Oxyloma salleanum (L. Pfeiffer, 1850 )
|
publication ID |
https://doi.org/ 10.5852/ejt.2021.757.1419 |
|
publication LSID |
lsid:zoobank.org:pub:7A947511-262D-49F1-96E8-63D83A75D03D |
|
DOI |
https://doi.org/10.5281/zenodo.5075700 |
|
persistent identifier |
https://treatment.plazi.org/id/03A95D0D-F144-D611-781E-FC30FDEEFD90 |
|
treatment provided by |
Felipe |
|
scientific name |
Oxyloma salleanum (L. Pfeiffer, 1850 ) |
| status |
|
Oxyloma salleanum (L. Pfeiffer, 1850) View in CoL
Figs 5–8 View Fig View Fig View Fig View Fig
Succinea salleana L. Pfeiffer, 1850: 133 View in CoL .
Succinea effusa L. Pfeiffer, 1853: 17 View in CoL . syn. nov.
Material examined
Syntypes
UNITED STATES OF AMERICA • 3 adult specs ( Succinea salleanum ); Louisiana, New Orleans; Cuming Collection ex Sallé; NHMUK 20170302 About NHMUK • 5 adult specs ( Succinea effusum ); Florida, East Florida; Cuming Collection, ex R.J. Shuttleworth; NHMUK 20170303 About NHMUK .
Additional material
UNITED STATES OF AMERICA • 26 adult specimens; Louisiana, intracoastal waterway at Lake Salvador , near Jean Lafitte; 29.741947° N, - 90.141741° W; 29 Jun. 2017; Marco Martinez Cruz and Kathryn E. Perez leg.; CM 164850 View Materials to CM 164975 View Materials (detailed in Supp. file 1) GoogleMaps • 19 adult specs; Florida, Spring Garden Lake ; 29.136254° N, - 81.36917° W; 27 Jun. 2017; Marco Martinez Cruz and Kathryn E. Perez leg.; CM 164905 View Materials to CM 164915 View Materials (detailed in Supp. file 1) GoogleMaps • 198 adult specs; Louisiana, Kenner , near river; 25 Nov. 1961; L. Hubricht leg.; FM 235556 • 30 adult specs; Louisiana, Alexandria, near Red River, foot of Monroe Street; 7 Apr. 1962; L. Hubricht leg.; FM 235557 • 40 adult specs; Louisiana, near Lake Providence, near Highland; 24 Sep. 1969; L. Hubricht leg.; FM 235558 • 8 adult specs; Louisiana, Lake Beresford ; FM 144852 • 6 adult specs; same collection data as for preceding; FM 103925 .
Original descriptions
Succinea salleana L. Pfeiffer, 1850
“S. testâ depressè ovatâ, tenuissimâ, striatulâ, lineis spiralibus, impressis irregulariter notatâ, pellucidâ, nitidâ, corneo-albidâ; spirâ brevissimâ, subpapillatâ; anfractibus 2½, penultimo convexo, ultimo ¾ longitudinis superante; columellâ subcallosâ, strictè recedente; aperturâaxis subparallelâ, angulato-ovali; peristomate submarginato, margine dextro vix arcuato. Long. 19, diam. 10, alt. 7 mill.; ap. 16 mill. longa, infra medium 9 lata. [Shell depressed ovate, very thin, weakly striate and marked with irregular spiral lines, transparent, glossy, corneous whitish; spire very short, subpapillate; whorls 2½, the penultimate convex, thelastexceeding threequarters the lengthof overall shell length; columellasomewhat calloused, strictly receding; aperture subparallel with the axis, strongly angulate, oval; peristome submarginate, the right margin slightly arcuate. Length 19 mm, diameter 10 mm, depth 7 mm, aperture 16 mm long, 9 mm wide below middle]. Hab. New Orleans [Louisiana, USA] (Mr. [Auguste] Sallé).”
Succinea effusa L. Pfeiffer, 1853
“T. depresso-ovata, tenuissima, striatula, parum nitens, diaphana, griseo-cornea; spira brevissima, acuta; anfr. 2½, ultimus magnus, depressus, 5/6 longitudinis aequans; columella vix arcuata, subrecedens; apertura ampla, obliqua, ovalis; perist. simplex, regulariter arcuatum, basi non incumbens. Long. 12, diam. 7, alt. 4½ mill. Ap. 10 mill. longa, 6 lata. [Shell depressed ovate, very thin, delicately striate, a little glossy, diaphanous, grayish-brown colored. Spire extremely short, acute; whorls 2½, the last large, depressed, five-sixth of shell length; columella scarely arcuate, subreceding; aperture ample, oblique, oval; peristome simple, regularly arcuate. Length 12 mm, diameter 7 mm, depth 4.5 mm, aperture 10 mm long, 6 mm wide] (Mus. Cuming.) Succinea effusa Shuttl. [Shuttleworth] mss. Habitat in Florida orientali.”
Redescription
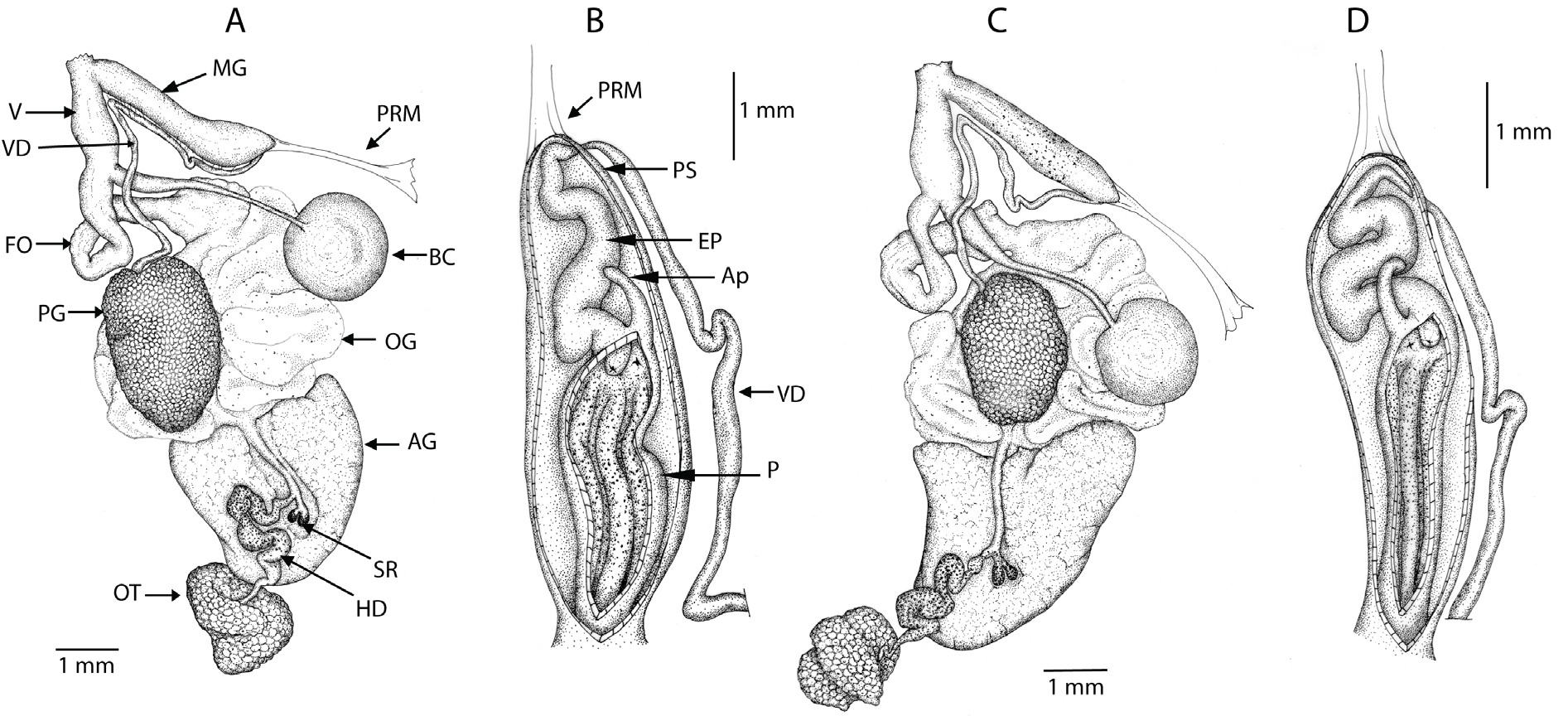
SHELL ( Fig. 5 View Fig ). Medium to large, succiniform, imperforate, oval shape, very thin and delicate, often fractures when handled, glossy, translucent, horn to straw-yellow, spire short to very short (0.75–0.83 of total shell height); entire shell with 2.5–3 whorls, body whorl very large, penultimate and early part of body whorl with small papillae, irregular and variable among individuals; growth lines visible on body whorl, as often are coarse wrinkles, and sometimes with traces of spiral lirae; embryonic whorl smooth until papillae begin (if present); some individuals with light variable spiral lines; aperture large, tear drop in shape, base of aperture flatter and receding, lip is not reflected, however along the columellar axis the shell edge rounds inwards. Shell capable of fully housing the retracted animal. Average shell height = 12.95 mm, average shell width = 6.91 mm, aperture height = 10.51 mm, aperture width = 5.85 mm; 2.54 whorls. Shells measured (n = 21 total) were from the DNA numbers 2813, 2812, 2958, 2757, 2956, 2754, 2752, 2756 and Field Museum of Natural History (FM) 235556 (n = 14).
ANIMAL EXTERNAL MORPHOLOGY. Anterior face of head bearing paired ocular peduncles, each with eye at the apex, and more ventrally paired inferior tentacles. Foot broad, tail extending behind shell in active animals. On each side of foot, a shallow pedal groove just above the margin with the sole; sole tripartite. Head, base of ocular peduncles and sides of foot not heavily pigmented, with brown, grey to black flecks; head flecking tending to be aggregated into posteriorly directed bands. Mantle collar and mantle pigmentation highly variable but never intense; speckling of brown and grey irregularly aggregated along mantle collar and adjacent anterior margin of mantle, becoming more diffuse over pallial cavity and very weak over kidney and viscera. Sole unpigmented in the examined animals.
REPRODUCTIVE ORGANS ( Figs 6–8 View Fig View Fig View Fig ). Lying predominately in the right side of the body cavity, more-orless close to and parallel to the columellar axis; in mature animals all other organs (other than pallial) are ventral or ventro-lateral to the reproductive system; genitalia extending diagonally across the body cavity. Ovotestis (gonad) largely confined within shell spire but extending into body whorl in mature animals, partially embedded in upper lobe of digestive gland, comprising a mass of densely clustered acini. Hermaphroditic duct running forward along columellar axis; expanded, highly convoluted as seminal vesicle in medial section; narrowing and less convoluted to reach carrefour region appressed to columellar surface of albumen gland; seminal receptacles paired, often unequally in length, their apices rounded and separate but otherwise the receptacles are united for the greater part of their length, opening into elongate saccular fecundation pouch to extend to origin of pallial gonoducts. Male and female gonoducts semi-diaulic in that spermiduct initially comprising a ciliated groove on the ventral floor of the female pallial gonoduct, forming a short spermoviduct before separating as a long slender duct, the vas deferens, running forward along the female pallial gonoduct before recurving to run along the face of the penial sheath into which it penetrates apically adjacent to the insertion of the penial retractor muscle. Prostatic gland a large more-or-less oval mass of fine acini partially enclosing and opening to the initial free part of the vas deferens. Vas deferens with ciliated lumen, but distally differentiated as an epiphallus with more muscular walls and folded internal lumen. Epiphallus convoluted, narrow proximally but broadening rapidly and then of more-or-less uniform width to run to penis into which it opens as a short but often distinct vergic papillum. Penial appendix opening at proximal end of penis, adjacent to vergic papillum; appendix digiform, short, more-or-less straight and uniform in width throughout length except rounded apex slightly dilated and reflected; appendix everting and functioning as a flagellate appendage during copulation. Penis more-or-less straight and of uniform width; wall of penial chamber thrown into several low longitudinal folds, smooth but for being weakly to densely studded with minute calcite rosettes; there is no proximal-distal differentiation in penial morphology. Penis and epiphallus entirely enclosed within a thin muscular sheath, which at the vas deferens-epiphallus junction gives rise to the penial retractor muscle that extends posteriorly to the right side of the body cavity, passing below the intestinal anterior loop to attach to the dorsal body wall. Albumen gland ovate in immature individuals, becoming large, linguiform on maturity, opening through a single channel to distal part of fecundation pouch. Female pallial gonoduct proximally an oviductal gland comprising sacculated, voluminous folds whose folds are more-or-less tangential to the long axis and regionally differentiated with several types of secretory cells. Distally, female gonoduct becomes narrower, free of secretory cells, with muscular walls; as free oviduct slender and folded above entry of the duct of the bursa copulatrix; as vagina stout, broader and straight below, opening into common reproductive orifice with male genitalia at anterio-lateral aspect of the animal behind the right ocular tentacle; male genitalia opening dorsal to the female vagina within this common orifice. Bursa duct distinctly broader, more muscular for initial part from free oviduct then slender, thin-walled extending to large oval bursa sac; bursa copulatrix duct extending diagonally across the body cavity, below the anterior loop of the intestine and the penial retractor muscle, such that the sac is ventral to and closely associated with the pericardium. Distal section of vagina, near genital orifice, equipped with thin retractor muscle running posterior-ventrally to body wall. Thin connective tissue encasing the reproductive organs; pigmented with grey-brown and black speckling over penis, distal section of vas deferens (some specimens), ovotestis, hermaphroditic duct and seminal receptacles, and less frequently and less intensely over prostatic gland; pigmentation most intense – approaching intense black in some individuals – over midsection (seminal vesicle) of hermaphroditic duct and the apices of the seminal receptacles.
FREE MUSCLE SYSTEM. Right branch of the columellar free retractor system, either free from near origin in columella or as a branch from the buccal retractor stem, anteriorly passes between male and female genitals, runs over the male genitalia to insert in the right ocular tentacle; smaller anterior branch passing under male genitalia to attach to right inferior tentacle and right floor of the body cavity lateral to buccal mass.
HAPLOID CHROMOSOME NUMBER. 19 ( Burch et al. 1966; Patterson 1971)
DIGESTIVE SYSTEM. Buccal mass equipped with jaw and radula. Jaw elasmognathous in having an accessory dorsal plate; cutting piece scribing a broad arc, with broad concave anterior margin, its lateral extremities rounded, and with small medial projection and less frequently with additional ribs. Radula tending to increase in size with animal size, reaching 90 to 130 transverse rows of teeth, each with 90 to 110 teeth; central tooth tricuspid, with large mesocone flanked on either side by a short ectocone; lateral teeth bicuspid with large mesocone and small ectocone; on transition to marginal teeth at the radula lateral edges, mesocone reducing in size and subdividing, and ectocone subdividing. Oesophagus arising from dorsal aspect of buccal mas, passes through CNS to dilate as esophageal crop before narrowing posteriorly to stomach. Stomach a simple curvature with oesophageal and intestinal openings at respective extremities; ducts of anterior and posterior lobes of digestive gland open into concave midregion; a small caecum associated with duct of the anterior lobe of the digestive gland. Intestine with one forward-directed loop, turning posterior after crossing over the aorta and running posteriorly to form a single posterior loop before running forward to the anus; intestine not coiling around oesophageal crop.
PALLIAL ORGANS. Pallial cavity short, broad, richly vascularized. Pericardium at left lateral aspect of pallial cavity, abutting kidney to which it communicates by a very short renopericardial duct; containing heart, with auricle anteriad receiving the pulmonary vascular network; ventricle posteriad, orientated more-or-less parallel with long axis of body, giving rise to aorta that passes over the anterior intestinal loop before dividing into anterior and posterior aortic branches. Kidney elongate, transverse in respect to body axis and orientated posteriad to reach right extremity of the pallial cavity. Ureter arising from left anterior extremity of kidney, elongate, tubular, extending along anterior face of kidney to reach posterior extremity of the pallial cavity on right, then recurving anteriorly to run more-or-less adjacent to and above rectum to excretory orifice immediately anterior to the pneumostome; terminal part of ureter, above the pneumostome, is continued forward as a blind bladder-like caecum.
Distribution and habitat
Distribution confirmed here with DNA sequence data includes USA: Florida, Louisiana, Washington D.C., Wyoming, Virginia; CAN: Alberta, Ontario.
Taxonomic remarks
From the Hugh Cuming Collection in NHMUK, Pfeiffer (1850) described his new species Succinea salleana , based on specimens collected from New Orleans by Auguste Sallé. Pfeiffer (1850) neither mentioned the number of specimens available to him in erecting S. salleana nor did he publish any illustration of the type material. Subsequently, Pfeiffer (1855: 49, pl. 5 figs 7–8) published two figures (apertural and reverse views) of a single shell. In an original illustration, Reeve (1873: pl. 6 fig. 40a–b) likewise depicted two views of a shell of S. salleana from NHMUK. The NHMUK material acquired by Cuming from Sallé likely formed the basis of the shell material illustrated by Pfeiffer (1855) and Reeve (1873). Three syntype (dry shell) specimens of Succinea salleana are presently in NHMUK 20170302. In an early American account of the species, Binney & Bland (1869: 270, text-fig. 486) had no material of S. salleana but reproduced Pfeiffer’s (1850) original description and provided an illustration of the shell based on Pfeiffer (1855). Later, Binney (1878: 42, pl. 79 fig. 18) and Binney (1885: 443, textfig. 488) also reproduced Pfeiffer’s (1850) original description. These authors made no mention of having examined specimen material of the species, yet the identical illustration of the shell was not reproduced from Pfeiffer (1855) but evidently based on examined specimens. Gratacap (1901) states that two specimens in the AMNH, namely 2137 and 2138 from Alexandria and New Orleans (Louisiana) respectively, correspond to the material of Binney (1878), with AMNH 2137 being the specimen illustrated. AMNH 2137 has no type status, but AMNH 2138 may be a syntype as part of Auguste Sallé’s original collection.As was common practice during the 19 th century, part of the lot of specimens collected by Sallé may have been dispersed on exchange or gift to other collectors and museums. Binney (1885: 498), in a tabulation of the Binney Collection, listed one specimen of S. salleana (Colln. No. 39783) from “New Orleans” ex “Sallé to Bland” as “original lot”. These specimens of possible syntype status, currently in NMNH 39783 and AMNH 2138, have not been sighted in the present work.
The original definition of the type locality of Succinea salleana was simply New Orleans. Additional material of Sallé were not found to establish a more precise type locality. Just south of New Orleans, west of the Missisippi River, we located large populations of ambersnails matching the description of S. salleana in Lake Salvador among cattails and on the banks of the lake. Pilsbry (1948) made no reference to the type material of S. salleana and thus it can be assumed that it was not examined. His referral of specimens from New Orleans to S. salleana , and others from Frierson Louisiana, Crève Coeur Lake Missouri, and Samburg Tennessee were thus evidently based on prevailing taxonomic wisdom founded on the conchological features described and illustrated by Pfeiffer (1850, 1855) and subsequent authors. Pilsbry (1948: 793, fig. 422d) was the first to anatomically examine material referred to S. salleana , based on preserved specimens from the west side of the Mississippi River, New Orleans (ANSP 60930 ex ANSP 60931, collected Pilsbry 1889) [ANSP 60931 is listed in error as ‘type’ in the museum database]. Features of the reproductive anatomy lead Pilsbry (1948) to transfer S. salleana to Oxyloma .
Pfeiffer (1853) described Succinea effusa as a new species based on specimen material in the Hugh Cuming Collection in NHMUK originating from Robert J. Shuttleworth. The collector of the specimens from “Florida orientali” [eastern Florida] is unproven, as is the precise type locality. In describing this species, Pfeiffer (1853: 17) explicitly stated that Shuttleworth was responsible for the specific name in an unpublished manuscript, list or specimen label (“ Succina effusa Shuttl. mss.”). Nonetheless, Pfeiffer (1853, 1855) explicitly attributes the species to Shuttleworth in the published work (e.g., as Succinea effusa Shuttleworth ) and in doing so caused considerable confusion as to the authority of the taxon. Several early workers (e.g., Binney 1885) considered Shuttleworth as the author. However, under ICZN Code, Art. 50, such attribution of authorship for the taxon name alone is not accepted; only authorship for the description is accepted. Accordingly, Pfeiffer (1853) is the author, and this has been recognized in more recent works on Succineidae (e.g., Pilsbry 1948: 788). The syntype material remaining in NHMUK 20170303 comprises five dry shell specimens. It is probable that additional specimens from the original collection in Florida are housed in the Shuttleworth collection in the Naturhistorisches Museum Bern, Switzerland, however, this cannot be documented. Naturhistorisches Museum Bern collection number 12772 is part of the Shuttleworth collection and listed as Succinea effusa , with the original label missing. In addition, a lot of Succinea effusa collected by Rugel is in the holdings of the Naturhistorisches Museum Basel and came to Basel via collection, Coll. Boissier and Bohny. This lot collected by Rugel had the locality of Spring Garden Lake, Florida and led to our sampling there for topotypic material. Further, that some of the syntype material was dispersed on exchange by H. Cuming after the publication of the species description by Pfeiffer has not been established by research.
The shell of S. effusa was first illustrated by Pfeiffer (1855: pl. 4 figs 18–20), evidently based on the NHMUK syntypes. Binney (1859: pl. 80 fig. 12; 1878: 429, text-fig. 306; 1885: 442, text-fig. 487) also described and illustrated the shell, which on account of the lots catalogued by Binney (1878: 498, NMNH 39780 and 39781) and Gratacap (1901: 402, AMNH 2135) was based not on type material but on other collections from eastern Florida and can be treated as topotypes. From this material, Binney (1878) described the jaw (p. 429), and described and figured the radula (p. 429 pl. 10 fig. n).
Pilsbry (1948) evidently did not examine the syntypes of S. effusa . He dissected specimens from Kissimmee River, Polk County, Florida, for which the reproduction tract was briefly described and fully illustrated ( Pilsbry 1948: 790, fig. 421a). The male genitalia were illustrated for further specimens from Bird Island, Florida ( Pilsbry 1948: fig. 422b). Based on the presence of an appendix opening to the proximal penis in these specimens, Pilsbry (1948) reassigned S. effusa to Oxyloma . Other specimens from across Florida state, and from North Carolina and New Jersey, evidently only available as dry shells, were similarly identified as O. effusum .
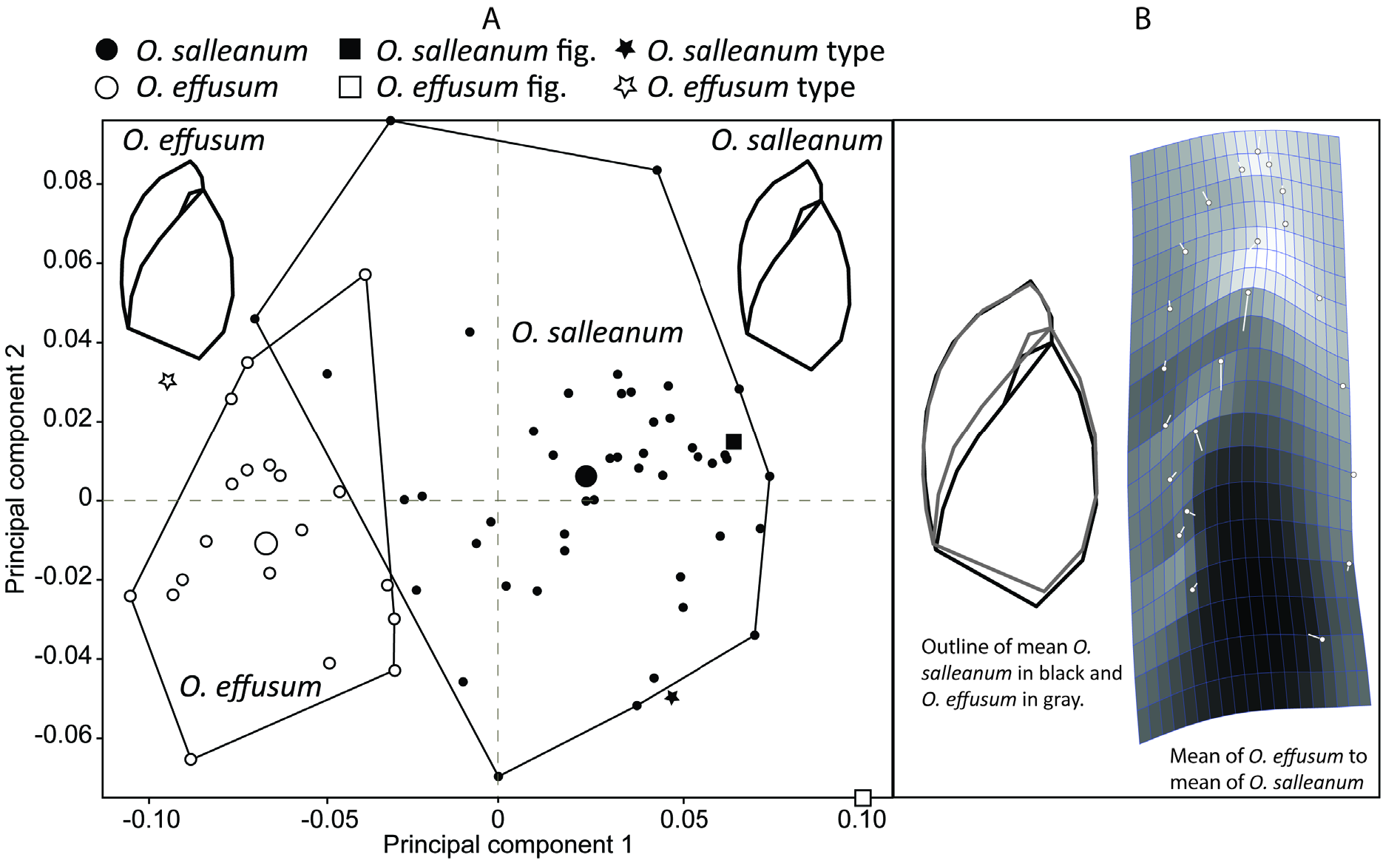
In comparing O. effusum with O. salleanum, Pilsbry (1948: 789) stated “The posterior part of the last whorl is more convex …, and the aperture is more ample.” Pilsbry (1948: 792–793) remarked on the conchological and male genital similarity between O. salleanum and O. effusum but suggested in respect to the shell “the spire is slightly longer in salleana ”. Pilsbry (1948) did not formalize the synonymy.
Our observations of the reproductive anatomy of O. salleanum and O. effusum from the respective type localities largely concur with that of Pilsbry (1948). However, contrary to Pilsbry, in our specimens, on opening the penial sheath the epiphallus was found to be generally strongly convoluted, while the penis more or less straight.
The establishment of Oxyloma effusum as a junior synonym of O. salleanum in this study is based on conchology, anatomy, and DNA. Populations from different parts of the range vary in conchological features that have been considered taxonomically important. For example, populations from the type locality of O. salleanum have a smaller aperture and consequently taller spire than those from the type locality of O. effusum ( Fig. 4 View Fig , morphometric figure). Individuals from the type locality of O. salleanum consistently had alarge bursacopulatrix sac, while those from eastern Florida (type localityof O. effusum ) possessed a distinctly smaller bursa copulatrix sac. Other anatomical features showed a similar range of variability across populations. We have not evaluated the status of Succinea salleana var. cordovana Von Martens, 1898 .
Oxyloma salleanum populations appear widespread in North America ( Fig. 1 View Fig ) but the details of the distribution remain uncertain due to difficulty with identification purely on shell specimens (e.g., most museum material). Using DNA, our study confirmed the species as present in Louisiana, Florida, Virginia, Washington D.C., Wyoming, and Alberta.
In addition to Louisiana, Pilsbry (1948) records O. salleanum from Tennessee, Missouri and Illinois, but all except that from New Orleans, Louisiana were evidently based on dry shell material. To this, we
can add Pilsbry’s (1948) recorded occurrences as O. effusum in Florida, New Jersey and North Carolina, although again only the records from Kissimmee, central Florida were evidently based on dissected animals. Pilsbry (1948) provides no comparative remarks as to how O. salleanum and O. effusum were distinguished from other species of Oxyloma recorded from Eastern USA.
Franzen (1966) considered O. salleanum a species of the lower Mississippi River valley, based on dissected animal material from Illinois and Louisiana. Franzen (1966) compared aspects of the shell morphology and anatomy of O. salleanum only with O. retusa and O. haydeni , based on earlier published works ( Franzen 1963, 1964).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Heterobranchia |
|
Order |
|
|
SuperFamily |
Succineoidea |
|
Family |
|
|
Genus |
|
|
SubGenus |
Neoxyloma |
Oxyloma salleanum (L. Pfeiffer, 1850 )
| Perez, Kathryn E., Martinez Cruz, Marco A., Steury, Brent W. & Barker, Gary M. 2021 |
Succinea effusa L. Pfeiffer, 1853: 17
| Pfeiffer L. 1853: 17 |
Succinea salleana L. Pfeiffer, 1850: 133
| Pfeiffer L. 1850: 133 |