Emesis ( Aphacites ) hypoaithos, Callaghan, Trujano-Ortega &Ríos-Málaver, 2024
|
publication ID |
https://doi.org/10.11646/zootaxa.5443.3.5 |
|
publication LSID |
lsid:zoobank.org:pub:D39BC251-9B14-4C00-8A34-280F97ED382F |
|
DOI |
https://doi.org/10.5281/zenodo.11068863 |
|
persistent identifier |
https://treatment.plazi.org/id/03A7878F-9851-FFAC-FF76-FCB3FBD3FD1C |
|
treatment provided by |
Plazi |
|
scientific name |
Emesis ( Aphacites ) hypoaithos, Callaghan, Trujano-Ortega &Ríos-Málaver |
| status |
sp. nov. |
Emesis ( Aphacites) hypoaithos, Callaghan, Trujano-Ortega &Ríos-Málaver , sp. nov.
( Figs. 1–4, 13–18 View FIGURES 1–14 View FIGURES 15–21 )
Emesis tegula DeVries 1997, p. 209 View in CoL , Pl. 17, Figs. 17–19 View FIGURES 15–21 ; Emesis tegula Warren et al. 2023 View in CoL , #G7300 (dorsal), #G7299 (ventral).
Diagnosis. Emesis ( Aphacites) hypoaithos sp. nov. males can be separated from other species of Emesis by the yellow orange ventral surface combined with a prominent postmedian row of connected crescent shaped spots on both wings; on the hindwing, these are proximal to a marginal row of small black spots, the first two and the last one of which are slightly larger. The females are yellow on both wing surfaces but can also be distinguished by the same prominent postmedian band, with the first two marginal spots being larger on the apex of the ventral hindwing.
Description. MALE: Forewing length HOLOTYPE 20.6 mm, paratypes: 19.07 mm (n=9), S= 1.92 mm.
Wing shape ( Fig. 1 View FIGURES 1–14 ): Forewing costal margin very slightly indented midway, before reaching a rounded, slightly falcate apex, distal margin slightly curved, proceeding to rounded tornus, inner margin straight; hindwing costa slightly curved to rounded apex, distal margin slightly rounded to pointed tornus, inner margin slightly convex to base.
Dorsal surface ( Fig. 1 View FIGURES 1–14 ): Ground color of both wings light brown with slight orange tinge. Forewing costal margin dark brown; forewing discal area infused with variable grey scaling, with short linear marks, discal cell with a slight reddish tinge, containing three linear black spots and one elongated at the end, with a similar set of elongated spots below in cell CuA 2; distad a post discal broken row of small, offset, irregular lines, those from cells R 1 to M 3 displaced proximally between M 1 and M 3, space between end of discal cell and cell CuA 1 darker brown, the irregular lines in cells CuA 1 and CuA 2 offset proximally; distad a parallel postmedian row of faint, crescent spots from costa to 1A+2A, space between these and the post discal row of irregular lines lighter yellow/brown forming a 2.3 mm wide median band; distad a row of small, faint submarginal dark brown spots, proximally with slightly reddish scaling, margin and fringe dark brown; anal margin below 1A+2A with some infusion of grey scaling.
Hindwing follows general pattern and shading of forewing. Discal area infused with light grey scaling, costa light yellow, cell Sc+R 1 with two small spots, discal cell with some reddish scaling and same configuration of marks as on forewing; cell CuA with two faint, linear spots directly below; a post discal row of short, unconnected, irregular black lines with those in cells M 2 and M 3 offset distally, distad of this, a row of post median crescent spots continues from forewing with the median band of similar lighter scaling, distad with the submarginal row of black spots as on forewing, proximally with reddish scaling; margin and fringe dark brown; anal margin light yellow; vein 3A accompanied by long, androconal scales increasing to tornus.
Ventral surface ( Fig. 2 View FIGURES 1–14 ): Ground color of both wings yellow orange. Costa of forewing above Sc+R 1 with some darker scaling, maculation of forewing reflected ventrally, but more strongly marked; space between end of discal cell to cell CuA 1 and post discal row of short lines darker; area occupied by median band of slightly lighter ground color; postmedian row of crescent brown spots more prominent, space between these and submarginal spots with slightly lighter scaling; margin distad of submarginal spots infused with darker scaling from apex to tornus; inner margin from middle of cell CuA 2 yellow.
Hindwing maculation continuation of that on forewing reflecting dorsal surface, with postmedian and submarginal black spots more prominent, the two submarginal spots on apex slightly larger with marginal area infused with darker scaling as on forewing; a row of androconal hairs found in middle of cell CuA 2; vein 1A+2A to inner margin yellow.
Head: Upper surface of frons and collar dark orange, lower frons around labial palpi white; labial palpi short, light orange, with short scaling, not extending beyond face when viewed dorsally, third segment short ( 0.4mm), rounded, 30% of length of second segment; proboscis light brown, long; antennal length 60% of forewing length, segments with two small, white points on each segment, club tips black.
Body: Dorsal surface color of thorax and abdomen dark brown, ventral surface light orange with random white scaling; forelegs trimerous, pubescent, with femur separating below midpoint of coxa; midlegs and hindlegs light orange with a few random spurs, final tarsus segments black.
Male genitalia ( Figs. 15–16, 18 View FIGURES 15–21 ), (n=5): Uncus with lobes joined, spread, uniting in a central peak with a variable notch in ventral view, in lateral view pointed distad at nearly 90°, ventrally slightly indented; tegumen dorsally long and slightly rounded, ventrally produces an extended point, cephalad indentation for attachment of vinculum extends higher than tips of vinculum; scaphium broad terminating in a point in ventral view ( Fig.16 View FIGURES 15–21 ); falces long, curved, pointed, and doubled inwardly; vinculum attached to cephalad margins of tegumen, not joined, and continues slightly curved to saccus with pointed triangular posterior flange in middle; saccus long, rounded in ventral view; valvae with a lateral plate on each valve in lateral view, valvae terminating in broad, slightly bifurcated tip, upper projection slightly smaller and rounded and lower rounded, short, and slightly turned outward in ventral view ( Fig. 16 View FIGURES 15–21 ); dorsally, valvae joined to opposite valve by a long transtilla; aedeagus short, wide, slightly curved with small ventral flange at exit of pedicel, posterior half from attachment of pedicel to tip sclerotized, caudad segment lightly sclerotized; tip pointed with vesica exiting dorsally, vesica with numerous small cornuti on tip, and with larger cornuti behind tip ( Fig. 18 View FIGURES 15–21 ).
FEMALE ( Figs. 3–4 View FIGURES 1–14 , 17 View FIGURES 15–21 ): Forewing length, 17.7 mm (n=1).
The female differs from the male in the following:
Wing shape ( Fig. 3 View FIGURES 1–14 ): Forewing costal margin more indented, distal margin more curved before rounded tornus; likewise, hindwing distal margin more rounded to more rounded tornus, inner margin slightly rounded.
Dorsal surface ( Fig. 3 View FIGURES 1–14 ): Female with same maculation as male, differing from the male in lighter yellow to brown ground color of both wings; a 3.6 mm wide median band of lighter yellow crosses both wings between a post discal row of short, curved lines and a fainter row of joined crescent shaped markings proximal to a marginal row of small black spots, the first two slightly larger; margin of forewing from apex to vein Cu1 variably infused with dark scaling; fringe black.
Ventral surface ( Fig. 4 View FIGURES 1–14 ): Ground color of both wings light yellow, maculation identical but more pronounced; apex of forewing with infusion of darker scaling to vein M 3; apex of hindwing with first two prominent submarginal black spots larger than male; fringe black.
Head: Same as male but with labial palpus narrow and light brown same length as male, but second segment broader, antennae as male.
Body: Dorsal surface color of thorax and abdomen yellow, abdomen with lighter scaling between segments, ventral surface white, appendages light yellow.
Female genitalia ( Fig. 17 View FIGURES 15–21 ), (n=1): Corpus bursae elongated with two sail-shaped invaginated signae of equal size, arising close to midpoint of corpus bursae, which tapers to exit of ductus bursae; ductus bursae long, with some small striations, broadening with increased striations and sclerotization before reaching the sclerotized sterigma slightly bent from left in ventral view, at the exit of membranous ductus seminalis; sterigma between ductus seminalis and ostium bursae tubular, tapering slightly to ostium bursae, dorsally opened with two small lateral flanges, and slightly bent to left viewed ventrally; lamella postvaginalis consists of a broad, irregular plate dorsad of ostium bursae; lobes of papillae anales rounded, pubescent ventrad with thicker sclerotization dorso-laterally.
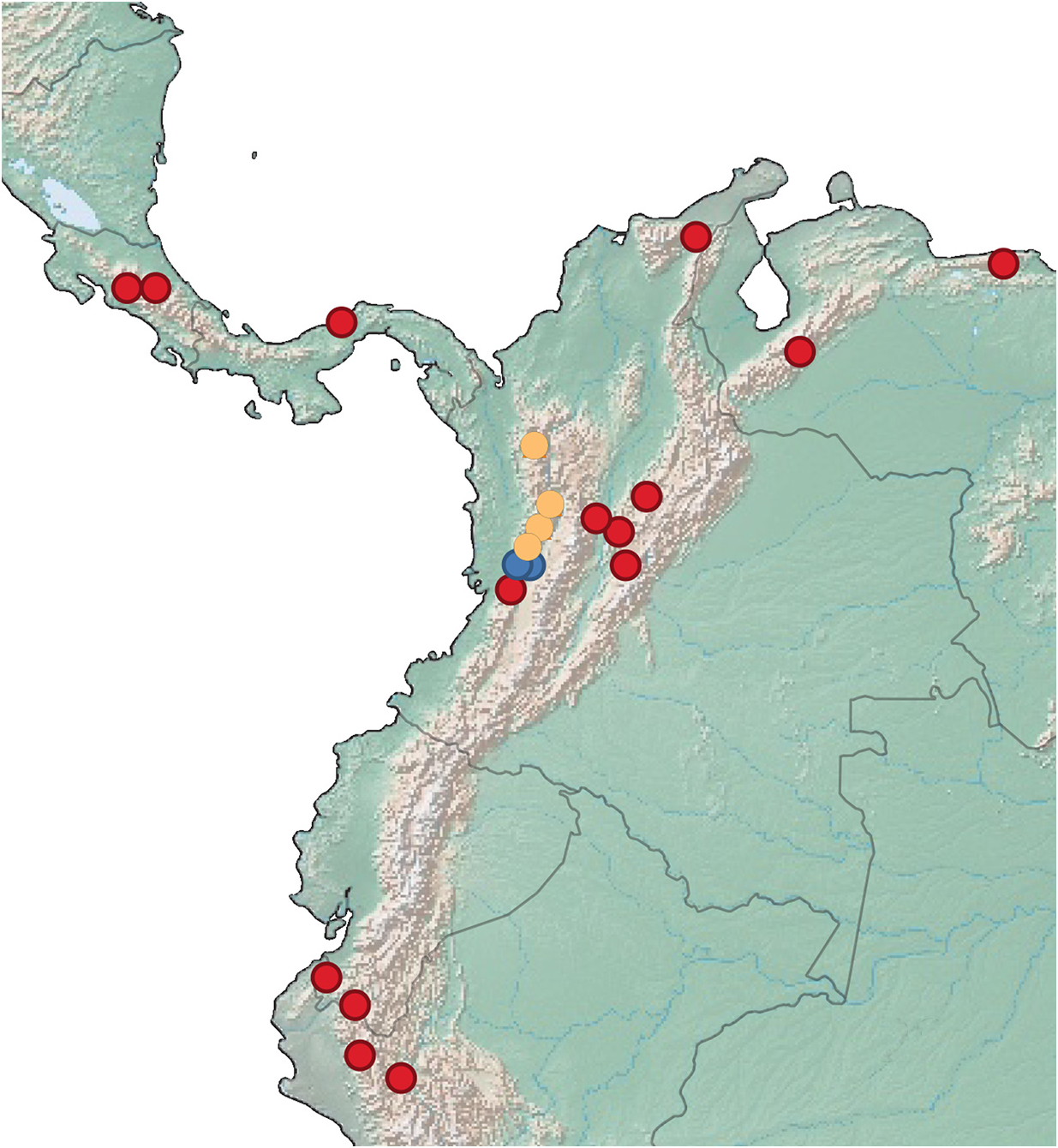
Distribution ( Fig. 29 View FIGURE 29 , red circles). The distribution of Emesis hypoaithos sp. nov. is from Costa Rica to Colombia west of the Serra Occidental to northern Peru and northern Venezuela. The species also most likely occurs in Ecuador due to the existence of similar habitats.
Etymology. The name for this species is a noun in opposition derived from the Greek words “hypo” (under) and “aithos” (dark red) referring to the color of the ventral surface.
Type Material. Holotype male with labels: HOLOTYPUS / COLOMBIA: Cundinamarca, Fusagasugá La Aguadita , 04° 23’26” N / 74° 19’40” W, 2200m, 2.ii.1958, E. Schmidt Mumm, leg. SM-5947. The type will be deposited in the Instituto Alexander von Humboldt, Colombia IAvH-E-9825 GoogleMaps . ALLOTYPUS / COLOMBIA El Cerrejón, Guajira, 1.v.81, Callaghan leg. /Genitalia #1505/ genitalia vial .
Paratypes: COSTA RICA: 1♂ ( CJC), Ciudad Colón , 10.x.93, Callaghan, leg. ; 1♂ ( CJC), Turrialba , Cartago, 28.viii.76, Serrano leg. PANAMA: 1♂ ( CJC), Rio Parté , Panamá Prov., 30.ix.78, Callaghan leg. COLOMBIA: 1♂ ( CJC), Villeta , Cundinamarca,10.ix.21, DNA sample 76, Callaghan leg. ; 1♂ ( CJC), Quichas , Santander, 400m, 21.i.95, F. Montero leg. ; 1♂ ( CJC), Vitoria , Caldas, 9.ii.69, DNA sample 77 ; 1♂ ( CJC) Bahía Concha, Tayrona , Atlántico, 22.ix.04 ; 1♂ ( CJC), Bahía Concha, Tyrona , 22.ix.04 . VENEZUELA: 1♂ ( CJC), Distrito Federal , Miranda, 30.vii.75, Costa leg. ; 1♂ ( CJC), Monte Zerpa , Merida, 2500m, 27.ix.07, Mielke & Orellana leg. PERU: 3♂ ( MUSM), Piura, Canchaque , 1200–1300m, 13.iv.81, Lamas, leg. ; 1♂ ( MUSM), 18.v.82, Lamas leg. ; 1♂ ( MUSM), Piura Ayabaca, Ambasal , 2900m, 04°35’S / 79°45’W, 9.vi.2007, Zelada leg. GoogleMaps ; 1♂ ( MUSM), Piura km 30 Olmos-Chamaya , 1300m, 05°54’S / 79°32’W, 17.vi.95, Lamas leg. GoogleMaps ; 1♂ ( MUSM), Piura Quebrada Ulunche , 1000m, 04°42’S / 79°50’W, 2.vi.00, Lamas leg. GoogleMaps ; 1♂ ( MUSM), Tumbes Cerros de Amotape, El Caucho , 3°49´S / 80°16´W, 340m, 30.ix.2019, Gamboa leg. GoogleMaps
Biology. Emesis hypoaithos sp. nov. inhabits a variety of vegetation types throughout its range, from sea level to 2200m in Colombia, but reaching 2900m in Peru. In Colombia it is found from the Dry Tropical Forest (Bs-T) in the Guajira to Pluvial Montane Rainforest (Bp-MB) on the Pacific coast. It is never common, and females are especially rare. The males perch in the late morning to early afternoon on leaf dorsal surfaces with wings outspread about 2 to 3m high in sunny localities along paths or inside the wood´s edge, usually close to streams where it has been observed on damp earth. DeVries (1997), in the section under “ Emesis tegula ”, gives information on the habits of this butterfly in Costa Rica. He reports a putative larval foodplant as Pisonia aculeata L. ( Nyctaginaceae ). The larva partially folds the leaf to provide a shelter in which it pupates. In Costa Rica, the species has been reported from 300 to 600m, occupying a range of habitats.
Discussion: In their study of the genomics of the tribe Emesidini, Zhang et al. (2019) defined a subgenus Aphacites Hübner, [1819] with Emesis lucinda, (Cramer [1775]) as the type species. This subgenus was divided into two clades with species close to E. lucinda separated from a group of three species, Emesis vulpina Godman & Salvin, 1886 , Emesis diogenia Prittwitz, 1865 and Emesis heteroclita Stichel,1929 , sharing similar genomic characteristics. A fourth species, Emesis eleanorae Gallardo & Grishin, 2021 , was described from Honduras ( Gallardo et al., 2021). An additional species belonging to this sub clade is Emesis tegula, Godman & Salvin, 1886 , placed by Zhang et al. (2019) erroneously in their subgenus Tenedia as the result of a misidentification. The basis for this is shown in Gallardo et al. (2021). In their figure 1- h, p. 54, the specimen identified as E. tegula is most likely Emesis tristis Stichel, 1910 , a taxon which indeed shares the genomic and morphological characteristics of Tenedia , as do Emesis lupina Godman & Salvin, 1886 and Emesis tenedia C. Felder & R. Felder, 1861 , also illustrated. Comparison of the genitalia of both E. tegula ( Figs. 19, 20 View FIGURES 15–21 ) and E. hypoaithos sp. nov. ( Figs. 15, 16 View FIGURES 15–21 ) and with that of E. eleanorae Gallardo & Grishin, 2021 , shows that these belong to the same subclade of Aphacites , as does E. eleanorae .
Emesis hypoaithos sp. nov. has been identified as E. tegula ( Figs. 9–12 View FIGURES 1–14 , 19–21 View FIGURES 15–21 ), with which it is sympatric by DeVries (1997) (plate 17, Figs. 17, 18 and 19 View FIGURES 15–21 ), and in the section under E. tegula , in Warren et al. (2023) (Figs. G7300, G7299 from Panama, Chiriqui). The males of both taxa share the light reddish-brown ventral surface but are separated by the strongly marked wing surfaces, especially the distal margins of the ventral surface of E. hypoaithos sp. nov. with well-marked crescent spots. The faint ventral surface maculation of E. tegula was the principal character cited by Godman & Salvin (1886, p. 444) to identify this species.Although there are considerable individual differences in the male genitalia of both species, E. hypoaithos sp. nov. can be separated by the longer valvae, the lateral plate of the genitalia rounder, the tips of the valvae with the dorsal of the two posterior processes smaller, and a stouter aedeagus. The width of the lobes of the uncus are usually more widely spread. Another sympatric species, Emesis ( Mandania) mandana (Cramer, 1780) has also been associated with E. hypoaithos sp. nov. in Seitz (1916) and DeVries (1997), but is separated by the lighter dorsal surface, lighter reddish tone to the ventral surface, distinct median band on both wings bordered distad with crescent spots and more rounded distal margins, as well as the genitalia.
The females of E. hypoaithos sp. nov. are more difficult to separate from other members of the genus, sharing the yellow shaded ventral surface and lighter median band of E. vulpina and E. tegula , as well as two slightly darker marginal spots on the apex of the ventral hindwing. However, the dorsal surface maculation is more pronounced and the ground color on both wing surfaces slightly darker than the other species ( Figs. 3–4 View FIGURES 1–14 ). While sharing the median band with E. tegula , the spots on the margin of the VHW of Emesis hypoaithos sp. nov. are more sharply defined, especially those on the apex. The female genitalia of Emesis hypoaithos sp. nov. can be separated from those of other members of the clade by the shorter sclerotized section before the entrance to the ostium bursae ( Fig. 17 View FIGURES 15–21 ).
Variation. In the populations of Emesis hypoaithos sp. nov. studied, the principal variation in the males was size and a slightly lighter shading of the ground color on the dorsal and ventral surfaces with a slightly different width of the median band. The male genitalia show considerable individual variation, especially in the separation of the lobes of the uncus from a ventral perspective. However, a population inhabiting the western cordillera and Chocó region of Colombia is quite distinct and we describe it here as a new subspecies.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Emesis ( Aphacites ) hypoaithos, Callaghan, Trujano-Ortega &Ríos-Málaver
| Callaghan, Curtis John, Trujano-Ortega, Marysol & Ríos-Málaver, Indiana C. 2024 |
Emesis tegula
| DeVries, P. J. 1997: 209 |