Megabalanus zebra ( Darwin, 1854 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4237.1.7 |
|
publication LSID |
lsid:zoobank.org:pub:908894A7-8C03-4182-A11C-0AF158F32D4D |
|
DOI |
https://doi.org/10.5281/zenodo.5612875 |
|
persistent identifier |
https://treatment.plazi.org/id/03A887AA-CA04-9025-FF4D-F963FFDEF941 |
|
treatment provided by |
Plazi |
|
scientific name |
Megabalanus zebra ( Darwin, 1854 ) |
| status |
|
Megabalanus zebra ( Darwin, 1854)
Balanus tintinnabulum var. (4) zebra Darwin, 1854 .
Balanus tintinnabulum var. 4. zebra .— Weltner 1897.
Balanus tintinnabulum var zebra .—Gruvel 1905.—1910.— Barnard 1924.— Stubbings 1961.— Karande & Pakelar 1966.
Balanus tintinnabulum zebra .— Pilsbry 1916.— Hiro 1939.— Davadie 1963.— Stubbings 1964.— 1967.
Megabalanus zebra .— Newman & Ross 1976.— Henry & McLaughlin 1986.— Foster & Willan 1979.— Pollard & Petherbridge 2002.— Liu & Ren 2007.
not Megabalanus zebra .— Chan et al. 2009: 265, fig. 232 (probably Megabalanus occator ( Darwin, 1854) ; see below).
Morphological characterization. Characterization is based on the specimens listed ( Table 3 View TABLE 3 ). Opercular plate measurements, mouthparts and cirri descriptions are based on 13 specimens, i.e. 11 from Chaguaramas ( Trinidad) and 2 from Piscadero ( Curaçao) ( MNRJ 25244, 25245 , 25246, 25247, 25248, 25249, 25250, 25251, 25252, 25254, 25255, 25256, 25257). See Appendix 1 for detailed information. Number of specimens (n) and standard deviations (±) are shown when necessary.
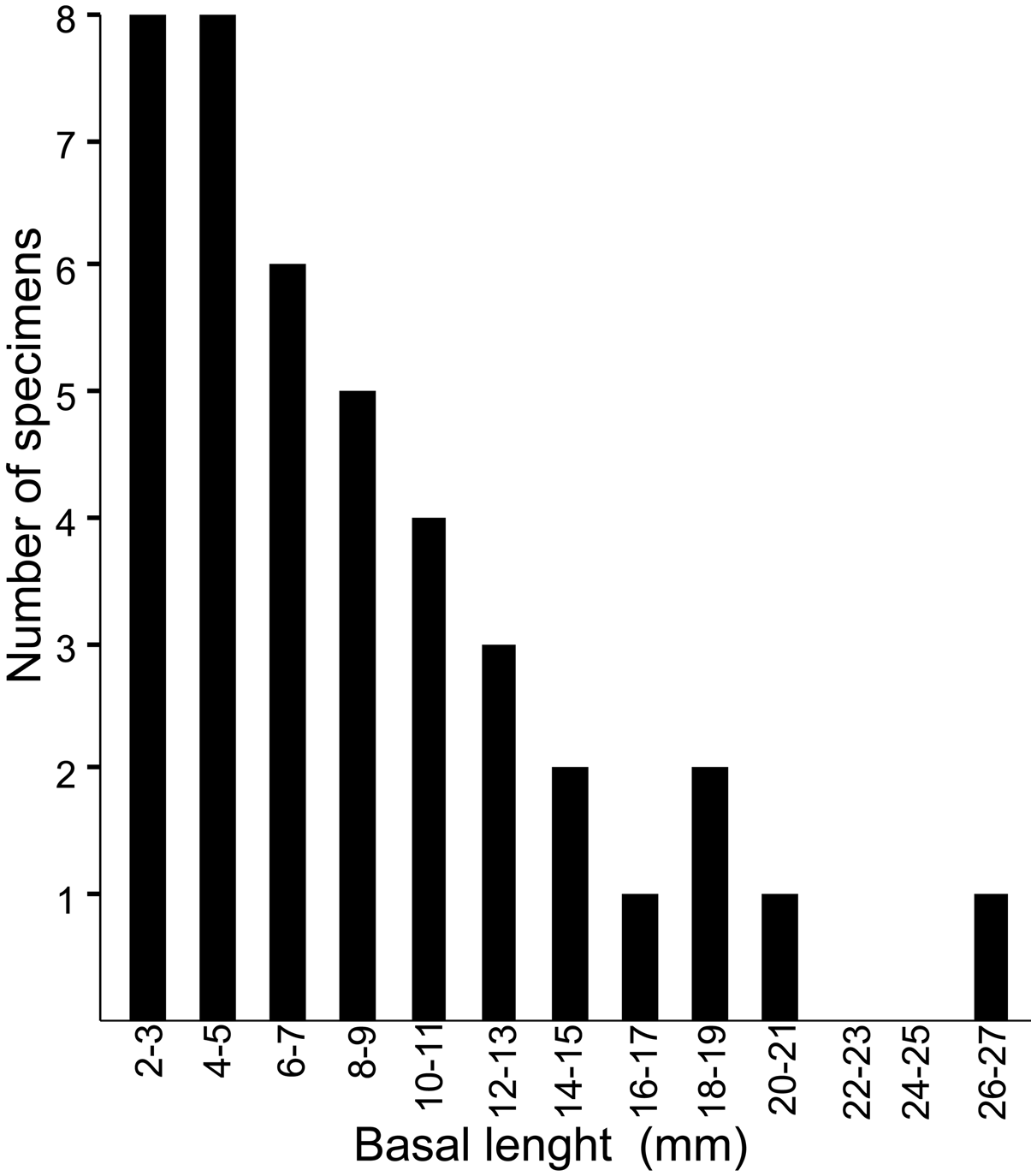
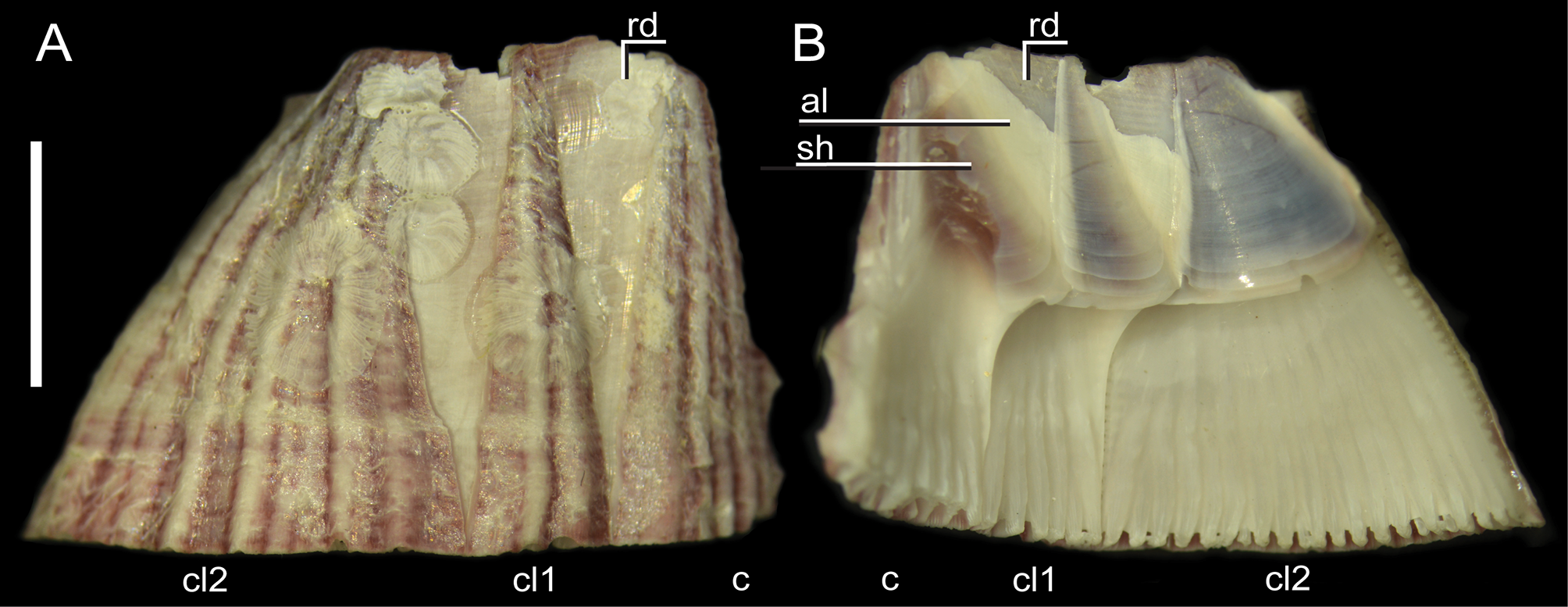
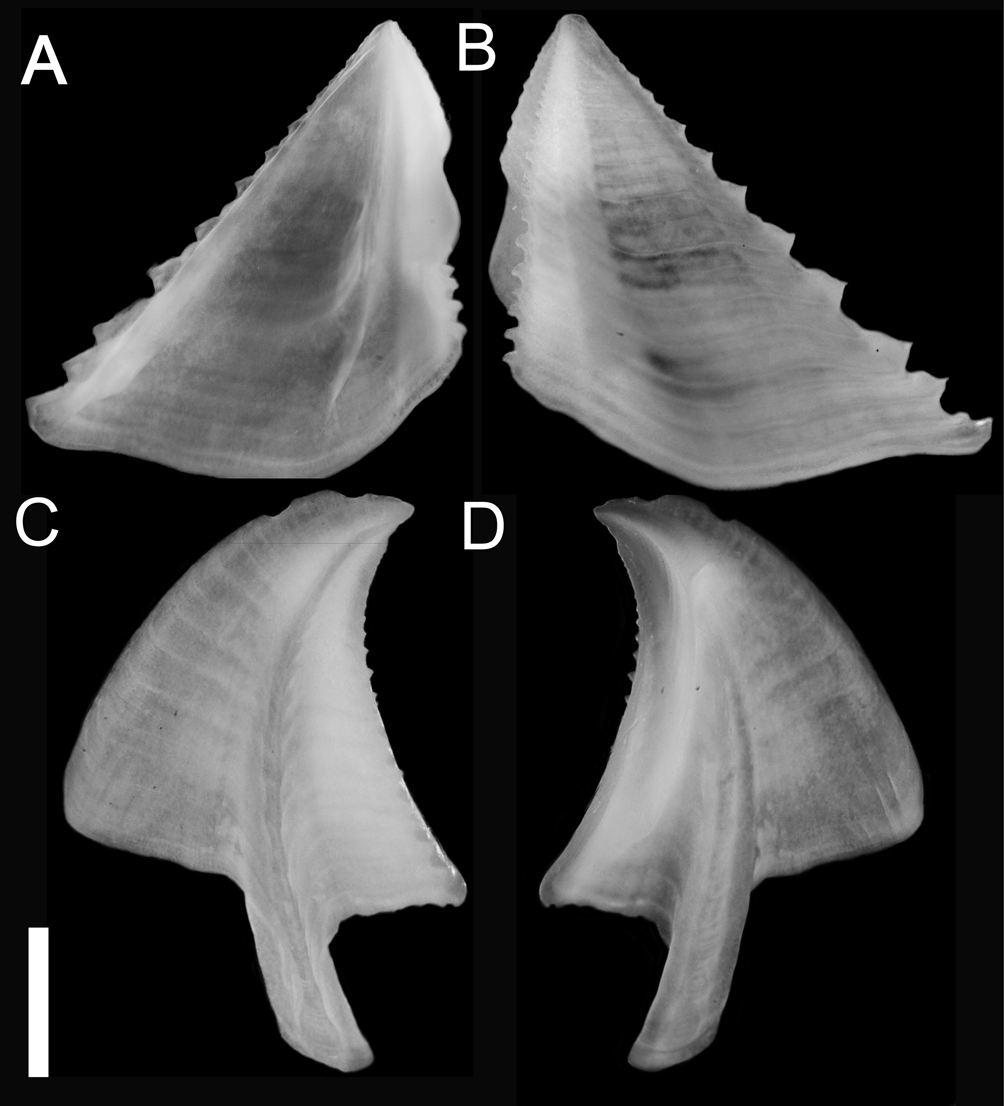
Shell conic with six plates, basal length varying from 2 to 26 mm and aperture from 0.7 to 12 mm; average aperture / basal length ratio 0.49 (±0.1, n=29). Although the aperture has a generally rounded profile, it is always angled at the carina ( Fig. 2 View FIGURE 2 ). Parietes externally with white and red intercalating longitudinal stripes, some with developed longitudinal white ribs, some with ribs nearly leveled; ribbing color varying from a contrasting white to pale white ( Figs. 2 View FIGURE 2 , 4 View FIGURE 4 , 6 View FIGURE 6 ). A horizontal banding pattern was also observed, with distinct degrees of intensity ( Figs. 2 View FIGURE 2 A D, 4A). Wall formed by 1 row of longitudinal tubes delimited by longitudinal (primary) septa connecting inner and outer laminae, with denticles at base; few transversal septa present near apex; some with secondary septa originating from outer wall with or without denticles. Radii horizontal, white with red spots, mostly on proximal side ( Figs. 2 View FIGURE 2 , 4 View FIGURE 4 ); tubiferous, sutural edge bearing denticles on upper and lower side. Alae with oblique growth lines related to adjoining radii, with beveled summits ( Figs. 4 View FIGURE 4 B and 6H). Sheath color brown, with distinct degrees of intensity; margins projected over adjacent alae ( Fig. 4 View FIGURE 4 B). Basis calcareous, formed primarily by 2 calcareous lamina connected by radiating septa forming radiating row of tubes; tubes interrupted by transversal septa; secondary row of tubes may occur, giving vesicular aspect to basis.
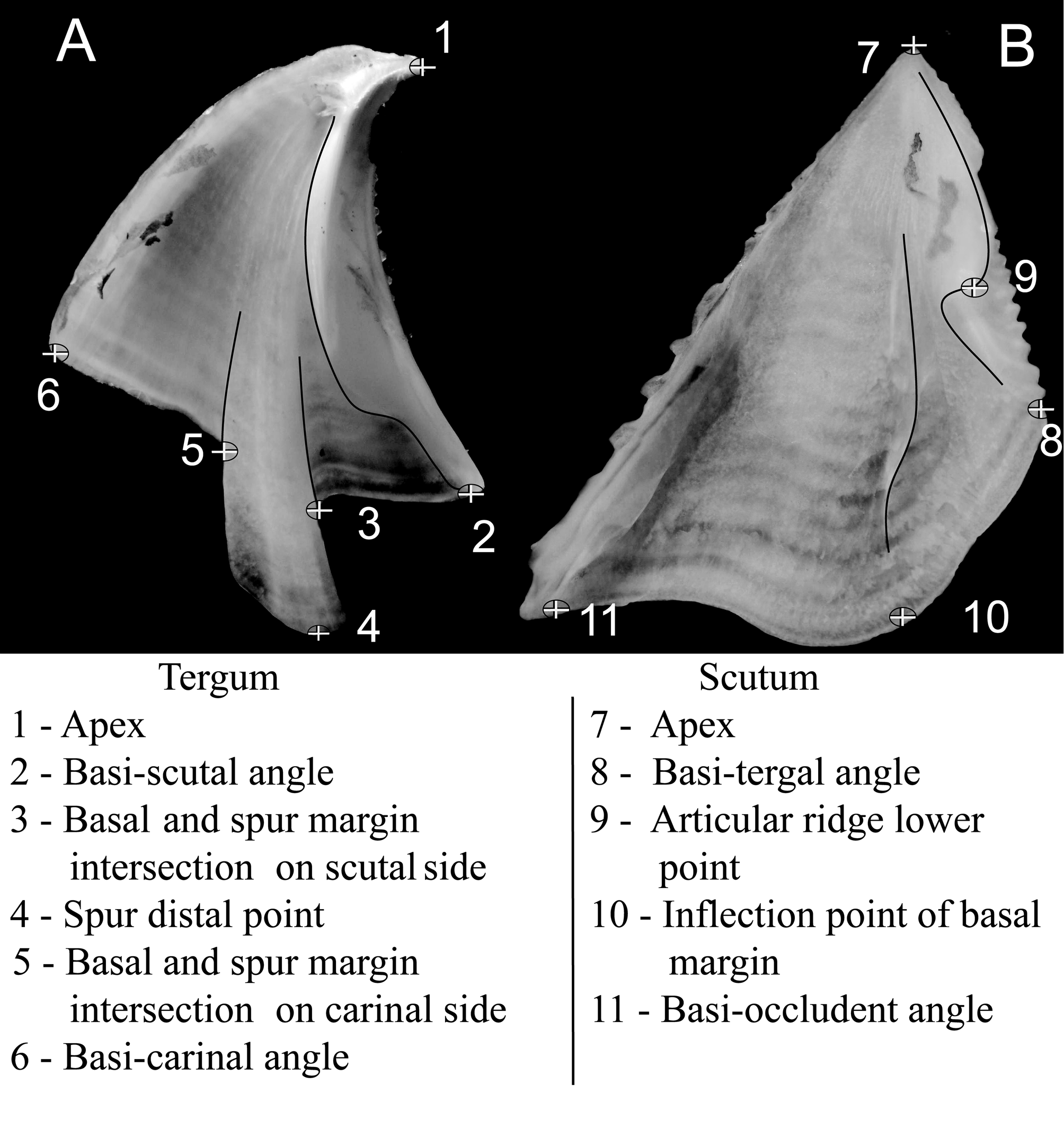
Scutum: triangular, wider than high with average basal margin 1.38 (±0.1) wider than tergal margin height ( Fig. 1 View FIGURE 1 B, 5A B, 6C D I J, 10C); tergal segment of basal margin inflected, with average of 0.43 (±0.03) of basal margin length ( Table 4); outer face with projected growth ridges with intercalating sizes, occludent margin toothed ( Figs. 5 View FIGURE 5 B, 6C I); longitudinal striae feeble, not affecting growth ridges. Articular ridge on average 0.7 (±0.03) shorter than tergal margin ( Figs. 5 View FIGURE 5 A, 6D I) ( Table 4). Adductor ridge conspicuous, not confluent with articular ridge, almost reaching basal margin, near lateral depressor muscle placement ( Figs. 1 View FIGURE 1 B, 5A, 6D).
Ratio description ANOVA Mcop Mzebra Mtint F p value avg ± std avg ± std avg ± std
Tergum
Basal margin segment, scutal side / spur width 82 <0.05 1.16 ±0.3 1.5 ±0.4 2.5 ±0.1 Spur length / spur width ns 1.4 ±0.2 1.5 ± 0.4 1.6 ± 0.4 Tergum triangular, a little taller than wide, with average basal margin of 0.94 (±0.09) of scutal margin length ( Table 4); scutal margin convex, sometimes with pointed beak on apex, formed by abrasion of soft external shell, exposing harder, reddish core ( Figs. 1 View FIGURE 1 A, 5C D, 6L), sometimes not visible in bigger (older) specimens ( Fig 6 View FIGURE 6 E,F); outer face smooth, with growth ridges more pronounced near scutal margin; spur with infolded margins, placed near scutal margin, at 1.5 (±0.4) its width from basi-scutal angle ( Table 4, Fig. 9 View FIGURE 9 C); spur width on average 0.8 (±0.1) smaller than basal margin length and, 1.5 (± 0.4) longer than wide ( Table 4); inner face with few delicate crests for depressor muscle attachment near basi-carinal angle ( Fig. 5 View FIGURE 5 D, 6E, K).
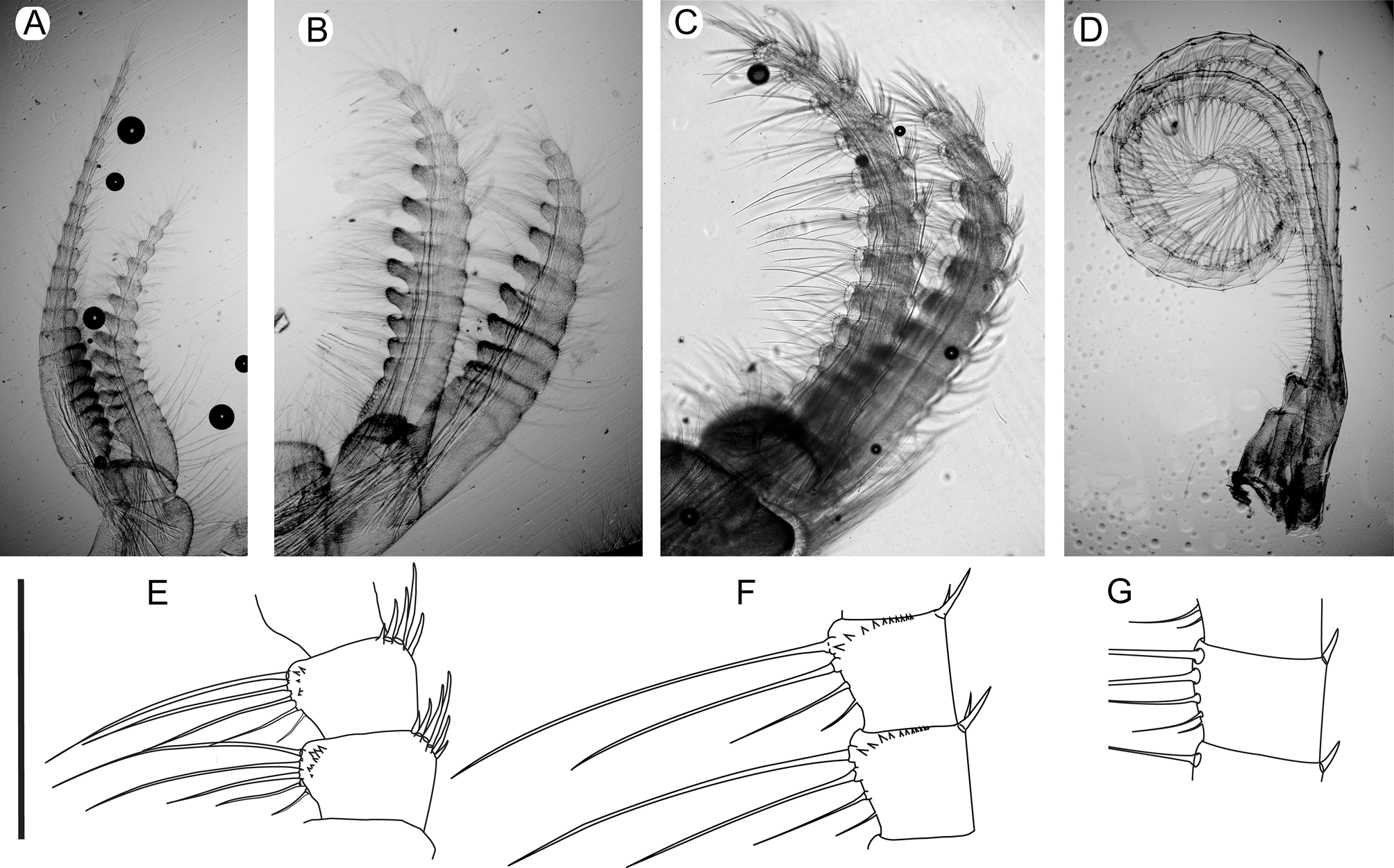
Labrum: crest straight bearing 3 denticles on each side of notch ( Fig. 8 View FIGURE 8 A,B). Palp with upper margin covered with coarsely serrulate setae, distal margin with long finely serrulate setae forming single row of long setae around inferodistal angle ( Fig. 8 View FIGURE 8 C, G). Mandible with 5 teeth excluding inferior angle, latter placed very close to fifth tooth; second tooth bifid in all examined specimens, some with third bifid tooth ( Fig. 8 View FIGURE 8 D, E, F, J).Maxilla I with 2 developed spines on upper and lower margins, with straight cutting edge; smaller spines between upper and lower pair, varying in number from 5-11, varying positively with size; short spines in small notch below upper pair of spines; below lower pair of spines, a row of short spines on straight margin ( Fig. 8 View FIGURE 8 H, I). Maxilla II with posterior margin of distal lobe covered with long, finely pinnate setae, anterior margin covered with long, smooth setae; anterior margin of proximal lobe covered with short coarsely serrulate setae ( Fig. 8 View FIGURE 8 K).
Cirrus I with right and left cirri composed of 12-15 (inner ramus) and 15-24 (outer ramus) articles; median segments on inner rami with protuberance ( Fig. 7 View FIGURE 7 A); inner face of both rami covered with serrulate setae; posterior margin of both rami with tuft of long pinnate setae on upper half. Cirrus II with right and left cirri composed of 10- 12 (inner ramus) and 12-17 (outer ramus) articles; median segments on both rami with protuberances ( Fig. 7 View FIGURE 7 B); long serrulate setae on protuberances and on distal suture of segments. Cirrus III with right and left cirri composed of 10-14 (inner ramus) and 13-18 (outer ramus) articles ( Fig. 7 View FIGURE 7 C); distal segments with row of short, conic teeth on outer face near anterior margin and on distal sutures ( Fig. 7 View FIGURE 7 E). Cirri IV to VI with anterior margins bearing 4-6 pairs of serrulate setae on median segments, of which 2 or 3 are longer near upper margin; posterior margin of each segment near distal suture with short cuspidate setae ( Fig. 7 View FIGURE 7 F, G). Cirrus IV with median and distal segments with row of short, conic teeth on outer face near anterior margin and distal sutures ( Fig. 7 View FIGURE 7 F). Penis with basidorsal point projected, with pair of setae, sometimes absent.
Remarks. All of the features noted by Darwin (1854: 195, quoted above) were also observed on the specimens we characterize here, but further descriptions are needed in order to establish a framework for within and among Megabalanus species comparisons. Shell color and shape are variable within the genus and their use, as diagnostic characteristics must be used with caution. Also, the “snow-white ribs” and the “rich chocolate purple” are features that show a gradient of color intensity and ribbing size, and we observed that the sheath color is more variable than Darwin’s (1854) description. Henry and McLaughlin (1986) also described a sheath of chestnut color for Megabalanus validus ( Darwin, 1854) , indicating that some previous identifications of M. zebra based on this feature could be incorrect. Darwin (1854: 200) described M. zebra as having oblique alae - “ in var zebra , however, in every specimen which I examined, the summits were oblique ”. Although this feature was not included in the species characterizations of Pilsbry (1916) or Henry & McLaughlin (1986), all of our examined samples showed oblique alae ( Figs 4 View FIGURE 4 B, 6H) (in accordance with Darwin’s description), seeming to be of systematic value. The last diagnostic feature described by Darwin was the almost circular orifice. The specimens we characterized had a relatively small orifice, with a rounded contour on the rostral side; this feature can be associated with Darwin’s perception of the rounded orifice, especially when compared with other species of Megabalanus .
Pilsbry (1916: 58) briefly characterized M. zebra (based mostly on samples from ships from the Philippines, Hong Kong, Java and India). He added information on the similarity with the opercular valves of M. tintinnabulum and the weak adductor ridge of the scutum, describing it as being “often very low, a mere convexity of the median part of the valve, but more emphatic in young individuals”. Based on an examination of Darwin’s material, Henry and McLaughlin (1986) provided a different description of the scutum adductor ridge of M. zebra , stating that it is “sometimes acute, often thickened, not confluent with articular ridge.” We suggest that the Pilsbry (1916) description needs to be used with caution, especially due to the variability of localities examined and the external resemblance of both M. zebra and M. validus , as described by Henry and McLaughlin (1986), and would urge that Pilsbry's original material be re-examined.
Some features of the cirri and mouth-parts might be of systematic value, and their use is dependent on more extensive morphological descriptions of other Megabalanus species. Few reports contain descriptions of the cirri and mouth-parts of Megabalanus , with the following a list of some papers that have furnished such information that can be used for further comparative work on Megabalanus: Darwin 1854 ; Pilsbry 1916; Nilsson-Cantell 1928; Ross 1968; Yamaguchi 1973; McLaughlin and Henry 1992; McLaughlin and Lacombe 1979; Chan et al. 2009; Pitombo 2010; Dionisio et al. 2012. Nilsson-Cantell (1928) described the “internal parts” from specimens identified as Balanus tintinnabulum antillensis (from Trinidad), and commented that the description could help “further identification of this subspecies”. The presence of a mandible with a bifid second tooth on all 11 specimens we characterized herein is a feature shared with the specimens described by Nilsson-Cantell (1928). However, at least four species of Megabalanus , including M. zebra , M. tintinnabulum , M. coccopoma , and Megabalanus occator ( Darwin, 1854) (FBP, personal observation) possess this feature, and the identification of Nilsson-Cantell's material will require examination of his specimens at the Natural History Museum in London.
The record of M. zebra from Taiwan of Chan et al. (2009) arose from two small (6 and 11.66 mm BL) specimens with scuta presenting radiating longitudinal marks on the upturned growth ridges (Fig. 232 c). This feature was not noted in the description by Henry & McLaughlin (1986) of specimens from the type series of M. zebra , nor in the specimens characterized here. We propose that these specimens are probably M. occator , which has these distinctive upturned growth ridges on the scuta. Henry and McLaughlin (1986) have suggested that the records of M. zebra from the Philippines of Pilsbry (1916) could be M. validus .
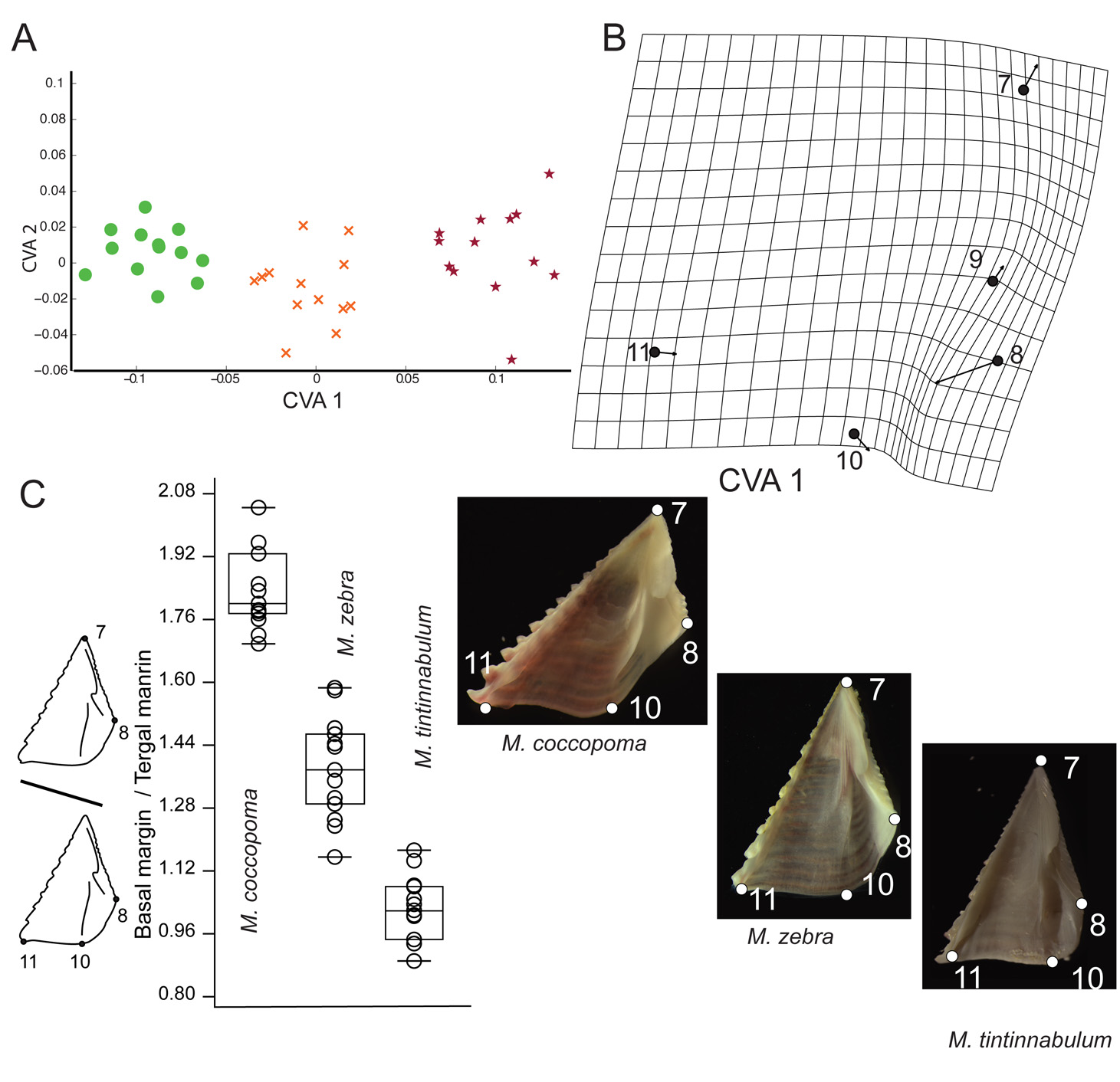
Morphometric characterization. Opercular plate ratios from selected length measurements obtained from landmarks ( Fig. 1 View FIGURE 1 ) of M. zebra , M. tintinnabulum and M. coccopoma are presented ( Table 4). Although our sample size might not reflect the full spectrum of variation of each species, it can be used in a comparative manner to aid further studies on the group. As an overall observation, M. zebra falls between the ratios of M. coccopoma and M. tintinnabulum , with some significant differentiation.
Some tergal ratios were established based on previous descriptions of megabalanids. For example, the degree of separation of the spur from the basi–scutal angle, using spur width as a scale, is a recurring feature of Megabalanus species characterization ( Henry and McLaughlin 1986). Here, we found the three studied species to be significantly distinct for spur separation (basal margin segment on scutal side / spur width) ( Table 4, Fig. 9 View FIGURE 9 ). Tergal height is expressed as a ratio between the apex and the intersection of the basal and spur margins and, for our studied samples, it significantly separates only M. coccopoma from M. tintinnabulum but not M. zebra , for which ratio values lay between these two ( Table 4). CVA of the tergum revealed two significantly discriminant axes (CVA1: Wilk’s λ = 0.0080, df=16 p<0.05 and CVA2: Wilk’s λ = 0.1643 df=7 p<0.05) with eigenvalues of 19.61 and 5.08 respectively ( Fig. 9 View FIGURE 9 A). The thin-plate spline diagram ( Fig. 9 View FIGURE 9 B) shows that the principal shape change implied by CVA1 is related to expansion of the spur width and a reduction of the basal margin segment on the scutal side; M. tintinnabulum having a narrower tergum and a wider scutal segment on the basal margin, M. zebra with an intermediate shape, and M. coccopoma having a wider spur placed closer to the basi-scutal angle. The CVA2 shape change mostly relates to a shift in the spur margin position (ldm 3 and 5) that discriminates M. zebra tergal shape from the other two species.
The scutal height and width relationship is a common aspect of scutum descriptions and, herein is represented by two significant ratios that relate the scutal basal margin with tergal and occludent margins ( Table 4 and Fig. 10 View FIGURE 10 C). The other two ratios (i.e. Articular ridge / Tergal margin and Tergal segment of basal margin / Basal margin) significantly separate only M. tintinnabulum from M. zebra and M. coccopoma . The scutum CVA resulted in one significant discriminant axis (Wilk’s λ = 0.0453 p<0.005) with an eigenvalue of 16.3 ( Fig. 10 View FIGURE 10 A). A thin-plate spline diagram ( Fig. 10 View FIGURE 10 B) shows that the shape change implied by scutal CVA1 is mostly associated with compression of the tergal segment of the basal margin (lmk 8-10) of the scutum, with Megabalanus coccopoma with a wider tergal segment, M. tintinnabulum a shorter segment and M. zebra on an intermediate stage of compression. This shape change can be associated with shell aperture outline, whereby the larger inflected tergal segment observed on M. coccopoma might be related to the round contour commonly observed in M. coccopoma , whereas the short tergal segment observed in M. tintinnabulum makes an angled association between the scutum and the tergum, leading to the typical triangular aperture of M. tintinnabulum . The aperture shape of Megabalanus zebra is intermediate between those two forms, with an angled carinal side and a rounded contour on the rostral side, more similar to the aperture of M. coccopoma .
Molecular Characterization. Two specimens of Megabalanus zebra from Curaçao, MNRJ 25256 View Materials and 25257 ( Fig. 6 View FIGURE 6 ), were sequenced for partial mitochondrial COX1, GenBank: KX538961 View Materials and KX538962 View Materials respectively. The alignment obtained for the sequences was 650 base pairs (bp) in length and represented a single haplotype. The frequencies of the bases were T=38.1, C=17.5, A=26.8 and G=17.5.
We used all available GenBank sequences of COX1 for the genus Megabalanus (see specimens list in Appendix II) in a 426 bp alignment including our M. zebra sequences. A total of 272 characters were constant, 4 were parsimony-uninformative and 150 were parsimony-informative. Table 5 View TABLE 5 summarizes the K2P distances between M. zebra from Curaçao and other species of Megabalanus . The interspecific distance varied from 0.11 to 0.199 and the net mean distance between this group was 0.16.
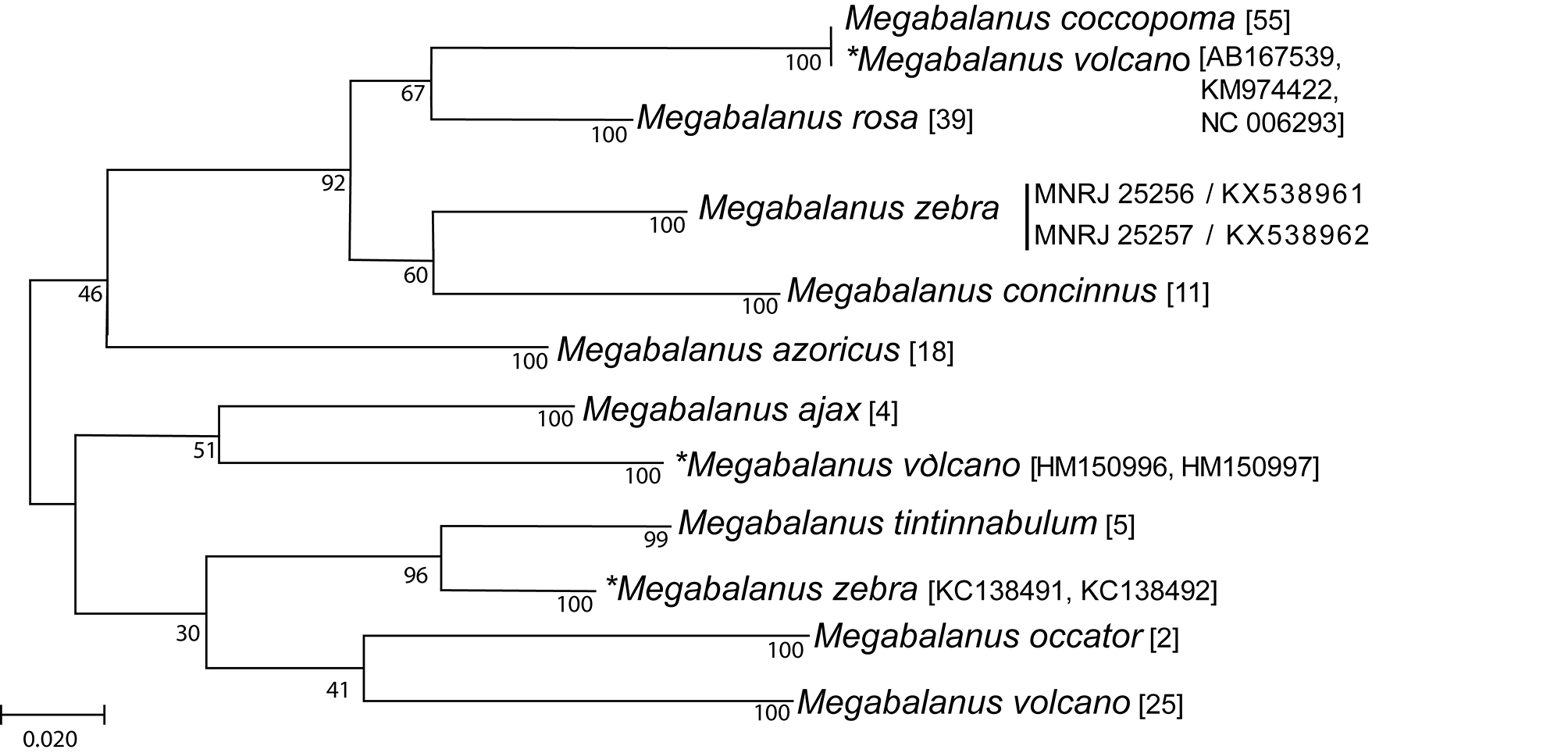
The relationships between specimens were obtained from COX1 sequences using Maximum Likelihood phylogenetic inference ( Fig. 11 View FIGURE 11 ). Eleven species-specific, divergent and well-supported clades (>96% bootstrap values) were recovered. Seven of those clades included all individuals belonging to their respective, presentlyrecognized species. Megabalanus zebra and M. volcano appeared in more than one clade.
*, divergent identified taxa, with GenBank accession numbers.
Megabalanus zebra is present in two clades, two sequences from Taiwan ( KC138491 View Materials , KC138492 View Materials ) ( Chen et al. 2013) and two sequences from Curaçao ( MNRJ 25256 View Materials and MNRJ 25257 View Materials ) . The sequences from Taiwan do not have voucher specimens and, according to the authors, the identification was based on Chan et al. (2009), which did not morphologically meet previous or current descriptions of M. zebra (see remarks above).
Megabalanus volcano appeared in three clades, one with 25 sequences and the other two with three and two sequences. The clade with most of the sequences are composed mostly from the sequences of Yamaguchi et al.
2009 (23) plus two sequences from Chen et al. (2013). This clade is the best related to M. volcano as Yamaguchi et al. (2009) furnished a morphological description of the sequenced species. The two M. volcano sequences nested with M. ajax were not included in the Yamaguchi et al. (2009) paper and are only found in the GenBank library, and thus needs to be treated as a distinct unknown taxa. The last three sequences labeled as M. volcano nested within the M. coccopoma clade are from two sources: Begum et al. (submission unpublished) and Tsang et al. 2004, and are probably an identification issue and thus need to be properly corrected.
Morphometric and molecular analyses. We demonstrate that morphometric tools are valuable for discriminating the three Megabalanus species presently reported in the Caribbean and Gulf of Mexico ( Pilsbry 1916, 1953; Southward 1975; Bacon 1976; Perreault 2004; Celis et al. 2007). However, the small sample and body size of most specimens of M. zebra examined here preclude any further generalizations on the shape of opercular plates as an aid to specimen discrimination within the genus Megabalanus . Further analysis with a larger sample size and a variety of body sizes will clarify the ontogenetic changes in early and late stages of development, and also if there is stability of shape among distinct growth forms.
Our molecular characterization shows that M. zebra is molecularly distinct from at least eight named species of Megabalanus (whose DNA sequences were obtained from GenBank) ( Fig. 11 View FIGURE 11 ), with most sequences grouped according to species assignment. However, as Cohen et al. (2014) pointed out, some incongruency exists for sequence identification of Megabalanus deposited with GenBank. Sequences associated with M. zebra and M. volcano appeared as distinct clusters ( Fig. 11 View FIGURE 11 ), highlighting issues with Megabalanus specimen identification and/ or the presence of cryptic species. We stress the importance of ensuring complete specimen characterization prior to publishing genetic sequences in order to address such incongruences. For most of the sequences we used herein, only a few have voucher specimens deposited with a scientific collection (Malay and Michonneau 2015; Pappalardo et al. 2015), or present or refer to morphological characterization of the studied specimen ( Yamaguchi et al. 2009; Quintero et al. 2015; Pappalardo et al. 2015). Such characterizations remains essential, especially for such a complex taxonomic group ( Ross 1999; Cohen et al. 2014).
Recently, Ashton et al. (2016) used GenBank sequences to “barcode” barnacle specimens found on ship hulls that were too small for definitive morphological identification. Of six molecularly identified Megabalanus , two had no match with the presently deposited sequences on GenBank. The currently small number of molecularly characterized species of Megabalanus when allied with conflicting sequences information is still a limiting factor for the use of molecular tools as an aid to identification. Although this approach can help with some identifications, the uncertainties still present on GenBank sequences may lead to conflicting species determinations if care is not taken. Thus, we suggest caution in the use of sequences as an aid to identification.
Establishment in the Western Atlantic Ocean. We suggest that M. zebra is established at least in the Southern Caribbean based on the following assumptions. The observation of small and newly-settled specimens on the hulls of vessels that had navigated solely within the Caribbean, and on specimens found on a navigation buoy in Trinidad and a pier in Curaçao ( Tables 2 View TABLE 2 , 3 View TABLE 3 ; Fig. 3 View FIGURE 3 ). The Pulsion had sailed outside the Caribbean—coming from the Mediterranean, passing through Senegal, Canary Islands, then Brazil and French Guiana—yet it presented only three specimens with an average size of 3.1 mm basal length, indicating a recent settlement in the Caribbean on the vessel. The specimens collected on pier pilings at Curaçao were covered by crustose coralline algae ( Fig. 6 View FIGURE 6 ), externally resembling other barnacles present on the same substrate, such as Newmanella radiata ( Bruguière, 1792) and Tetraclita stalactifera ( Lamarck, 1818) .
Our earliest material of M. zebra is from 2002. Thus, a thorough search of museum material will be required to establish possible earlier records. For example, in describing the Cirripedia of Trinidad, Bacon (1976) listed only Megabalanus tintinnabulum (as Balanus tintinnabulum antillensis ) but was intrigued by some samples that externally resembled Megabalanus zebra : two large specimens (28 and 32 mm BL) from oil-drilling platforms in the Gulf of Paria, as well as smaller ones “on rocks and Perna perna ” from Tyrico Bay . However, he could not distinguish these completely from M. tintinnabulum . This was understandable, since Henry and McLaughlin’s (1986) Megabalanus revision was not yet available. Compelling, too, is Davadie's 1963 report of M. zebra from French Guiana, from a sample collected by Gruvel, probably in the early 20th century. It suggest an earlier presence in the tropical Western Atlantic Ocean, but whether this material was from a vessel hull or from a natural substrate is not known, as he ( Davadie 1963) only furnished a photo of the shell without opercular plates.
Origin of Megabalanus zebra . The first step in establishing the endemic area for M. zebra is a clear taxon delimitation associated with its range of occurrence. The endemic area of Megabalanus zebra has not yet been established and the lack of information on its presence in natural areas precludes any initial hypothesis for its natural distribution. There is also a record of M. zebra on naturally-mobile substrata, such as whales ( Barnard 1924) and our observation of M. zebra fixed to L. anatifera ( Fig. 2 View FIGURE 2 B), highlighting the potential of this species to be attached to such basibionts. Extending the possibility of “natural” range extension demonstrates the complexity in establishing the native range for this species.
As noted earlier, M. zebra' s long history is linked, curiously, to reports of it largely as a fouling species on ships' hulls. A global map ( Fig. 12 View FIGURE 12 ) reveals that records of what we interpret here as resident, established populations occur in relatively few locations in the Western and Eastern Atlantic and in the Western Pacific. As M. zebra is a relatively recent (we presume 20th century) addition to the Western Atlantic, and as it is absent from the Eastern Pacific, this leaves either (1) the Eastern Atlantic Ocean or (2) the Indo-West Pacific Ocean as its origin. We note that the only material available to Darwin (1854) in the first half of the 19th century was from ships arriving from Bengal and China, perhaps suggesting that it was not yet present and available to foul ships in the Eastern Atlantic (the west coast of Africa) at that time. Finally, we are compelled by the numerous historical reports of M. zebra that seem to place the species commonly in the Indo-Pacific region , both from ships returning there from Bengal , China, Hong Kong, and Java, in Darwin (1854) and Pilsbry (1916), and by the many records presented by Henry and McLaughlin (1986, p. 68), including Japan , Indonesia, Sumatra , New Caledonia, and Australia. While these latter reports are distinguished by Henry and McLaughlin from their "from ships" category, we do not include these on our global map, because of the lack of substrate details at this time. Liu and Ren (2007), also reported at least four localities where M. zebra was found in the South China Sea (Zhelang Province, Pearl River estuary, Paracel Islands and Hainan Province), living in the intertidal zone of coral reefs and attached to buoys. Further resolution of the origin of M. zebra must await more extensive review of early museum material, along with a global examination of population genetics that may identify regions with the highest genetic diversity
TABLE 5. Estimates of Evolutionary Divergence using a Kimura 2 - parameter model between the two sequenced specimens of Megabalanus zebra (MNRJ 25256 / GenBank KX 538961, MNRJ 25257 / GenBank KX 538962) and other sequences of Megabalanus taken from GenBank. The values of average (avg), standard deviation (std), maximum (max) and minimum (min) and number of specimens (n) are provided. Appendix II lists the GenBank accession numbers of each specimen used.
| Specie | avg | sdv | max | min | n |
|---|---|---|---|---|---|
| Megabalanus rosa | 0.115 | 0.002 | 0.118 | 0.110 | 39 |
| Megabalanus ajax | 0.191 | 0.000 | 0.191 | 0.191 | 3 |
| Megabalanus azoricus | 0.154 | 0.004 | 0.16 | 0.148 | 18 |
| Megabalanus coccopoma | 0.138 | 0.0030 | 0.145 | 0.13 | 55 |
| Megabalanus occator | 0.187 | 0.007 | 0.192 | 0.182 | 2 |
| Megabalanus concinnus | 0.121 | 0.004 | 0.127 | 0.116 | 11 |
| Megabalanus volcano | 0.182 | 0.017 | 0.199 | 0.133 | 25 |
| Megabalanus zebra | 0.17 | 0.0 | 0.173 | 0.173 | 2 |
| Discussion |
| MNRJ |
Museu Nacional/Universidade Federal de Rio de Janeiro |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Megabalanus zebra ( Darwin, 1854 )
| Pitombo, Fabio Bettini, Gobin, Judith, Abreu, Nivia Maria Nunes & Jute, Alana 2017 |
Megabalanus zebra
| Chan 2009: 265 |