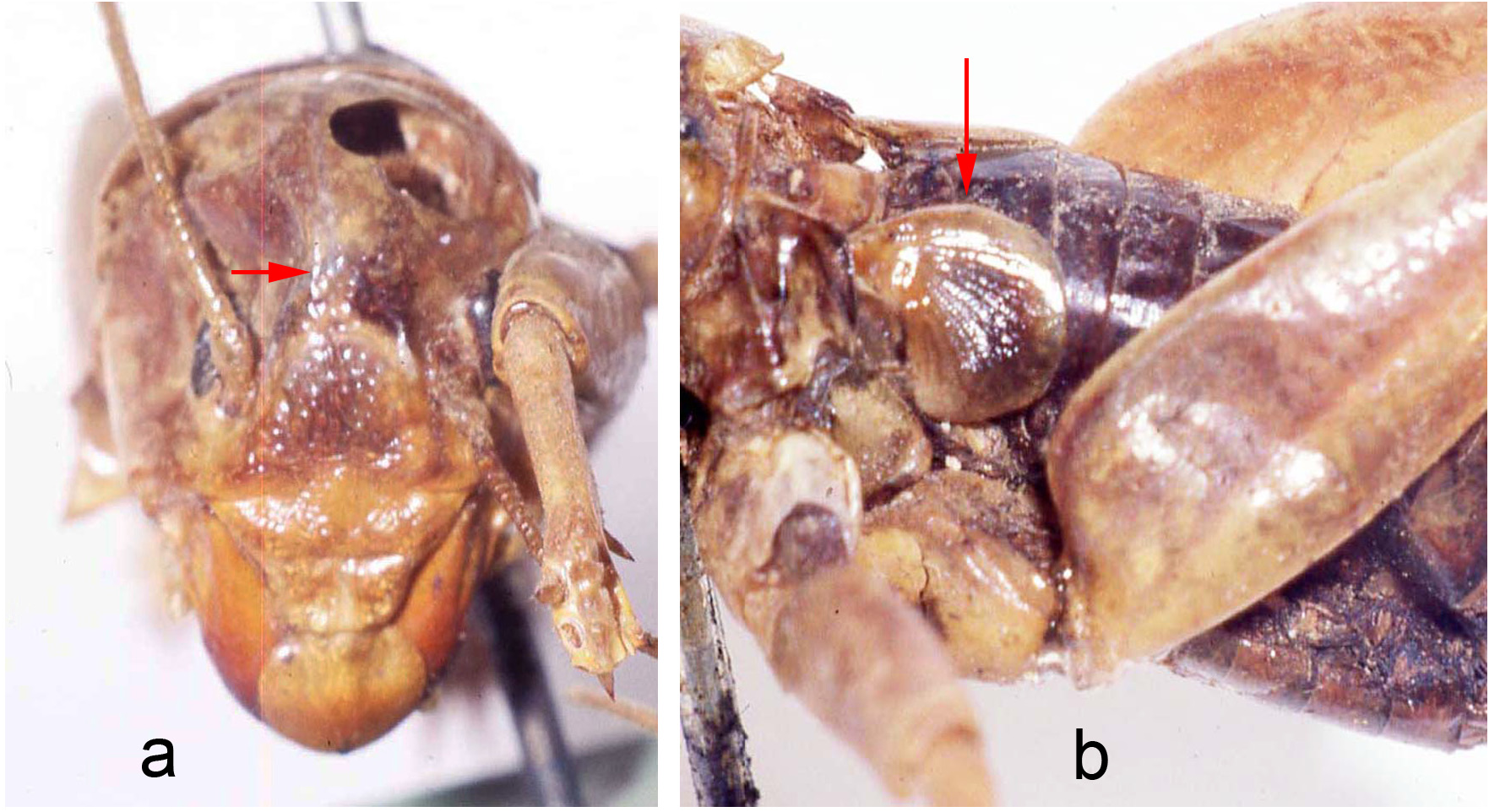
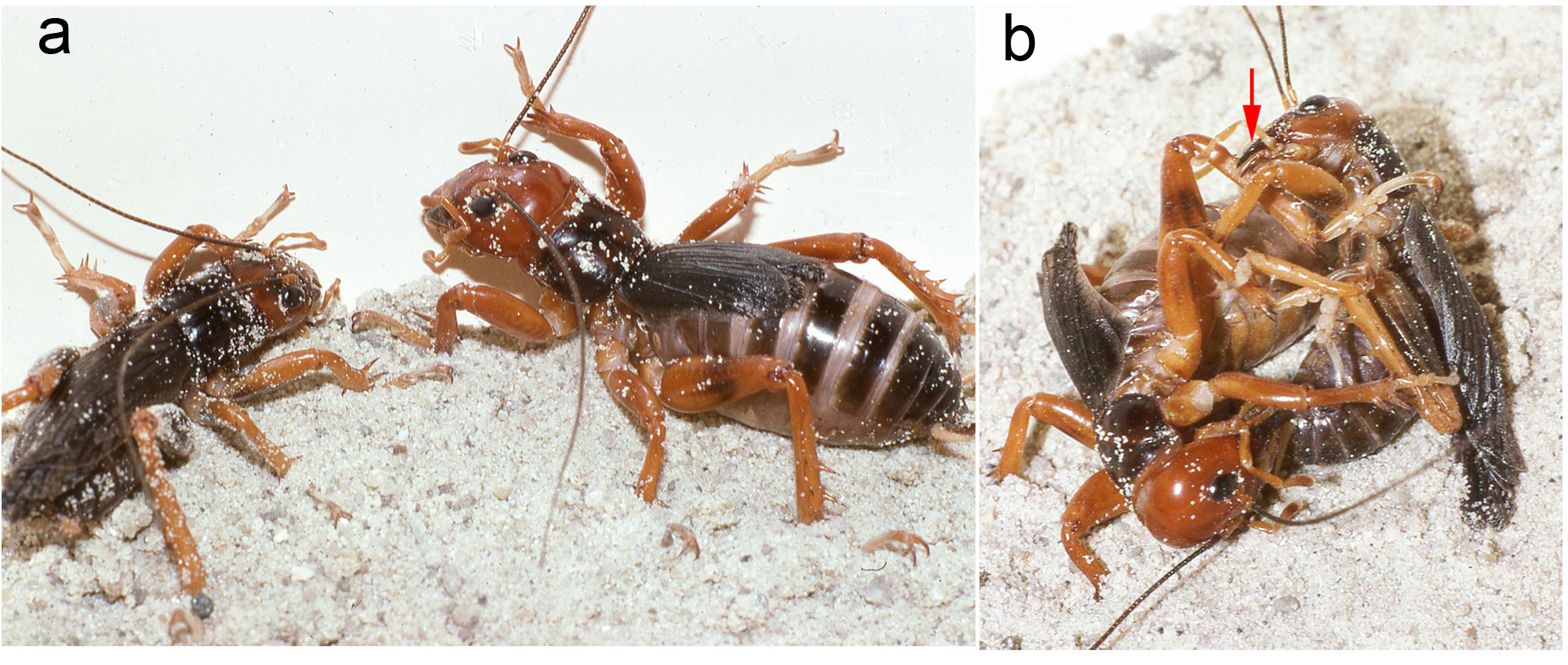
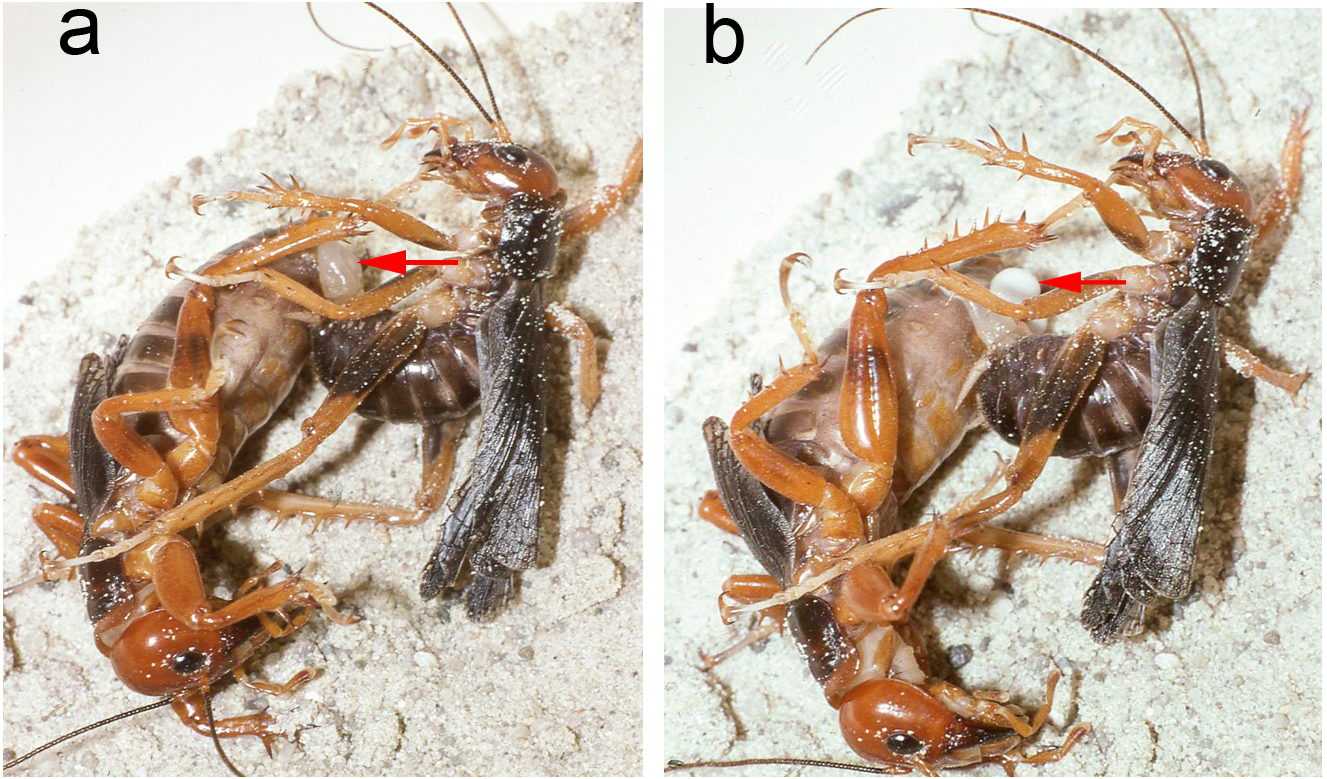
Mating trials. Virgin adults, both individuals having molted to adult shortly after capture, from Metlac Canyon (S06-39), Veracruz, were enclosed together on 19-vii-2006, initially under incandescent light. While the basic mating details are the same as discussed and documented, for Ammopelmatus sp., in Weissman (2001b), Weissman et al. (2008), and filmed in 4K video (https://www.youtube.com/watch?v=mHbwC-AIyTE), there are similarities and differences worth noting because this is the first time we have documented mating in Stenopelmatus . Four minutes after being placed together ( Fig. 155 View FIGURE 155 ), the male approached the female and touched her antennae with his antennae. One minute later, the female tremulated (moved her abdomen up and down without striking the substrate) and moved closer to the male. We then switched from incandescent to red light. At 29 minutes, the female tremulated and performed audible abdominal-femoral stridulation. The male tremulated but no stridulation was heard. At 33 minutes, the male quickly got into a “bite-back” position, biting the female’s left rear leg tibia ( Fig. 155 View FIGURE 155 ), curling his abdomen toward the female’s genitalia while extruding his internal genitalia ( Fig. 156 View FIGURE 156 ), until he could anchor his hooks and quickly pass a spermatophore ( Fig. 156 View FIGURE 156 ). Note absence of any obvious, clear spermatophylax, which is normally easily visible in a mating involving a virgin male Ammopelmatus (see Fig. 1j View FIGURE 1 in Weissman et al. 2008). They stayed adjacent ( Fig. 157 View FIGURE 157 ) for 2 minutes and then separated. Yet within 30 seconds, the male was again “onto” the female, a situation never seen in some 90 matings involving various species of Ammopelmatus . Over the next 10 minutes, this male repeatedly showed interest in the female by approaching her, rolling onto his side, biting her, and trying to physically get her to engage with him. She showed no interest, kicking him off several times. Such a refractory period is typically seen in just-mated female Ammopelmatus . At 11 minutes post coitus, the male ceased his sexual aggressiveness. Around that time, the female curled her abdomen 3 times, in 5 minutes, as if trying to get to the spermatophore. She never succeeded and the sticky spermatophore quickly accumulated a layer of substrate sand ( Fig. 157 View FIGURE 157 ).
Four months later, this now once mated male was enclosed with a second virgin adult female from S06-39. While there was no consummation, the male made 2 novel motions not seen in his previous mating or in any previous Stenopelmatinae pairing: (1) a silent, oscillating back and forth motion, along his long body axis, and (2) a rapid “shivering” motion that was faster than (1) and with less total body movement than (1). Whether these unique body movements are widespread in this species is unknown given our small sample size. We also don’t know if such motions are ancient, or recently derived, within this group, although we subsequently saw oscillations, as described in (1) above, in S. perote (p. 69).
Weissman et al. (2008) noted how “the [bite-back] positions assumed during copulation are so characteristic and unique [in the Stenopelmatinae ] that they may be used, perhaps for the first time, to delimit an entire subfamily of insects.” It will be interesting to see if members of the other two extant Jerusalem cricket subfamilies use such a bite-back mechanism.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Stenopelmatoidea |
|
Family |
|
|
SubFamily |
Stenopelmatinae |
|
Genus |