Kalanchoe gideonsmithii N.R.Crouch & Figueiredo, 2022
|
publication ID |
https://doi.org/ 10.11646/phytotaxa.566.2.8 |
|
DOI |
https://doi.org/10.5281/zenodo.7119244 |
|
persistent identifier |
https://treatment.plazi.org/id/03A5E427-FFDF-BB4F-63FF-F86DFCF2FCF8 |
|
treatment provided by |
Plazi |
|
scientific name |
Kalanchoe gideonsmithii N.R.Crouch & Figueiredo |
| status |
sp. nov. |
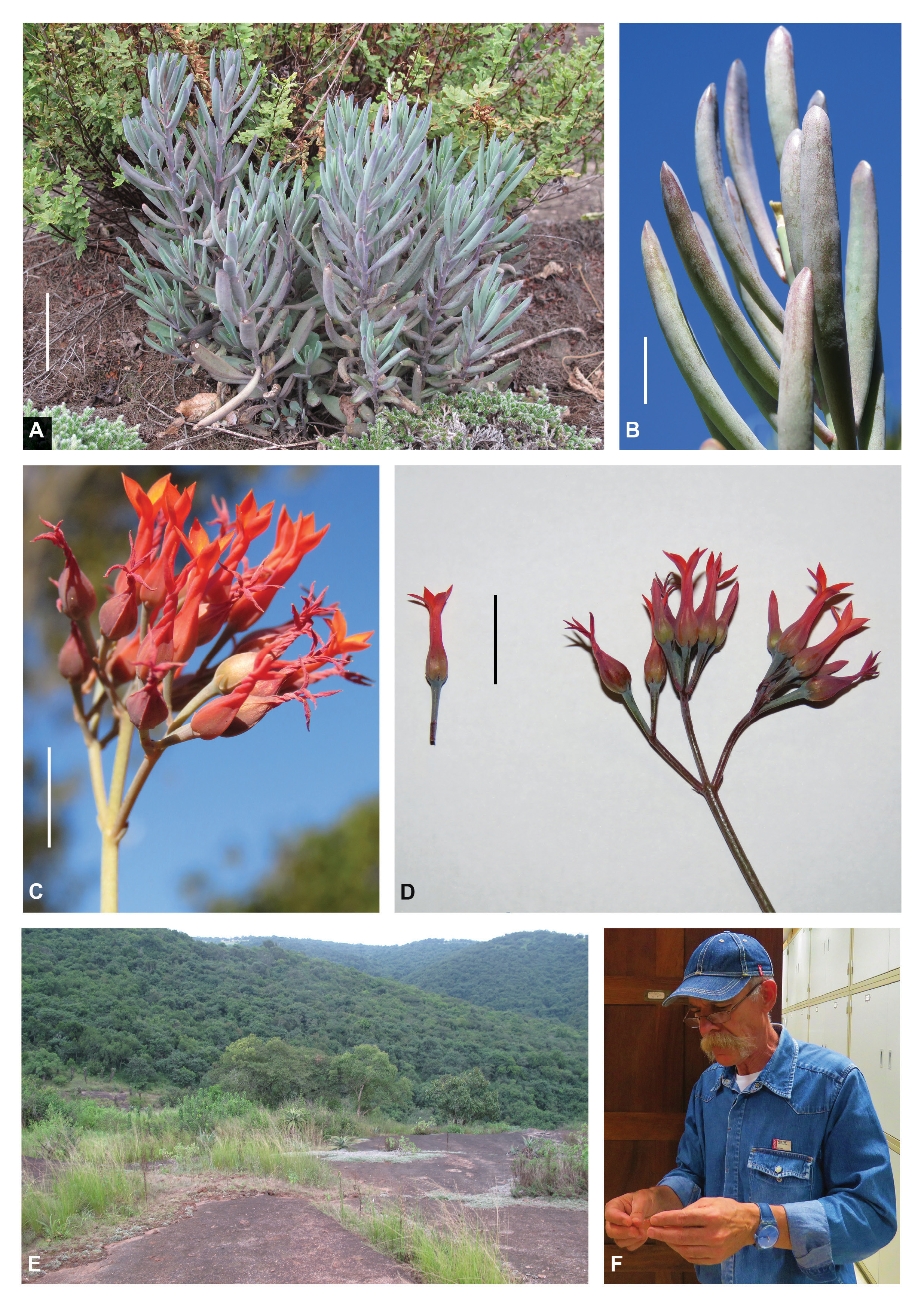
Kalanchoe gideonsmithii N.R.Crouch & Figueiredo View in CoL , sp. nov. ( Fig. 1A–D View FIGURE 1 )
Type:— SOUTH AFRICA. KwaZulu-Natal province.—2930 (Pietermaritzburg): Bishopstowe (– DA); Collected on 3 March 2010 by Neil R. Crouch; Flowered in cultivation in Pretoria on 26 June 2021, G. F. Smith 1153 (holotype PRU) .
Diagnosis:—The overall colour of the leaves and stems of K. gideonsmithii is pale bluish purple or in shady positions somewhat pale light green with a blue sheen, while K. decumbens plants are more uniformly light green and K. rotundifolia mid- to light green to glaucous. The leaves of K. gideonsmithii are narrowly oblong-terete to somewhat clavate rather than round or oblanceolate to obovate as in K. rotundifolia . At the level of the ovary the corolla tubes of K. gideonsmithii are dark orange-red and purplish blueinfused, rather than somewhat green-infused, as in the case of K. rotundifolia and, to a lesser extent, K. decumbens .
Description:—Perennial, few- to many-leaved, sparsely to densely branched from base, glabrous, low-growing small to medium-sized succulent, with creeping stems 0.2–1.0 m long when in flower. Stems one to few arising from rootstock, usually simple or sometimes producing obovate-leaved branchlets near base, main stems very rarely branched higher up, erect or leaning to creeping, rooting along the way, thin, green to strongly bluish purple-infused. Leaves 50–75(–80) × (4–)5.0– 7.5 mm, opposite-decussate, sessile, erect, uniformly densely carried throughout, pale bluish green to pale light green, with slight to distinct bloom, finely purple-dotted in distal ¼ and towards base; petiole absent; blade succulent, spathulate when young, narrowly oblong-terete to somewhat clavate at maturity, often involute when desiccated, then appearing grooved above, sometimes recurved or incurved in distal ⅓ to ½; apex rounded to obtuse, slightly apiculate; base gradually tapering to narrow slightly thickened insertion on stem; margins entire. Inflorescence a corymbose cyme, 10–30(–40) cm tall, with leaf-like bracts at nodes, floriferous only at top, erect or leaning, apically sparsely branched, few- to many-flowered, rather round in outline when viewed from above, branches opposite, subtended by small, persistent leaf-like bracts, leafy branchlets sometimes developing in axils, axis light green to strongly bluish purple-infused with slight waxy bloom; pedicels slender, 7–9(–11) mm long. Flowers 11–14 mm long, erect; calyx dull bluish green, distally red, covered with slight bloom; sepals 4, 0.75–1.00 × 0.75– 1.00 mm, ± separate, basally very slightly fused, deltoid-triangular, acute, somewhat contrasting against basal part of corolla; corolla 10–13 mm long, prominently enlarged basally around carpels, distinctly and tightly anti-clockwisetwisted apically after anthesis; corolla tube 9–12 mm long, terete to 4-angled, narrowly urceolate, strongly globose basally to somewhat cubic when viewed from below, narrowing above carpels, distally consistently bright orange-red with bluish purple infusion, bright orange-red to distinctly bluish purple-infused around ovaries, yellowish green basally at level of sepals; lobes 4–5 × 1.5–2.0 mm, at 30°–45° angle to rarely patent, narrowly elliptic, distinctly acute apically, showing diurnal movement, bright orange-red, sometimes slightly yellowish-infused in centre. Stamens inserted in two ranks just above and just below middle of corolla tube, included; filaments 1.0– 1.5 mm long, thin, yellow; anthers 0.25–0.30 mm long, yellow. Pistil consisting of 4 carpels; carpels 4–5 mm long, mid-green, distinctly purple-infused; styles ca. 1 mm long; stigmas very slightly capitate, yellowish green; scales 2.0– 2.5 mm long, linear, light yellow. Follicles 5–6 mm long, grass spikelet-like, dull mid-green, enveloped in dry, purple to purplish white remains of corolla, eventually brittle. Seed 0.75–1.00 mm long, somewhat banana-shaped to ellipsoid, striated, brown. Chromosome number: unknown.
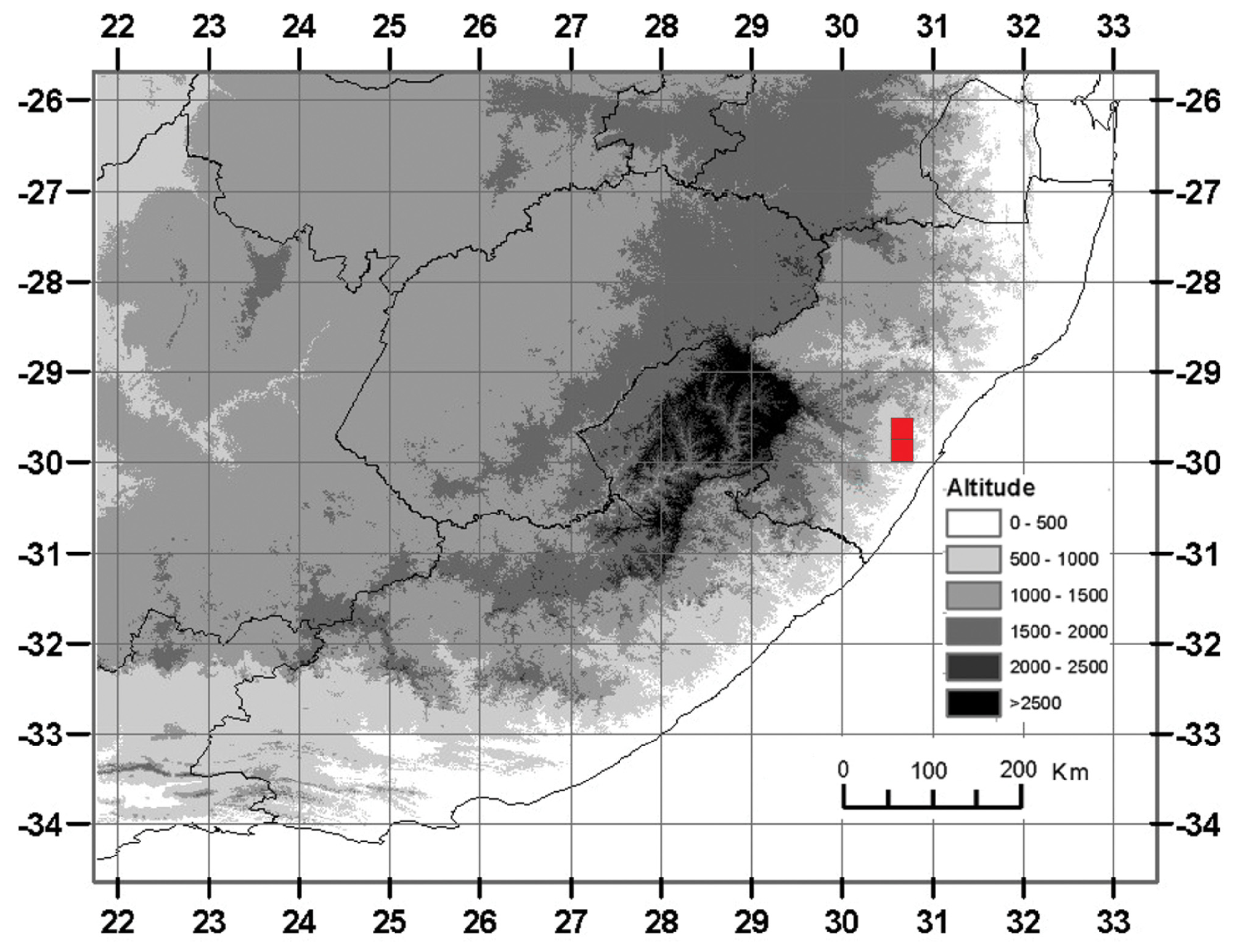
Distribution and habitat:—The type locality of Kalanchoe gideonsmithii is at Bishopstowe in South Africa’s KwaZulu-Natal province ( Fig. 1E View FIGURE 1 ). Plants grow exposed to full sun in the thin soils that accumulate in pockets overlying granitic rock sheets, where it co-occurs with the succulents Aloe candelabrum Berger (1906: 246) (Asphodelaceae) , Kalanchoe rotundifolia (Crassulaceae) , and Kleinia fulgens Hooker (1866 : t. 5590) ( Asteraceae ). Several hundred plants are known from the (formally unprotected) type locality, which occurs at an elevation of ca. 540 m, in KwaZuluNatal Hinterland Thornveld (SVs 3) ( Rutherford et al. 2006). At the second known locality to the south of Cato Ridge, plants occur on sandstone-derived soils at an elevation of ca. 565 m. The entire known natural geographical distribution range ( Fig. 3 View FIGURE 3 ) of K. gideonsmithii falls within the Maputaland-Pondoland Region of Endemism, to which it accordingly is endemic ( Van Wyk & Smith 2001: 82–85).
Flowering time:— Kalanchoe gideonsmithii flowers mainly in the winter months, May to August in the southern hemisphere, although in cultivation there as early as March.
Eponymy:— Kalanchoe gideonsmithii is named for Prof. Dr Gideon François Smith (Kariega, Eastern Cape province, South Africa, 20 October 1959 –) ( Fig. 1F View FIGURE 1 ). Gideon has held several tenured and temporary senior management positions at the South African National Biodiversity Institute, and simultaneously the John Acocks Professorial Chair at the University of Pretoria. He has a research interest in Old and New World succulents and is at present attached to the Nelson Mandela University in Gqeberha [Port Elizabeth], South Africa. Gideon recently co-authored a book on southern African kalanchoes ( Smith et al. 2019) and has published well over 100 papers on the genus.
We here combine Gideon’s first and surname in the specific epithet to prevent confusion with ‘ Kalanchoe smithii ’ Raym. -Hamet in sched., a designation not validly published (see https://science.mnhn.fr/institution/mnhn/collection/ p/item/p03380700?lang=en_ US). Although Jacobsen (1986: 629) referenced this designation, as far as could be determined Raymond-Hamet never described such a species ( Smith 2020b; see Turland et al. 2018: Rec. 23A.3.(i)).
Discussion:— Kalanchoe rotundifolia occurs sympatrically with both K.decumbens and K.gideonsmithii . However, although these species, like most kalanchoes, generally flower simultaneously during the winter months, hybrids or intermediate forms that show introgression have yet to be observed between K. rotundifolia and K. decumbens , and K. rotundifolia and K. gideonsmithii .
With 19 of the 22 hitherto known kalanchoes from southern Africa occurring to the east of the Drakensberg massif, that region is evidently a regional centre of diversity for the genus. Additionally, about 50% of these eastern southern African kalanchoes are endemic to the Flora of Southern Africa region ( Namibia, Botswana, Eswatini [formerly Swaziland], Lesotho, South Africa) (Smith & Figueiredo 2021).
| R |
Departamento de Geologia, Universidad de Chile |
| G |
Conservatoire et Jardin botaniques de la Ville de Genève |
| F |
Field Museum of Natural History, Botany Department |
| PRU |
University of Pretoria |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.