Carollia perspicillata (Linnaeus, 1758)
|
publication ID |
https://doi.org/ 10.5281/zenodo.6458594 |
|
DOI |
https://doi.org/10.5281/zenodo.6727873 |
|
persistent identifier |
https://treatment.plazi.org/id/03A687BC-FF84-FF87-16BC-FC19FDE1FB89 |
|
treatment provided by |
Plazi |
|
scientific name |
Carollia perspicillata |
| status |
|
110. View Plate 40: Phyl
Seba’s Short-tailed Bat
Carollia perspicillata View in CoL
French: Carollia commune / German: Brillenblattnase / Spanish: Carolia comuna
Other common names: Common Short-tailed Bat
Taxonomy. Vespertilio perspicillatus Linnaeus, 1758 ,
“America.” Restricted by 0, Thomas in 1911 to: “ Surinam [= Suri name].”
R. H. Pine in 1972 and L. J. McLellan and K. F. Koopman in 2008 listed names previously used for C. perspicillata . Although Pine’s review did not formally recognize subspecies, morphometric analyses by McLellan in 1984 validated these subspecific names and believed that they were widely intergraded. Three subspecies recognized.
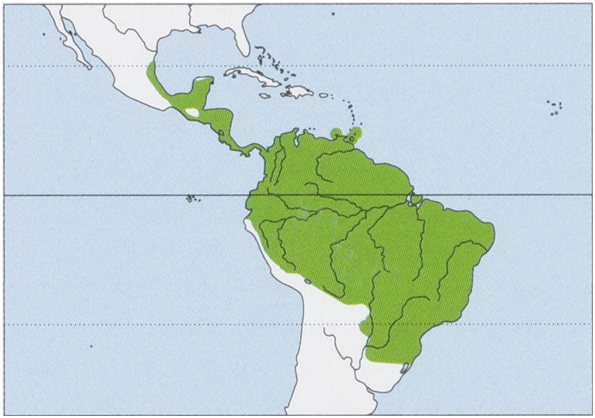
Subspecies and Distribution.
C. p. tricolor G. S. Miller, 1902 — Parana River drainage, including S Bolivia, Paraguay, S Brazil, and N Argentina. View Figure
Descriptive notes. Head—body 48-70 mm, tail 8-16 mm, ear 12-22 mm, hindfoot 12-17 mm, forearm 41-45 mm; weight 15-25 g. Seba’s Short-tailed Bat is the largest species of Carollia , although some measurements overlap with those of the Silky Short-tailed Bat (C. brevicaudum) and Sowell’s Short-tailed Bat (C. sowellr) in specific parts of their distributions. Dorsal fur of Seba’s Short-tailed Bat varies from blackish to various browns, grays, and bright orange. Fur appears shorter (5-6 mm), coarser, and sparser than in similar species. Hair on nape of neck has three bands, dark basal band, lighter middle band, and darker terminal band (sometimesfaintly frosted), but bands do not contrast sharply. Forearm is sparsely haired and looks naked in most populations. Longer tibia and feet as compared to other Carollia species. Wing membranes are dark brown to blackish and attached to ankles. Uropatagium is wide, enclosing shorttail (about one-third the length of uropatagium), with a deep notch. Horseshoe of noseleaf is free on sides and fused below nostrils. Lower lip has central papilla surrounded by smaller warts in a U-shape. Ears are moderately large, broad, and triangular, with pointed tips. Rostrum is elongated, braincase is robust, and interorbital region is slightly inflated. Upper tooth rows are fairly straight and more crowded than in the Silky Short-tailed Bat, with reduction of diastema between premolars. Lower jaw is Vshaped, and occlusal surface of I, is concealed by canine cingula. Chromosomal complement has 2n = 20 (females) or 21 (males) and FN = 36. Females are XX and males XY. additional Y is the homolog of an autosome translocated to the X element.
Habitat. Typically humid tropical forests commonly at elevations below 1700 m and less frequently up to 3000 m. Seba’s Short-tailed Bat also is tolerates different habitats. It is found in tropical evergreen and deciduous forests and cleared areas covered with only grass, thickets, low shrubs, and a few trees. It might have a preference for degraded areas, where humans have transformed original forests into agricultural clearings. It can be found in open formations like savannas, cerrado, Pantanal wetlands, dry forests, deciduous forests, urban areas, and human-disturbed forests, but at lower densities.
Food and Feeding. Seba’s Short-tailed Bat is perhaps one of the most studied phyllostomids relative to its ecology, almost as much or greater than Jamaican Fruit-eating Bat ( Artibeus jamaicensis ) or Pallas’s Long-tongued Bat ( Glossophaga soricina ). Foraging patterns have been studied in detail in Costa Rica and Panama. More than 50% of dietary fruits are produced on low trees and shrubs, categorizing this bat in the “ground-story” frugivore guild. Fruit is usually carried off to be consumed in a temporary night roost. Individuals have spatially well-defined feeding patterns, with little overlap between individuals; Piper spp. ( Piperaceae ) is the preferred food during rainy seasons. Diet also includes other available fruits and some insects. Insect material constituted as much as 40% of stomach contents of Panamanian individuals in April-May. Insects constitute 10% of stomach contents in wet season and 40% in dry season in Costa Rica. It seems Seba’s Short-tailed Bat purposely supplements its diet with insects. Being a lowland species,it Is an important frugivore, consuming as many as 42 species offruits from 13 genera in twelve families of plants. As other species of Carolia ,it is strongly associated with species of Piper and Solanum (Solanaceae) but also species of Cecropia (Urticaceae) , Vismia (Hypericaceae) , and Anthurium (Araceae) . It supplements its diet with nectar and pollen during dry season when fruit availability is low and flower production peaks. Due to its abundance, Seba’s Short-tailed Bat can be an important pollinator of Ochroma lagopus ( Malvaceae ), Hymenaea courbaril ( Fabaceae ), Bombacopsis quinata ( Malvaceae ), Ceiba pentandra ( Malvaceae ), Pseudobombax septenatum ( Malvaceae ), Crescentia sp. ( Bignoniaceae ), and Manilkara zapota ( Sapotaceae ) in Costa Rica.
Breeding. Seba’s Short-tailed Bat shows bimodal polyestry, with birth peaks in February—May andJune-August in Panama butearlier in Colombia, suggesting a geographically variable pattern that adjusts to local rainfall regimes. In Costa Rica, pregnant and lactating females were observed in February—June and one pregnant female in December. Like other frugivores, Seba’s Short-tailed Bat could have two birth periods: one in the last one-half of dry season and other in middle of wet season. One young is born per pregnancy.
Activity patterns. Seba’s Short-tailed Bats leave day roosts to forage soon after sunset, creating an early evening activity peak. No other clear peaks follow. Females and bachelor males commute to feeding areas, with exploratory flights constituting only 1-5% of 393 km collectively flown by 24 tagged individuals. They then search, harvest, and carry a fruit to a feeding roost and repeat these feeding passes 40-50 times per night. They return to day roosts between 03:00 h and 05:00 h, after ingesting at least their own weight in fruit pulp and seeds. They might carry fruits to day roosts at the end of the night. Most individuals forage within 2 km oftheir day roosts. Mean recapture distances vary from 167 m to 310 m and are correlated with body size. Seba’s Shorttailed Bats roost at caves, tunnels, mines, culverts, hollow trees, hollow logs, and houses and under bridges. There is a record of two individuals roosting under banana leaves. When found in caves, Seba’s Short-tailed Bats roost in the darkest or well-lit areas.
Movements, Home range and Social organization. Seba’s Short-tailed Bats use understory vegetation, where they concentrate feeding activity on fruits, especially slender, green, candle-like fruits of Piper plants. They are gregarious; groups of ten to more than 100 individuals commonly roost together. Studies in captivity and in the wild indicate that they have a polygynous (harem) social organization. Males defend protected roosting sites where females aggregate, suggesting a resource defense system. Two types of roost sites exist: harem sites used by a single territorial adult male, one or severalfemales, and juvenile offspring and bachelorsites used by adult and subadult males that do not have harems. Young femalesjoin these latter groups seasonally. Only 12-17% of adult males have harems. In captive colonies, harems have 1-5 females; males actively recruit females by hovering and vocalizing, suggesting that females choose harems on the basis of male quality. Territorial males are faithful to a given roost for up to nine months, and harem males defend their territory even in the absence of females. They actively defend their harem from intruders by nosing, wing shaking, and vocalizing. Seba’s Short-tailed Bat has low fecundity. Annual mortality rate is 53% for the first two years of life, and 22% for the following years. Life expectancy is 2-6 yearsat birth, and maximum life expectancy is nearly ten years. Survivorship of males and females is similar. Mother-young communication is mediated through sounds, but olfactory cues might also be important. Seba’s Short-tailed Bat has been observed roosting with at least 35 other bat species in eight families.
Status and Conservation. Classified as Least Concern on The IUCN Red List. Seba’s Short-tailed Bat is locally abundant, has a wide distribution,is very tolerant of various habitats, and occurs in several protected areas throughout its distribution.
Bibliography. Baker & Bleier (1971), Bonaccorso et al. (2006), Castano et al. (2018), Charles-Dominique (1991), Cloutier & Thomas (1992), Durant et al. (2013), Fleming (1988, 1991), Fleming & Heithaus (1986), Fleming et al. (1972), Genowayset al. (1998), Goodwin & Greenhall (1961), Gorchov et al. (1995), Heithaus & Fleming (1978), Hoffmann & Baker (2003), Hsu et al. (1968), Maguina et al. (2012), McLellan (1984), McLellan & Koopman (2008), Pine (1972), Reid (2009), Seba (1734), Smith & Genoways (1974), Stoner (2001), Thies & Kalko (2004), Thomas (1911a), Wilson (1979).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Carollia perspicillata
| Don E. Wilson & Russell A. Mittermeier 2019 |
Vespertilio perspicillatus
| Linnaeus 1758 |