Rhigonema naylae, Morffe, Jans & Hasegawa, Koichi, 2017
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4269.2.6 |
|
publication LSID |
lsid:zoobank.org:pub:66864253-16A0-4BEA-B237-088A29AF5C12 |
|
DOI |
https://doi.org/10.5281/zenodo.6053173 |
|
persistent identifier |
https://treatment.plazi.org/id/03A787F9-4E10-6A41-FF19-FD66FCCC67FC |
|
treatment provided by |
Plazi |
|
scientific name |
Rhigonema naylae |
| status |
sp. nov. |
Rhigonema naylae n. sp.
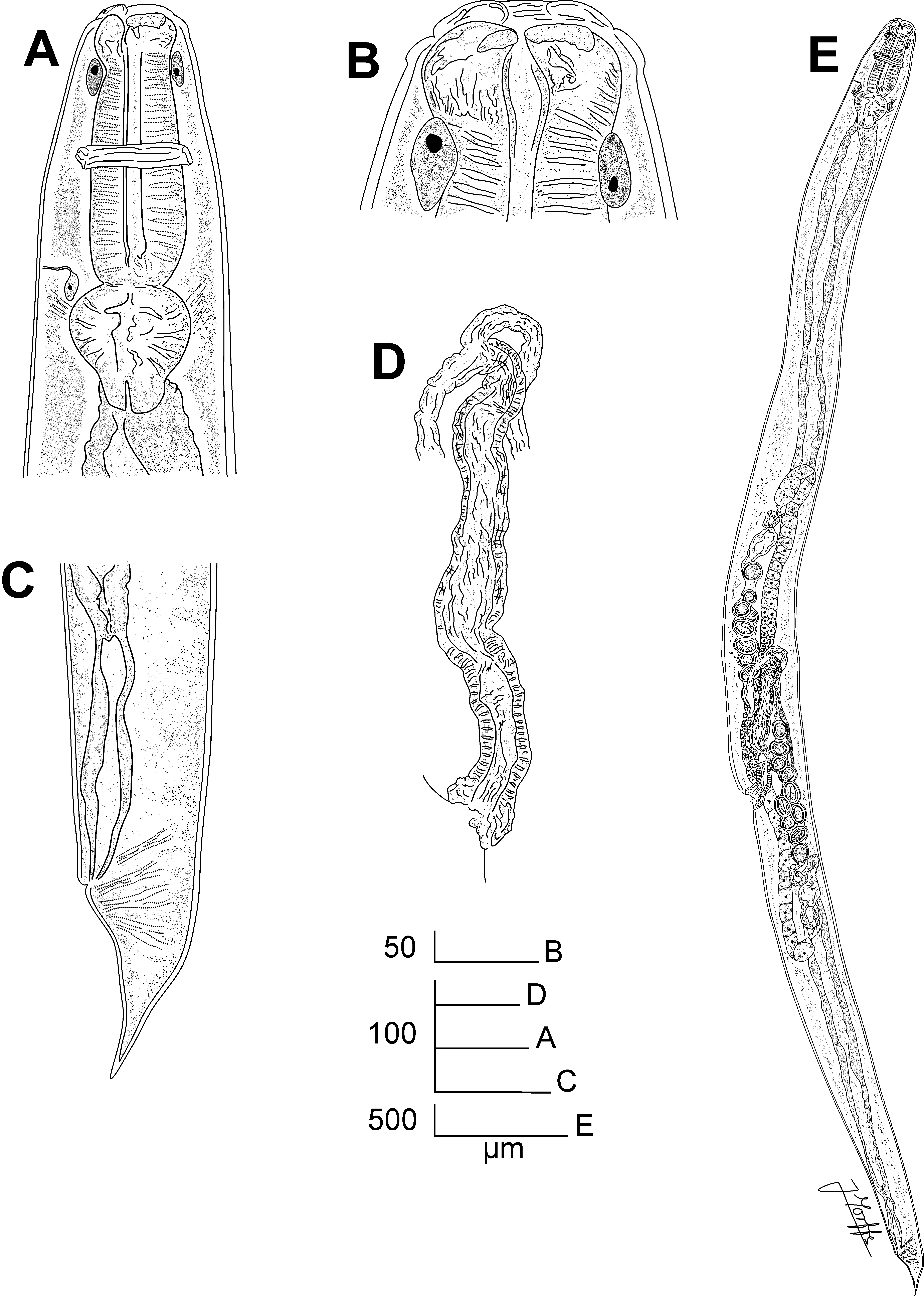
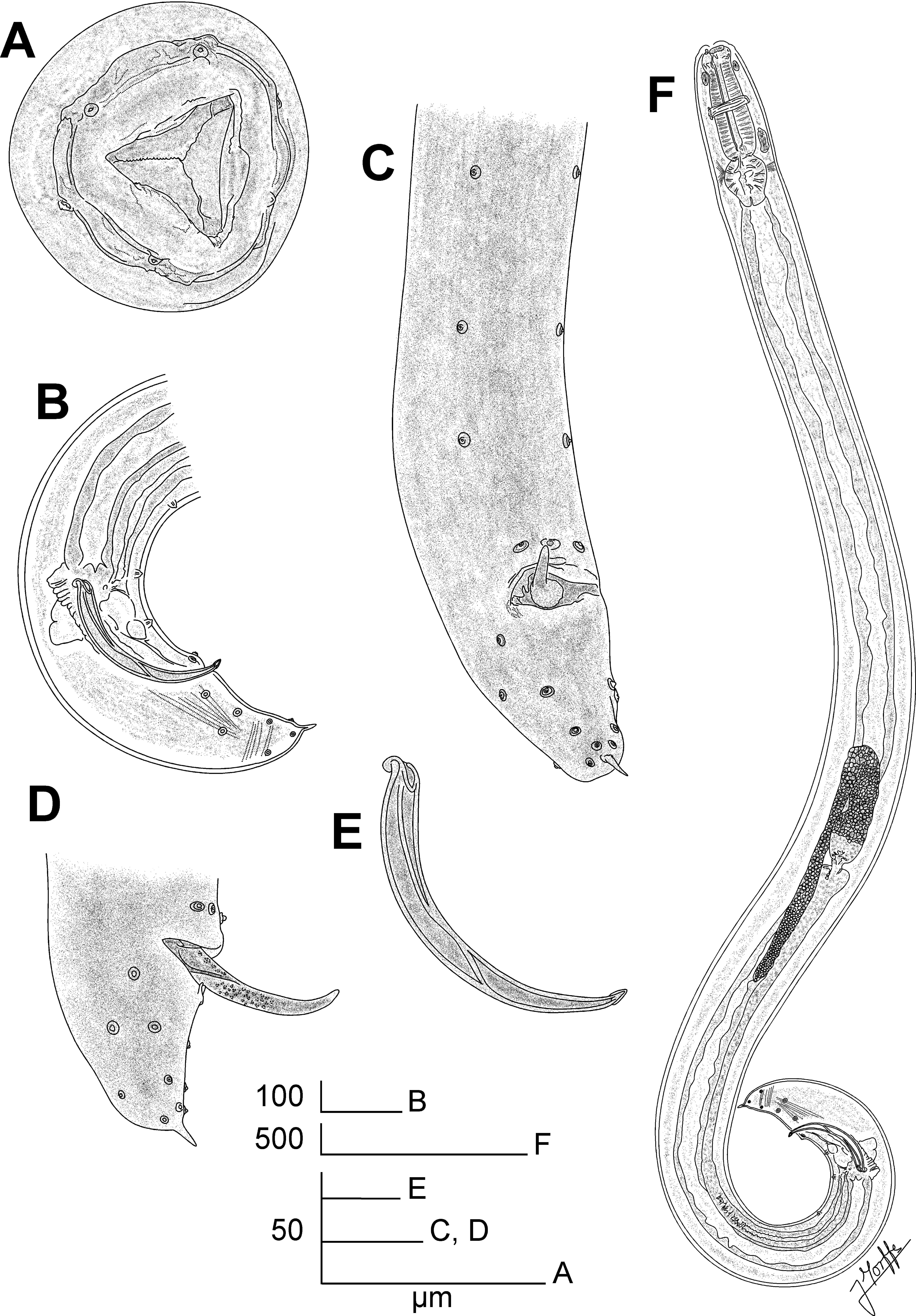
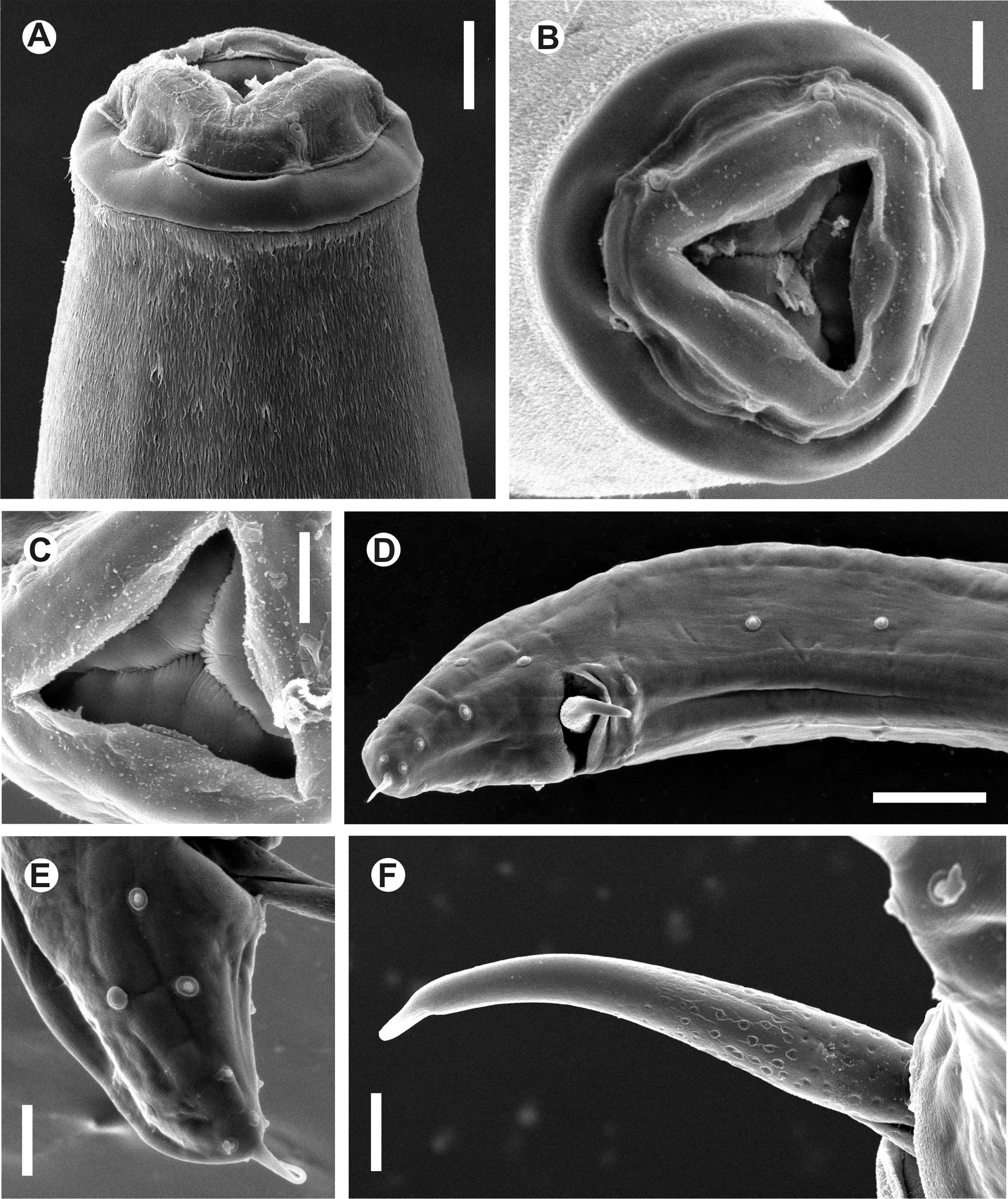
Fig. 1 View FIGURE 1 A–E, Fig. 2 View FIGURE 2 A–F, Fig. 3 View FIGURE 3 A–F
Type material. Holotype: ♀, Japan, Aichi prefecture, Kasugai, Chubu University Campus ; in Parafontaria laminata ; 20/VI/2016; S. Ozawa, K. Sato, J. Morffe coll.; CZACC 11.7000 View Materials . Paratypes: 8♀♀, same data as the latter; CZACC 11.7001–11.7008 . 8♂♂, same data as the latter; CZACC 11.7009–11.7016 . 2♀♀, same data as the latter; NCHL-A9, A10 . 2♂♂, same data as the latter; NCHL-A11, A12.
Description. General. Medium sized body. Cephalic region heavily cuticularised, consists of a well-developed cephalic cap and a wide cephalic collar, its posterior margin partially fused to the body cuticle, set-off from the body cuticle by a shallow but well-defined groove, but not projecting from the body contour. Cuticle with fine, transverse striae. Cervical region densely covered with numerous, fine and short microtrichs, extending from the base of the cephalic collar to ca. 1.5 oesophagus lengths posterior to the base of the basal bulb. Microtrichs are ca. 2–3 µm long near the cephalic collar and becoming shorter (ca. 1–2 µm) and more scattered near the point where they disappear. Cephalic cap circular in en face view, with four mammilliform, equidistant papillae; two sub-dorsal and two sub-ventral. Amphids pore-like, wide, lateral in the junction of the cephalic cap and the cephalic collar. Oral aperture triangular, with three isometric sectors, one dorsal and two sub-ventral. Three cuticularized plates articulate to form a jaw-like apparatus in the anterior end of the oesophagus. Plates isomorphic and isometric, arranged as one dorsal and two sub-ventral, coinciding with the sectors of the oral aperture and present ca. 30 minute, rounded teeth on their margins. Oesophagus characteristic of the genus, with a robust, powerful corpus with a distal expansion. Isthmus not evident. Basal bulb anchored to body wall by muscles attached to its equatorial point. Three cardia are evident, extending from the base of the basal bulb into the intestine, which is simple, subrectilinear, with its fore region slightly inflated. Brown arcade cells surrounds the corpus posterior at its distal expansion. Nerve ring encircling oesophageal corpus ca. its midpoint. Excretory pore ventral, located at the level of the junction of the oesophageal corpus and the basal bulb.
Female. Body narrowed immediately posterior the level of the vulvar region. Vulva a ventral transverse slit, slightly displaced to the posterior half of the body and presenting an irregular brown-yellowish plaque. Type 2 genital tract ( Adamson 1987), consisting of a muscular, forwardly directed, thick-walled vagina vera which leads to a longer vagina uterina (ca. two vagina vera lengths) thin-walled and less muscular. The distal end of vagina uterina divides into the two uterine ducts. Ovaries reflexed, arranged in a didelphic-amphidelphic pattern. Eggs rounded to ellipsoidal, their shell thick and smooth. In some specimens a few eggs were observed in the distal end of the vagina uterina or immersed in the vulval plaque. Rectum comparatively long, with depressor ani muscles well developed, extending from the sub-dorsal body wall. Tail very short, conoid, slightly subulate, ending in a fine point.
Male. Body slightly shorter than females, posterior end ventrally curved. Monorchic. Testis distally reflexed at the level of the posterior half of body, flexure ca. three body-widths long. The distal flexure of the testis is filled with rounded, granular spermatocytes. Posterior to the distal flexure the testis has three regions: a short, wide segment (ca. half of the distal flexure length) filled with spermatids, set-off from a second, narrow region by a strait constriction and a third, posterior area that opens in the cloaca, with wider and more muscular walls. Spicules isomorphic and isometric, ventrally arcuate. Shaft broad, its surface sculptured with randomly scattered punctations that extend through most of its length and disappear near the tip, which has a smooth surface. Capitulum dorsally hamate. An inconspicuous velum is observed in protruded spicules. Twenty-three copulatory papillae present. Pre-cloacal papillae arranged as four pairs, the first, second and third sub-ventral and the fourth ventral, flanking the single median papilla located in the anterior lip of cloaca. Second, third and fourth pre-cloacal pairs almost equidistant, the distance between them is slightly shorter than the distance between the first and second pairs. Post-cloacal papillae consist of seven pairs, the anteriormost sub-lateral, located a short distance posterior to the cloaca. Second post-cloacal pair lateral, followed by a third sub-ventral pair, almost at the same level than the second pair, its papillae closer to each other than in the first pair. A fourth pair is sub-dorsal. Three terminal pairs clustered near the tail tip, one sub-ventral, one ventral (its papillae very close) and the last subdorsal, almost at the same level as the previous pair. Tail short, conoid, ending in a short, sharp mucron. Phasmids inconspicuous, lateral, located at the level of the fifth pair of post-cloacal papillae.
Type locality. Chubu University Campus , Kasugai, Aichi prefecture, Japan.
Type host. Parafontaria laminata (Attems, 1909) (Diplopoda: Polydesmida : Xystodesmidae ).
Etymology. Specific epithet dedicated to Nayla García Rodríguez, Cuban parasitologist and author of several species of rhigonematomorphs and thelastomatoids from the Neotropics and Africa. This is a modest homage for being the mentor, best friend and colleague of the first author.
Differential diagnosis. R. naylae n. sp. belongs to the African/Asian species group, by possessing the characteristic arrangement of copulatory papillae in the males, which have 23 papillae, including one post-cloacal pair lateral and two post-cloacal pairs sub-dorsal in position. That feature differentiates it from the Australasian/ American species, with all the post-cloacal papillae ventral or sub-ventral in position (Hunt 1995). In this group, we can include two Asian species: R. flabellifer Hunt, 1999 from Vietnam and R. golovatchi Hunt, 1999 from Philippines, whose copulatory papillae are all sub-ventral ( Hunt 1999a).
The type of female genital tract distinguishes R. naylae n. sp., which has a Type 2 genital tract, from R. multipapillatum (Skrjabin, 1916) from Eastern Africa, R. peziphorum Hunt, 2002 from Zimbabwe and R. voratum Hunt, 1999 from Vietnam ( Hunt 1999a; 2002b, c) each of which have a Type 1 female genital tract.
R. naylae n. sp. can be distinguished from several African taxa with a Type 2 female genital tract ( R. oxydesmi Hunt, 2002 ; R. rostrellum Hunt, 2002 ; R. spicatum Hunt, 2002 and R. xiphiurus Hunt, 2002 ) by their posterior rim of the cephalic collar, which joins with the body cuticle at an angle ( Hunt 2002c). Additionally, the posterior margin of the cephalic collar of R. naylae n. sp. does not overhang the body cuticle. R. spicatum and R. xiphiurus also present a different tail shape: longer and attenuate-subulate in females and with a ventral subulate projection in males. R. naylae n. sp. differs from R. disparovis Van Waerebeke, 1991 and R. madecassum Van Waerebeke, 1984 (from Ivory Coast and Madagascar, respectively) in the shape of the spicules, which are robust, arcuate and with a hamate capitulum. R. disparovis has long, slender spicules and lacks a conspicuous capitulum ( Van Waerebeke 1991). In R. madecassum the spicules are shorter and more robust, but lack the hamate capitulum ( Van Waerebeke 1984). Moreover, both R. disparovis and R. madecassum are longer than R. naylae n. sp. (body length of females = 5.58–7.82 vs. 7.68–8.38 vs. 4.45–5.33; body length of males = 4.08–5.36 vs. 6.68–7.51 vs. 2.81–4.20). R. pachyboli Adamson, 1983 has an unusual arrangement of copulatory papillae: 25 in number and asymmetrical ( Adamson 1983), instead of the 23 and symmetrical arrangement of R. naylae n. sp. R. fecundum Hunt, 2002 and R. seychellarum Adamson, 1987 have a longer body length than R. naylae n. sp. in both sexes (body length of females = 4.45–7.02 vs. 6.63 vs. 4.45–5.33; body length of males = 4.25–4.69 vs. 5.62 vs. 2.81–4.20). In both of the latter species, the anterior lip of the vulva forms a vulval flap ( Adamson 1987; Hunt 2002c) that is absent in R. naylae n. sp.
Several Asian Rhigonema have Type 2 female genital tract. From these, R. naylae n. sp. differs from R. erringtoni Van Waerebeke, 1986 and R. longicorpus Rao, 1973 (from Malaysia and India, respectively) whereby the males possess four pairs of pre-cloacal papillae as opposed to three ( Rao 1973; Van Waerebeke 1986). In addition, the tail of both sexes of R. erringtoni is unusually long and subulate whereas the Indo-Malayan species are short and conoid. A similar case exists for R. malayae Hunt, 1998 in which the females have a long conoid tail and the males have a conoid tail with the end bluntly rounded ( Hunt 1998b), and lacking the short mucron present in R. naylae n. sp. Another Indian species, R. neyrae Singh, 1955 has microtrichs extending up to ca. half of the body length ( Singh 1955) vs. R. naylae n. sp. that possesses pilosity extending from the posterior margin of the cephalic collar to ca. 1.5 oesophagus lengths posterior to the level of the basal bulb.
R. ornatum Majundar, 1967 from India resembles R. naylae n. sp. in body length of both sexes (body length of females = 3.70–5.00 vs. 4.45–5.33; body length of males = 2.90–4.30 vs. 2.81–4.20) and the spicules length, which according to Hunt (2002a) were presumably measured along the cord rather than the arc (200–230 vs. right spicule = 187–248, left spicule = 212–245). However, the tail of the males in R. ornatum is longer than R. naylae n. sp. (160–200 vs. 119–157) and presents a well-developed spicate process instead of a short mucron. R. indicum ( Gupta & Tewarson, 1977) from India present the shape of the tail in both sexes similar to R. naylae n. sp.: short, conoid and subulate in the females and conoid with a short mucron in the males ( Gupta & Tewarson 1977). Both species can be clearly differentiated by having R. indicum a longer body than R. naylae n. sp. (body length of females = 6.40–7.44 vs. 4.45–5.33; body length of males = 5.20–7.05 vs. 2.81–4.20). Similarly, R. raoi Adamson, 1983 also from India is larger than R. naylae n. sp. (body length of females = 5.70–6.30 vs. 4.45–5.33; body length of males = 3.80–4.70 vs. 2.81–4.20). R. molitor Hunt, 2002 from Sri Lanka differs from R. naylae n. sp. by its longer body in both sexes (body length of females = 5.32–6.58 vs. 4.45–5.33; body length of males = 4.71–5.66 vs. 2.81–4.20) and lacking the body narrowed immediately posterior to the vulva (Hunt 2002). Moreover, accordingly with the figures of Hunt (2002a), the vagina uterina of R. molitor is comparatively longer than R. naylae n. sp. (ca. three vs. two vagina vera lengths long).
R. trichopeplum Hunt & Moore, 1995 from Myanmar can be distinguished from R. naylae n. sp. by its longer body (body length of females = 5.56–7.23 vs. 4.45–5.33; body length of males = 4.14–5.08 vs. 2.81–4.20), the cephalic collar overhanging the body cuticle, the anterior lip of vulva forming a long flap-like structure, and the vagina reflexed, with a proximal portion anteriorly directed and a distal portion posteriorly directed ( Hunt & Moore 1995). Additionally, in R. naylae n. sp. the cephalic collar does not overhang the body cuticle, lacks a vulval flap and the vagina is straight and anteriorly directed. R. rigonanae Hunt, 1999 from the Philippines also presents unusual features in the vulval region and genital tract that differentiates it from the present new species: the posterior lip of the vulva is more prominent than the anterior lip and the first portion of the vagina vera is posteriorly directed or forms an angle of 90° with the body axis ( Hunt 1999a). In addition, both sexes of R. rigonanae are longer than R. naylae n. sp. (body length of females = 6.30–7.34 vs. 4.45–5.33; body length of males = 5.15–5.79 vs. 2.81–4.20). R. nepalense Hunt, 1998 can be differentiated from R. naylae n. sp. by having the cephalic collar with its posterior margin thickened and slightly overhanging the body contour and by the shape of the female tail, which is conoid and attenuate vs. conoid and subulate ( Hunt 1998b).
Three species from Vietnam present the female genital tract of Type 2, namely R. euprepeia Hunt & Spiridonov, 1995 ; R. ingens Hunt, 1997 and R. spiridonovi Hunt, 1999 ( Hunt 1997, 1999a; Hunt & Spiridonov 1995). The Japanese species differs from R. euprepeia by having the body longer in both sexes (body length of females = 4.45–5.33 vs. 2.50–3.50; body length of males = 2.81–4.20 vs. 2.10–2.70) and the vagina vera straight vs. elbowed. R. ingens is one of the largest species of the genus, with about twice the body length of R. naylae n. sp. (body length of females = 8.88–9.32 vs. 4.45–5.33; body length of males = 7.01–7.52 vs. 2.81–4.20), but its tail is comparatively shorter (female c = 41.50–51.20 vs. 22.83–32.72; male c = 31.20–35.40 vs. 23.60–29.25). R. spiridonovi is similar to R. naylae n. sp. in several of the morphometrics, namely body length (body length of females = 4.57–5.38 vs. 4.45–5.33; body length of males = 3.29–4.71 vs. 2.81–4.20) and relative length of the oesophagus (female b = 12.60–14.50 vs. 11.43–15.28; male b = 9.00–13.90 vs. 9.36–11.06) and the tail (female c = 21.30–26.50 vs. 22.83–32.72; male c = 21.80–35.90 vs. 23.60–29.25) in both sexes. It differs mainly in the shape of the cephalic collar, which is more closely fused to the body cuticle, and the shape of the tail in females, which is also conoid but not markedly subulate and the single ventromedian papilla is hypertrophied, and larger than the papillae that form the posteriormost pre-cloacal pair ( Hunt 1999a). In R. naylae n. sp. the cephalic collar is partially fused to the body cuticle, and a shallow, narrow groove is still visible delimitating the joint; the female tail is conoid, but subulate and the single ventromedian papilla is less evident.
R. pilosum , from Okinawa, is the only species of the genus described from Japan ( Hunt 1998a). From the latter, R. naylae n. sp. differs by having a Type 2 female genital tract ( Adamson 1987). In R. pilosum the genital tract is considered as Type 1, but with the vagina uterina dilated, as a reminiscence of the diverticulum typical of Type 4 genital tracts ( Hunt 1998a). Additionally, in R. pilosum the cephalic collar is not fused and overhanging the body cuticle whereas the cephalic collar of R. naylae n. sp. is partially fused and not overhanging the body cuticle. Males of both species differ by the arrangement of the copulatory papillae, with five pre-cloacal pairs plus a single, median papilla and six post-cloacal pairs in the Okinawan species. R. naylae n. sp. has fewer pre-cloacal papillae: four pairs plus the single papilla and seven post-cloacal pairs.
| CZACC |
Coleccion Zoologia, Academia de Ciencias de Cuba |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Rhigonema naylae
| Morffe, Jans & Hasegawa, Koichi 2017 |
R. oxydesmi
| Hunt 2002 |
R. rostrellum
| Hunt 2002 |
R. spicatum
| Hunt 2002 |
R. xiphiurus
| Hunt 2002 |
R. fecundum
| Hunt 2002 |
R. molitor
| Hunt 2002 |
R. rigonanae
| Hunt 1999 |
R. spiridonovi
| Hunt 1999 |
R. malayae
| Hunt 1998 |
R. nepalense
| Hunt 1998 |
R. ingens
| Hunt 1997 |
R. trichopeplum
| Hunt & Moore 1995 |
R. euprepeia
| Hunt & Spiridonov 1995 |
R. disparovis
| Van Waerebeke 1991 |
R. seychellarum
| Adamson 1987 |
R. erringtoni
| Van Waerebeke 1986 |
R. madecassum
| Van Waerebeke 1984 |
R. pachyboli
| Adamson 1983 |
R. raoi
| Adamson 1983 |
R. indicum (
| Gupta & Tewarson 1977 |
R. longicorpus
| Rao 1973 |
R. ornatum
| Majundar 1967 |
R. neyrae
| Singh 1955 |
Parafontaria laminata
| Attems 1909 |