Agramma abruptifrons Golub, 1990
|
publication ID |
https://doi.org/ 10.37520/aemnp.2020.035 |
|
publication LSID |
lsid:zoobank.org:pub:5F67ED70-3632-48DF-AF4A-2A1D8D6F4232 |
|
persistent identifier |
https://treatment.plazi.org/id/03A5879C-FFFC-FFCF-5BAA-6936FD30FB9A |
|
treatment provided by |
Carolina |
|
scientific name |
Agramma abruptifrons Golub, 1990 |
| status |
|
Agramma abruptifrons Golub, 1990
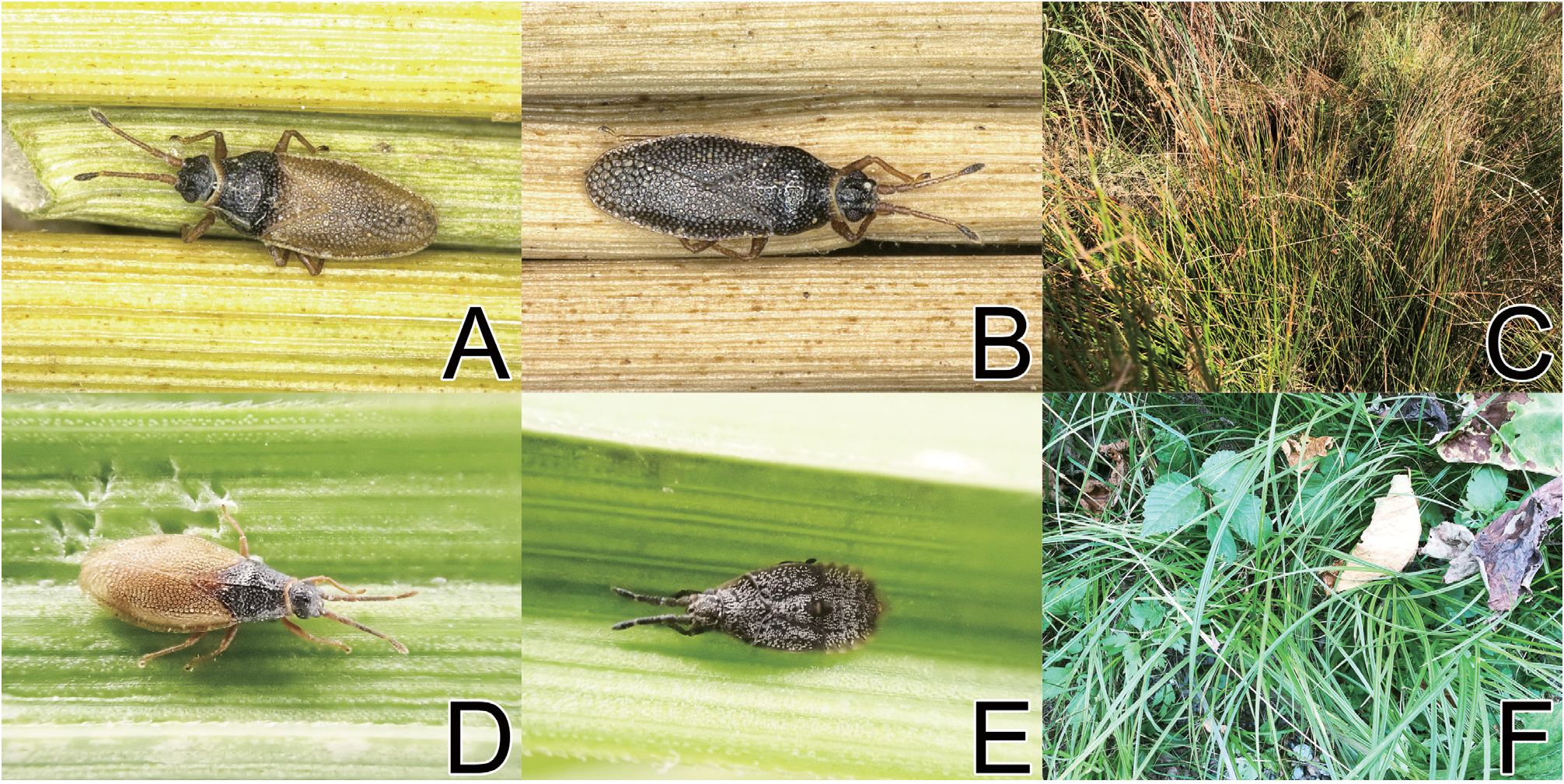
( Figs 1A–E View Fig 1 , 2A, C, F, G View Fig , 3A–C View Fig , 4A, B View Fig 4 )
Agramma nexile (non Drake, 1948): Kൾඋඓπඇൾඋ (1972): 291 (distribution); Gඈඅඎൻ (1977): 252 (distribution). Misidentifications (Gඈඅඎൻ 1990: 48).
Agramma gibbum (non Fieber, 1844): JංඇǤ (1981): 280 (distribution). Misidentification (Gඈඅඎൻ 1990: 51).
Agramma abruptifrons Golub, 1990:41 . Holotype:J (macropterous), Mongolia: E Aimak, Khalkh-Gol River , 33 km SE of Khalkh-Gol (ZIAS).
Agramma abruptifrons : Pඣඋංർൺඋඍ & Gඈඅඎൻ (1996): 12 (check-list, Palaearctic); Vංඇඈκඎඋඈඏ et al. (2010): 151 (check-list: eastern Russia).
Material examined. Non-types (9 macropterous JJ 3 macropterous
♀♀ 1 submacropterous J 8 submacropterous ♀♀): JAPAN: Hඈඇඌflඎ:
Mie-ken, Tsu-shi, Hakusan-chô, Kaminomura, 13.iv.2019, leg.Y.Yazaki
(1 macropterous J, ELKU); Mie-ken, Tsu-shi, Hakusan-chô, Hattaino,
34.662034N 136.309636E, 13.vii.2019, leg. Y. Yazaki (2 macropterous
JJ 1 macropterous ♀ 1 submacropterous ♀, ELKU); as above but
1.x.2019, leg. J. Souma (2 macropterous JJ, ELKU); Mie-ken, Tsu-shi,
Hakusan-chô, Hattaino, 34.657823N 136.315497E, 17.vii.2019, leg. Y.
Yazaki (4 macropterous JJ 2 macropterous ♀♀ 1 submacropterous J
7 submacropterous ♀♀, TUA) ( Fig. 5 View Fig ).
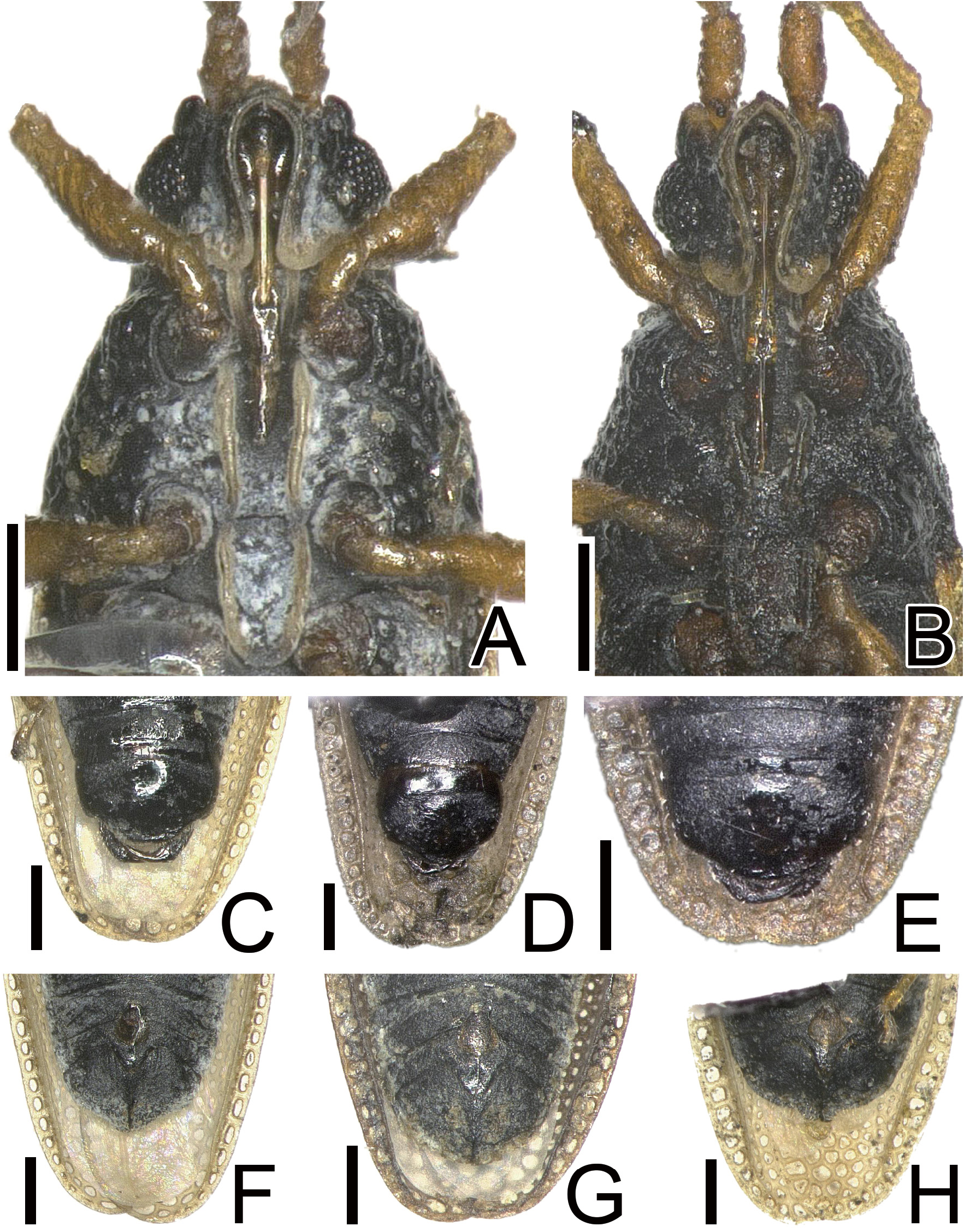
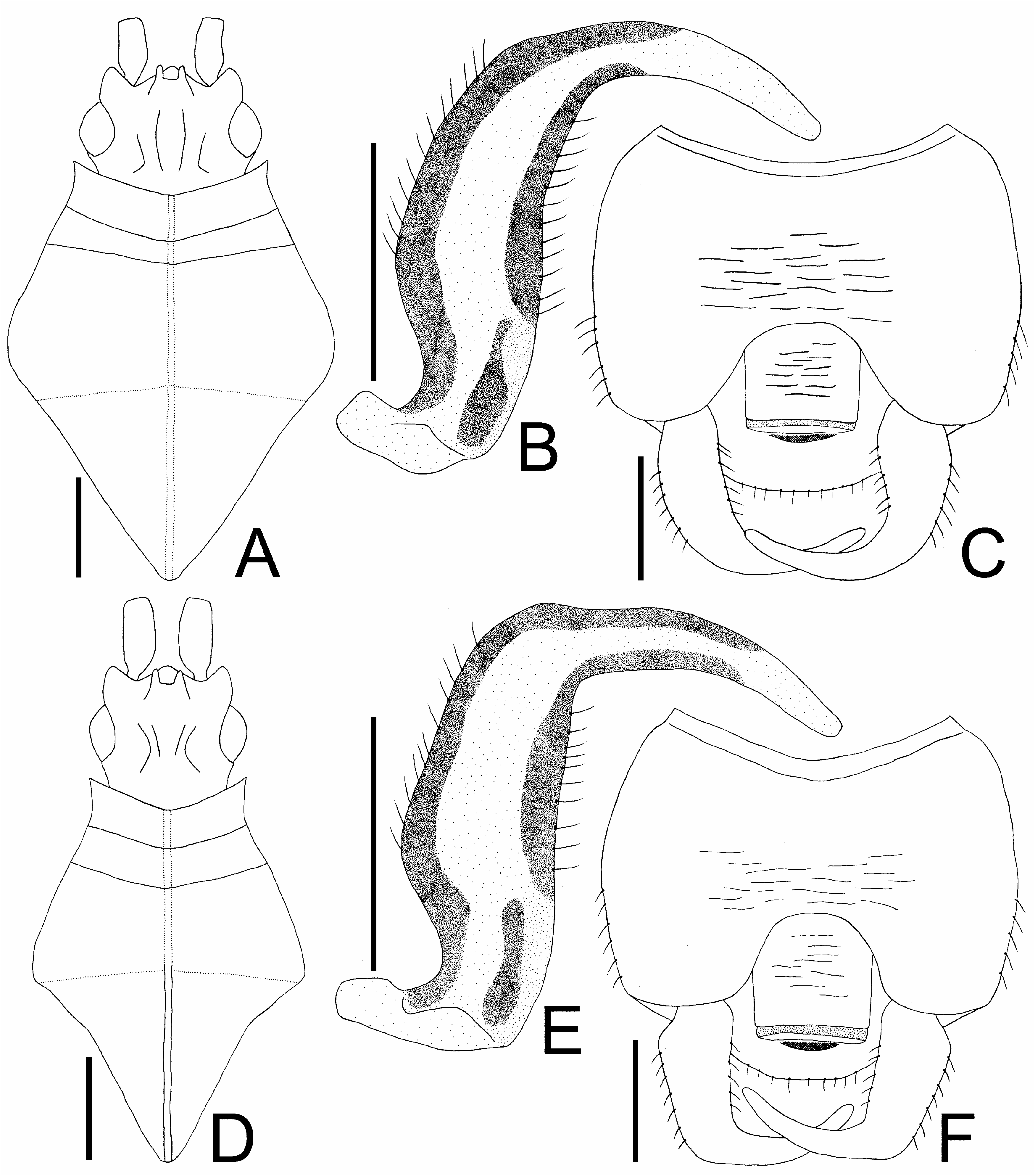
Diagnosis. Recognized among other species of Agramma by a combination of the following characters:frons convex, descending to base of clypeus; a pair of frontal spines obliquely protruding downward, touching clypeus at apices, separated from each other at apices; distance between apices of frontal spines as long as their length; pubescence on body less than 0.5 times as long as diameter of compound eye; rostrum reaching middle part of mesosternum;median carina of pronotum indistinct throughout its length; costal area with a single row of areolae throughout its length, narrower than tibia; areolae of costal area less than 2 times as long as its maximum width at middle of hemelytron; subcostal-discoidal boundary vein present in basal part and absent in remaining parts; subcostal area with 4 rows of areolae at widest part; discoidal-sutural boundary vein absent; discoidal-sutural area with 8 rows of areolae at widest part; outer and inner margins of paramere gently curved in middle part ( Figs 3B, C View Fig ); female terminalia pentagonal in ventral view ( Figs 2F, G View Fig ); and posterior margin of female terminalia not protruding posteriad in middle part.
Redescription. Coloration. Head, antennal segment IV, calli, pronotal disc, basal part of posterior process, thoracic pleura, apical part of tarsi and abdomen black; antennal segments I to III, bucculae, rostrum, collar, sternal laminae, legs except apical part of tarsi brown; apical part of posterior process, hemelytron brown or black; compound eyes dark red; pubescence on body yellowish ( Figs 1A–E View Fig 1 , 2A, C, F, G View Fig ).
Macropterous morph. Body ( Figs 1A, B, E View Fig 1 ) approximately 2.6 times as long as maximum width across hemelytra. Ratios of lengths from antennal segments I to IV as 1.3: 1.0: 2.9: 1.6. Bucculae 2.5 times as long as its maximum height, with 2 rows of areolae throughout its length. Rostrum ( Fig. 2A View Fig ) 0.6 times as long as antennae.
Pronotum ( Figs 1A, B, E View Fig 1 , 3A View Fig ) 1.2 times as long as maximum width across humeri, without paranota. Pronotal disc as high as hemelytron at highest part of each. Collar lower than pronotal disc at highest part of each; anterior margin gently curved inward. Median carina indistinct throughout its length. Posterior process 0.8 time as long as its maximum width.
Hemelytron ( Figs 1A, B, E View Fig 1 ) 2.9 times as long as its maximum width; maximum width across hemelytra 1.2 times as wide as maximum width across humeri; apices of hemelytra overlapping each other in rest.
Abdomen 1.5 times as long as its maximum width. Pygophore ( Figs 2C View Fig , 3C View Fig ) compressed dorsoventrally, semicircular in ventral view, reaching beyond apex of subcostal area of hemelytron; anterior margin of dorsum gently curved inward. Paramere ( Fig. 3B View Fig ) expanded in middle part. Female terminalia ( Fig. 2F View Fig ) reaching apex of subcostal area of hemelytron.
Submacropterous morph. General appearance very similar to that of macropterous morph except for the following characters: body ( Figs 1C, D View Fig 1 ) approximately 2.5 times as long as maximum width across hemelytra; hemelytron 2.7 times as long as its maximum width; female terminalia ( Fig. 2G View Fig ) reaching beyond apex of subcostal area of hemelytron.
Measurements (12 macropterous and 9 submacropterous morphs). Body length with hemelytra 1.8–2.1 mm; maximum width across hemelytra 0.7–0.8 mm; pronotal width across paranota 0.6–0.7 mm.
Remarks. The above-recorded specimens well match the original description (Gඈඅඎൻ 1990) of Agramma abruptifrons described from Mongolia in terms of general appearance.
Segmental oligomery of antenna is confirmed in A. abruptifrons ( Fig. 1E View Fig 1 ), and this individual lack antennal segment III. In other species of Agramma , similar teratological form has been known in A. atricapillum ( Spinola, 1837) and A. confusum (Puton, 1879) (Šඍඎඌගκ & Sඍൾπඅටκ 1978). Therefore, segmental oligomery of antenna may be common in the genus Agramma .
InJapan, Agramma abruptifrons inhabits wetlands with a warm-temperate climate.
Host plant. Juncus sp. (Juncaceae) ( Fig. 4C View Fig 4 ) has been confirmed as a host plant for Agramma abruptifrons in Japan by me and one of my colleagues Mr. Yoichi Yazaki.
Biology. Although many tingids generally feed on the abaxial surface of leaves of host plants (Sർπඎπ & Sඅൺඍൾඋ 1995), a number of individuals of A. abruptifrons were collected from stems of the host plant Juncus sp. ( Fig. 4C View Fig 4 ) in Japan. In Japan, adults were collected in April, July and October.
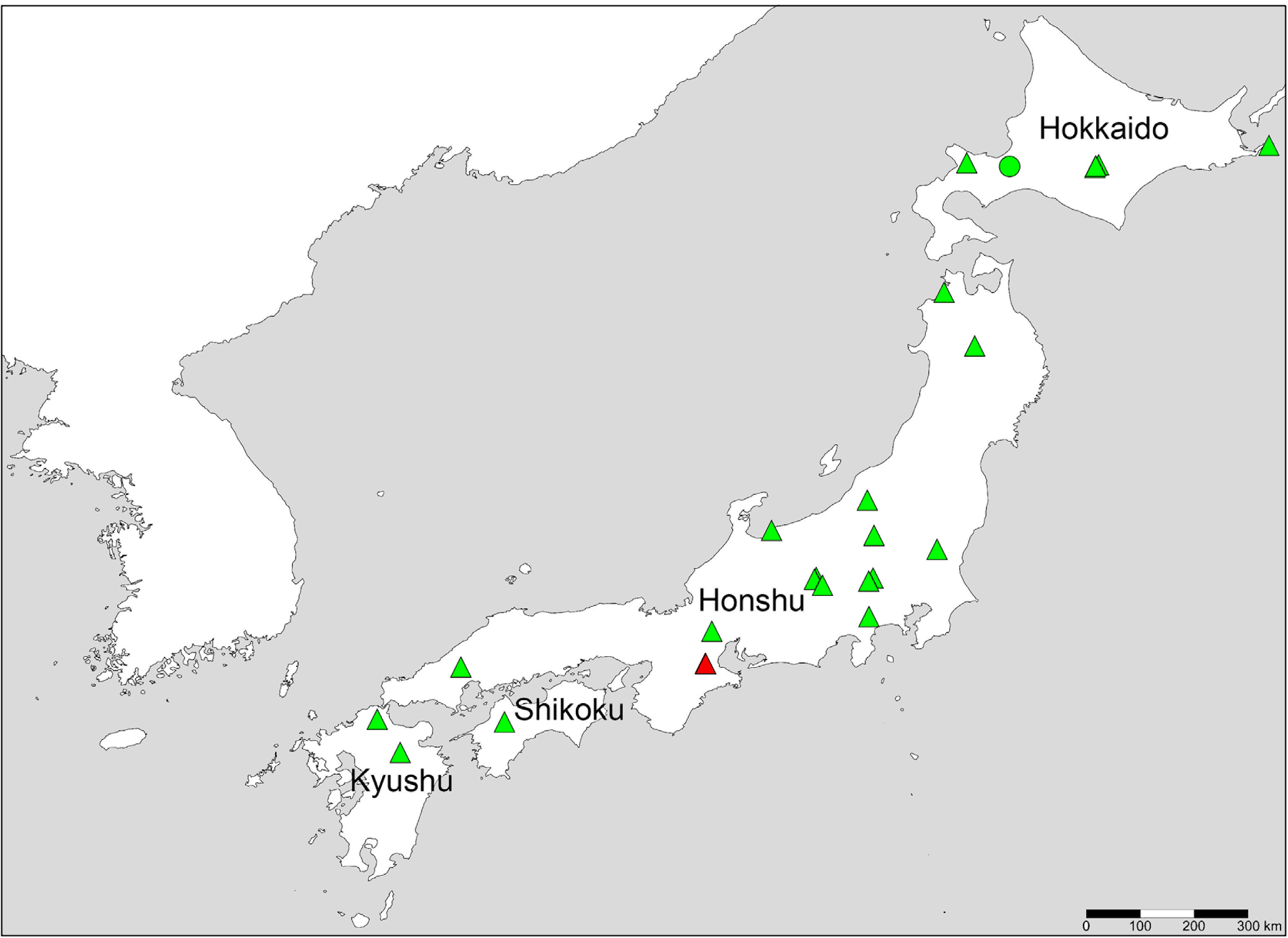
Distribution. China: Beijing City, Hebei Province, Shanxi Province, Inner Mongolia Autonomous Region, Ningxia Hui Autonomous Region (JංඇǤ 1981; Gඈඅඎൻ 1990; Pඣඋංർൺඋඍ & Gඈඅඎൻ 1996); Mongolia (Gඈඅඎൻ 1990); Russia: East Siberia (Gඈඅඎൻ 1990); Japan (Honshu) (new record).
Agramma japonicum ( Drake, 1948)
( Figs1F–J View Fig 1 , 2B, D, E, H View Fig , 3D–F View Fig , 4D, E View Fig 4 )
Serenthia japonica Drake, 1948: 174 . Holotype: ♀ (submacropterous), Japan:Sapporo [= Japan: Hokkaido, Sapporo-shi] (USNM:see Hൾඇඋඒ 2020b).Synonymized with Agramma nexile ( Drake, 1948) View in CoL by Tൺκൾඒൺ (1962: 52) and with A. ruficorne (Germar, 1835) View in CoL by Pඣඋංർൺඋඍ (1983: 531). Restored by Gඈඅඎൻ (1990: 48).
Agramma nexile View in CoL (non Drake, 1948): Dඋൺκൾ (1948): 174 (paratypes); Tൺκൾඒൺ (1951): 23 (check-list: eastern Asia); Eඌൺκං (1952): 238 (distribution); Tൺκൾඒൺ (1953): 167 (distribution); Mංඒൺආඈඍඈ (1965): 90 (distribution); Lൾൾ (1967): 92 (distribution); Lൾൾ (1969): 162 (nymph, male genitalia); Kൾඋඓπඇൾඋ (1978): 47 (distribution); Tඈආඈκඎඇං (1979): 135 (distribution); Tඈආඈκඎඇං (1987): 115 (distribution); Tൺκൺπൺඌπං (1990): 2 (distribution); Tඈආඈκඎඇං & Iඌπංκൺඐൺ (2002): 170 (distribution); Mංඒൺආඈඍඈ (2008): 156 (distribution); Yൺආൺൽൺ & Tඈආඈκඎඇං (2012): 188 (monograph); Yൺඇඈ et al. (2013): 24 (distribution); Mൺൾπൺඋൺ (2014): 58 (distribution); Tඈආඈκඎඇං (2014): 362 (distribution); Yൺආൺൽൺ & Iඌπංκൺඐൺ (2016): 429 (check-list: Japan); Oκඈർπං (2019): 1 (distribution). Misidentifications.
Agramma ruficorne (non Germar, 1835): Pඣඋංർൺඋඍ (1983): 531 (monograph); Gඈඅඎൻ (1988): 148 (key to species); Mංඒൺආඈඍඈ & YൺඌඎඇൺǤൺ (1989):167 (check-list: Japan);Tඈආඈκඎඇං (2005):400 (distribution). Misidentifications (Gඈඅඎൻ 1990: 48).
Agramma japonicum : Tൺκൾඒൺ (1951): 23 (check-list:eastern Asia); Dඋൺκൾ & Mൺൺ (1953): 23 (distribution); Gඈඅඎൻ (1988): 148 (key to species); Mංඒൺආඈඍඈ & YൺඌඎඇൺǤൺ (1989): 167 (check-list: Japan); Kൾඋඓπඇൾඋ & Mൺඋඎඌංκ (1996): 26 (check-list:Kuril Islands); Pඣඋංർൺඋඍ & Gඈඅඎൻ (1996): 14 (check-list: Palaearctic); Kඐඈඇ et al. (2001): 186 (checklist: Korea); Kൾඋඓπඇൾඋ et al. (2004): 239 (check-list: Kuril Islands); Kൺඇඒඎκඈඏൺ & Mൺඋඎඌංκ (2006): 171 (check-list: Kuril Islands); Tඈආඈκඎඇං (2006): 60 (distribution); Vංඇඈκඎඋඈඏ et al. (2010): 151 (check-list: eastern Russia); Yൺආൺൽൺ & Tඈආඈκඎඇං (2012): 187 (monograph);Aඎκൾආൺ et al. (2013): 59 (check-list: Palaearctic);Yൺආൺൽൺ & Iඌπංκൺඐൺ (2016): 429 (check-list: Japan); Cπඈ et al. (2020): 738
(check-list: Korea). Material examined. Non-types (1 macropterous J 4 macropterous ♀♀
118 submacropterous JJ 138 submacropterous ♀♀ 8 N 5 4 N 4), JAPAN: Hඈκκൺංൽඈ: Sapporo-shi,Toyohira-ku, Hitsujigaoka, 18.vi.2007, leg. M. Hayashi (7 submacropterous JJ 12 submacropterous ♀♀, TUA);Nemuro City, Habomai Marsh, 7.vii.2017, leg. M. Hayashi (1 submacropterous J 7 submacropterous ♀♀, TUA); Iwanai-gun, Kyowa-cho, Kunitomi, Inaho Mountain Pass, 43°03′28.1″N 140°41′05.2″E, 1.x.2017, leg. T. Ban (1 submacropterous J 2 submacropterous ♀♀, TUA); Kamikawagun, Shimizu-cho, Kitashimizu For. Rd., 43°00′17.4″N 142°50′35.9″E, 5.viii.2019, leg. J. Souma (6 submacropterous JJ 2 submacropterous ♀♀ 6 N 5 4 N 4, TUA); Kamikawa-gun, Shimizu-cho, Route 274, 42°58′45.3″N 142°49′44.8″E, 6.viii.2019, leg.J. Souma (1 macropterous J 2 macropterous ♀♀ 11 submacropterous JJ 12 submacropterous ♀♀, TUA); Kamikawa-gun, Shimizu-cho, Shimizu Baseline, 43°01′18.3″N 142°53′29.2″E, 7.viii.2019, leg. J. Souma (2 submacropterous JJ 1 submacropterous ♀, TUA); Kamikawa-gun, Shimizu-cho, Shimizu east line 1, 43°01′16.7″N 142°53′47.4″E, 30.vii.2020, leg. J. Souma (2 submacropterous JJ 2 submacropterous ♀♀, NMPC). Hඈඇඌflඎ: Aomori-ken, Tsugaru-shi, Kidukuritateoka, Hiratakinuma, 28.ix.1992, leg. M. Hayashi (1 submacropterous ♀, TUA); Akita Pref., Kazuno, Hachimantai, Fukenoyu, 1,120 m, 25.viii.2000, leg. M. Hayashi et al. (3 submacropterous JJ 4 submacropterous ♀♀, TUA); as above but 13.ix.2000 (36 submacropterous JJ 27 submacropterous ♀♀, TUA); as above but 10.ix.2001 (2 submacropterous ♀♀, TUA); Tochigi-ken, Haga-gun, Motegi-machi, Mt.Kamakura, 36°34′17.8″N 140°11′14.4″E, 11.vii.2019, leg. J. Souma (1 submacropterous ♀, TUA);Gunma-Pref,Katashina-Vil, Mt. Hotaka, 10.viii.1999, leg. S. Nagashima (1 macropterous ♀ 1 submacropterous J, TUA); Saitama-ken, Chichibu-shi, Shimoyoshida, Torikata, 26.iv.1996, leg. M. Hayashi (2 submacropterous JJ 3 submacropterous ♀♀, TUA);Saitama-ken,Chichibu-gun,Minano-machi, Shimotano, 36°05′23.8″N 139°07′00.1″E, 29.v.2019, leg. J. Souma (1 submacropterous J 1 submacropterous ♀, TUA); Kanagawa-ken,Ashigarakami-gun, Yamakita-machi, Nakagawa, 35°26′45.5″N 139°02′52.4″E, 30.vi.2018, leg. J. Souma (1 submacropterous ♀, TUA); Nîgata-ken, Nagaoka-shi, Kuriyamazawa, alt. 373 m, 37.395468 N 139.020894 E, 15.v.2016, leg. Gô Mashima (1 submacropterous J 1 submacropterous ♀, TUA); Nagano Pref., Suwa-gun, Hara-mura, 4.vii.1998, leg. J. Narukawa (1 submacropterous ♀, TUA); Shinano, Kamisuwa, 18.vii.1951, leg. M. Takahashi (1 submacropterous ♀, ELKU); Shinano, Kirigamine, 10.viii.1959, leg.S. Miyamoto (2 N5, ELKU); Toyama, Ikuji, 4.viii.1960, leg. T. Hidaka (1 macropterous ♀ 1 submacropterous ♀, ELKU); Mie Pref., Inabe-shi, Fujiwara-chô, Kurakake-tôge Pass, 30.vi.2012, leg. N. Tsuji (1 submacropterous J 1 submacropterous ♀, ELKU);Aki, Sandankyô, 10–11.vi.1939, leg. Teiso Esaki (2 submacropterous ♀♀, ELKU). Sflංκඈκඎ: Iyo, W. Kuma, Kawasemura, 22.v.1949, leg. M. Miyatake (1 submacropterous ♀, ELKU). Kඒඎඌflඎ: Fukuoka, Fukuchi, 29.iv.1956, leg.T.Hidaka (1 submacropterous ♀, ELKU); Ôita Pref., Kokonoe-machi, Yutsubo, Jizôbaru Marshland, 17.viii.2019, leg. R. Ito (43 submacropterous JJ 51 submacropterous ♀♀, ELKU) ( Fig. 5 View Fig ).
Note. The label data of two specimens collected by Teiso Esaki corresponds to a single paratype of Agramma nexile from Honshu described by Dඋൺκൾ (1948). Six specimens from Honshu and Shikoku collected in the 1930s–1950s, which are deposited at ELKU, had been recorded as A. nexile by Tൺκൾඒൺ (1951, 1953, 1962). The collection site of 19 specimens from Sapporo, Hokkaido provided by M. Hayashi corresponds to type locality of A. japonicum and one of paratype localities of A. nexile described by Dඋൺκൾ (1948).
Diagnosis. Recognized among other species of Agramma by a combination of the following characters: frons convex, descending to base of clypeus; a pair of frontal spines obliquely protruding downward, touching clypeus at apices, separated from each other at apices; distance between apices of frontal spines as long as their length; pubescence on body less than 0.5 times as long as diameter of compound eye; rostrum reaching middle part of mesosternum; medi- an carina of pronotum distinct throughout its length ( Fig. 3D View Fig ); costal area with a single row of areolae throughout its length, wider than tibia; areolae of costal area less than 2 times as long as its maximum width at middle of hemelytron; subcostal-discoidal boundary vein present throughout its length; subcostal area with 4 rows of areolae at widest part; discoidal-sutural boundary vein absent; discoidal-sutural area with 8 rows of areolae at widest part; outer and inner margins of paramere angularly curved in middle part ( Figs 3E, F View Fig ); female terminalia hexagonal in ventral view ( Fig. 2H View Fig ); and posterior margin of female terminalia protruding posteriad in middle part.
Redescription. Coloration. Head, calli, pronotal disc, basal part of posterior process, thoracic pleura, sternal laminae, apical part of tarsi and abdomen black; antenna, bucculae, rostrum, collar, apical part of posterior process, hemelytron, legs except apical part of tarsi brown; compound eyes dark red; pubescence on body yellowish ( Figs 1F–J View Fig 1 , 2B, D, E, H View Fig ).
Macropterous morph. Body ( Figs 1F, G View Fig 1 ) approximately 2.8 times as long as maximum width across hemelytra. Ratios of lengths from antennal segments I to IV as 1.4: 1.0: 2.7: 1.7. Bucculae 2.8 times as long as its maximum height, with 2 rows of areolae throughout its length. Rostrum ( Fig. 2B View Fig ) 0.6 times as long as antennae.
Pronotum ( Figs 1F, G View Fig 1 , 3D View Fig ) 1.3 times as long as maximum width across humeri, without paranota. Pronotal disc higher than hemelytron at highest part of each. Collar lower than pronotal disc at highest part of each; anterior margin gently curved inward. Median carina of pronotum distinct throughout its length. Posterior process 0.8 time as long as its maximum width.
Hemelytron ( Figs 1F, G View Fig 1 ) 3.3 times as long as its maximum width; maximum width across hemelytra 1.3 times as wide as maximum width across humeri; apices of hemelytra overlapping each other in rest.
Abdomen 1.7 times as long as its maximum width. Pygophore ( Figs 2D View Fig , 3F View Fig ) compressed dorsoventrally, semicircular in ventral view, reaching level far remote from apex of discoidal-sutural area of hemelytron; anterior margin of dorsum gently curved inward. Paramere ( Fig. 3E View Fig ) expanded in middle part. Female terminalia ( Fig. 2H View Fig ) reaching level far remote from apex of discoidal-sutural area of hemelytron.
Submacropterous morph. General appearance is very similar to that of macropterous morph except for the following characters: body ( Figs 1H–J View Fig 1 ) approximately 2.6 times as long as maximum width across hemelytra; pronotal disc as high as hemelytron at highest part of each; hemelytron 3 times as long as its maximum width; pygophore ( Fig. 2E View Fig ) reaching apex of discoidal-sutural area of hemelytron.
Measurements (5 macropterous and 256 submacropterous morphs). Body length with hemelytra 1.8–2.3 mm; maximum width across hemelytra 0.7–0.9 mm; pronotal width across paranota 0.5–0.6 mm.
Remarks. The above-recorded specimens well match the photographs of the holotype (Hൾඇඋඒ 2020b) and the original description (Dඋൺκൾ 1948) of A. japonicum described from “Sapporo, Japan ” [= Japan: Hokkaido, Sapporo-shi] in terms of general appearance.
Agramma japonicum strongly resembles A. nexile in general appearance, and the identification of these two species in Japan has long been challenging (e.g. Dඋൺκൾ 1948; Tൺκൾඒൺ 1962; Yൺආൺൽൺ & Tඈආඈκඎඇං 2012). However, based on a comparison between hundreds of specimens together with the photographs of the holotype (Hൾඇඋඒ 2020b) of A. japonicum and the photographs of the holotype (Hൾඇඋඒ 2020a) of A. nexile , a single main characteristic was recognized that permits easy differentiation between A. japonicum and A. nexile , i.e., the areolae of the costal area are less than 2 times as long as its maximum width at middle of hemelytron. In contrast, A. nexile has the areolae of costal area more than 3 times as long as its maximum width at middle of hemelytron.
Host plants. Carex spp. (Cyperaceae) ( Fig. 4F View Fig 4 ) have been confirmed as host plants for A. japonicum (Mංඒൺආඈඍඈ 1965, 2008; Yൺආൺൽൺ & Tඈආඈκඎඇං 2012; Mൺൾπൺඋൺ 2014; Tඈආඈκඎඇං 2014; Oκඈർπං 2019; present study). They have also been collected from the following monocotyledonous herbs of four families but without any data on its development: Ophiopogon japonicus (Thunb.) Ker Gawl. (Asparagaceae) (Tඈආඈκඎඇං 1979); Liriope sp. (Asparagaceae) (Tඈආඈκඎඇං 1979); Cyperus sp. (Cyperaceae) (Yൺආൺൽൺ & Tඈආඈκඎඇං 2012); Scirpus wichurae Boeck. f. wichurae (Cyperaceae) (Tඈආඈκඎඇං 1979); Miscanthus sp. (Poaceae) (Tൺκൾඒൺ 1962; Yൺආൺൽൺ & Tඈආඈκඎඇං 2012); poaceous herb (Tඈආඈκඎඇං 1987).
Biology. A number of individuals of A. japonicum were collected from the leaves of Carex sp. ( Fig. 4F View Fig 4 ) in Japan proper, suggesting that this lace bug appears to feed on the leaves as do many tingids (Sർπඎπ & Sඅൺඍൾඋ 1995). This lace bug is often collected in dimly lit environments and it has rarely been found in sunny environments (Mൺൾπൺඋൺ 2014).
In Japan, adults were collected from April to October (Dඋൺκൾ 1948; Tൺκൾඒൺ 1953; 1962; Kൾඋඓπඇൾඋ 1978; Tඈආඈκ ඎඇං 1979, 1987, 2005, 2014; Tൺκൺπൺඌπං 1990; Tඈආඈκඎඇං & Iඌπංκൺඐൺ 2002; Yൺආൺൽൺ & Tඈආඈκඎඇං 2012; Mൺൾπൺඋൺ 2014; Oκඈർπං 2019; present study); nymphs were observed in July and August (Tඈආඈκඎඇං 1987, 2014; Tඈආඈκඎඇං & Iඌπංκൺඐൺ 2002; Yൺආൺൽൺ & Tඈආඈκඎඇං 2012; present study); the overwintering form is unknown.
Distribution. Japan (Kunashiri Island, Hokkaido, Honshu, Hachijo Island, Sado Island, Shikoku, Kyushu) (Kൾඋඓπඇൾඋ 1978; Yൺආൺൽൺ & Iඌπංκൺඐൺ 2016; new record from Kyushu); China: Fujian ((Dඋൺκൾ & Mൺൺ 1953); Korea (Lൾൾ 1967); Russia: Far East (Vංඇඈκඎඋඈඏ et al. 2010); Taiwan (Tඈආඈκඎඇං 2006).
In Japan, A. japonicum inhabits the wetland and forest floor with cool- and warm-temperate climates.
A total of 273 specimens from Japan including “ A. nexile ” recorded by the previous authors (Dඋൺκൾ 1948; Tൺκൾඒൺ 1951, 1953, 1962) were examined, and all of these are A. japonicum . In addition, the illustrations and photographs of A. nexile from Japan provided by the previous authors (Eඌൺκං 1952; Mංඒൺආඈඍඈ 1965, 2008; Lൾൾ 1969; Yൺආൺൽൺ & Tඈආඈκඎඇං 2012; Tඈආඈκඎඇං 2014) in fact represent A. japonicum . Therefore, at least most of, possibly all previous distributional records of A. nexile from Japan (e.g. Dඋൺκൾ 1948; Tൺκൾඒൺ 1962; Yൺආൺൽൺ & Tඈආඈκඎඇං 2012) probably correspond to A. japonicum , and “true” A. nexile described from Taiwan appears not to be present in Japan.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.