Capromys pilorides lewisi, Morgan & Macphee & Woods & Turvey, 2019
|
publication ID |
https://doi.org/ 10.1206/0003-0090.428.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/03AA87B0-FFCA-FF8F-FD34-10CAFEAFFBAE |
|
treatment provided by |
Carolina |
|
scientific name |
Capromys pilorides lewisi |
| status |
subsp. nov. |
Capromys pilorides lewisi , new subspecies
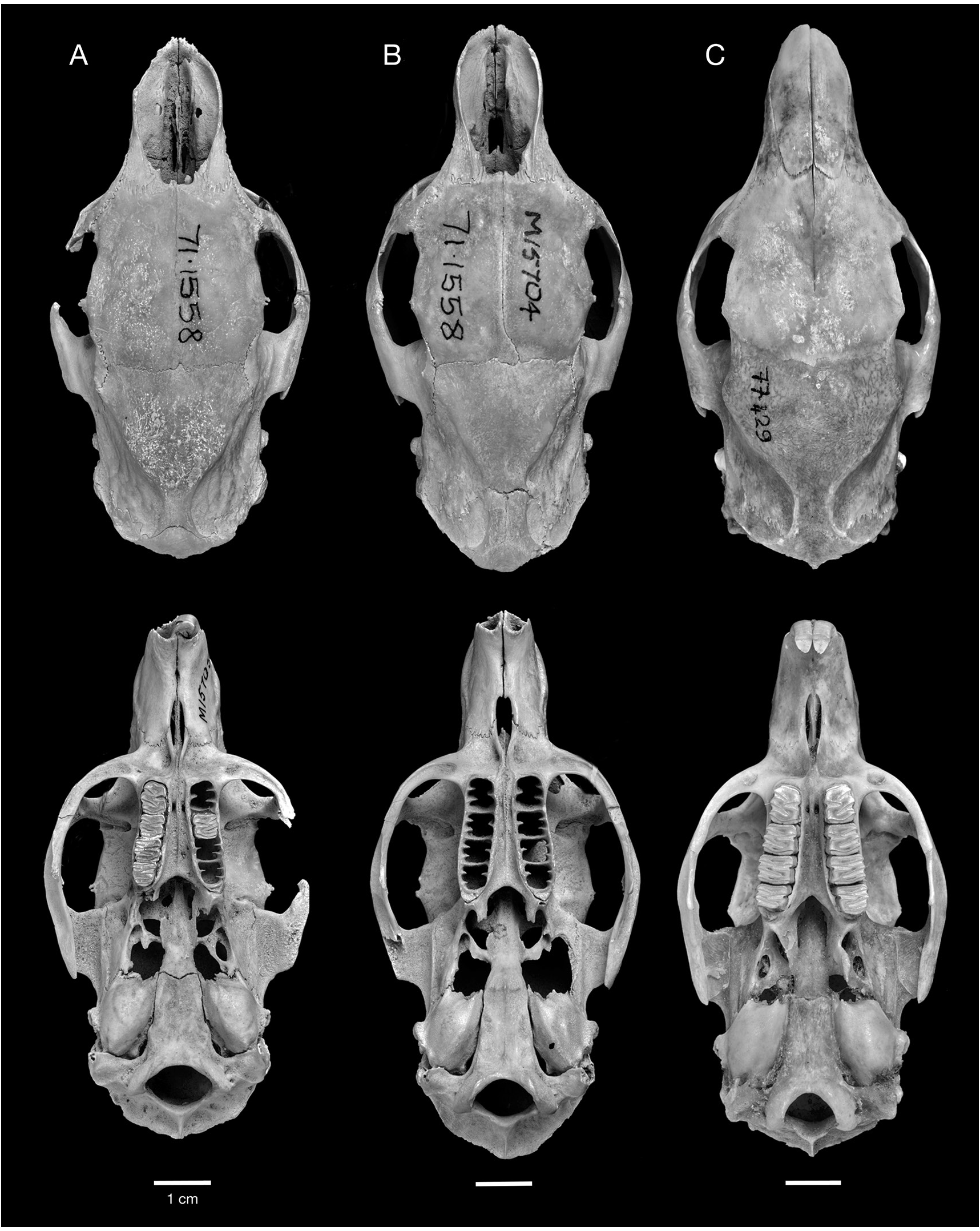
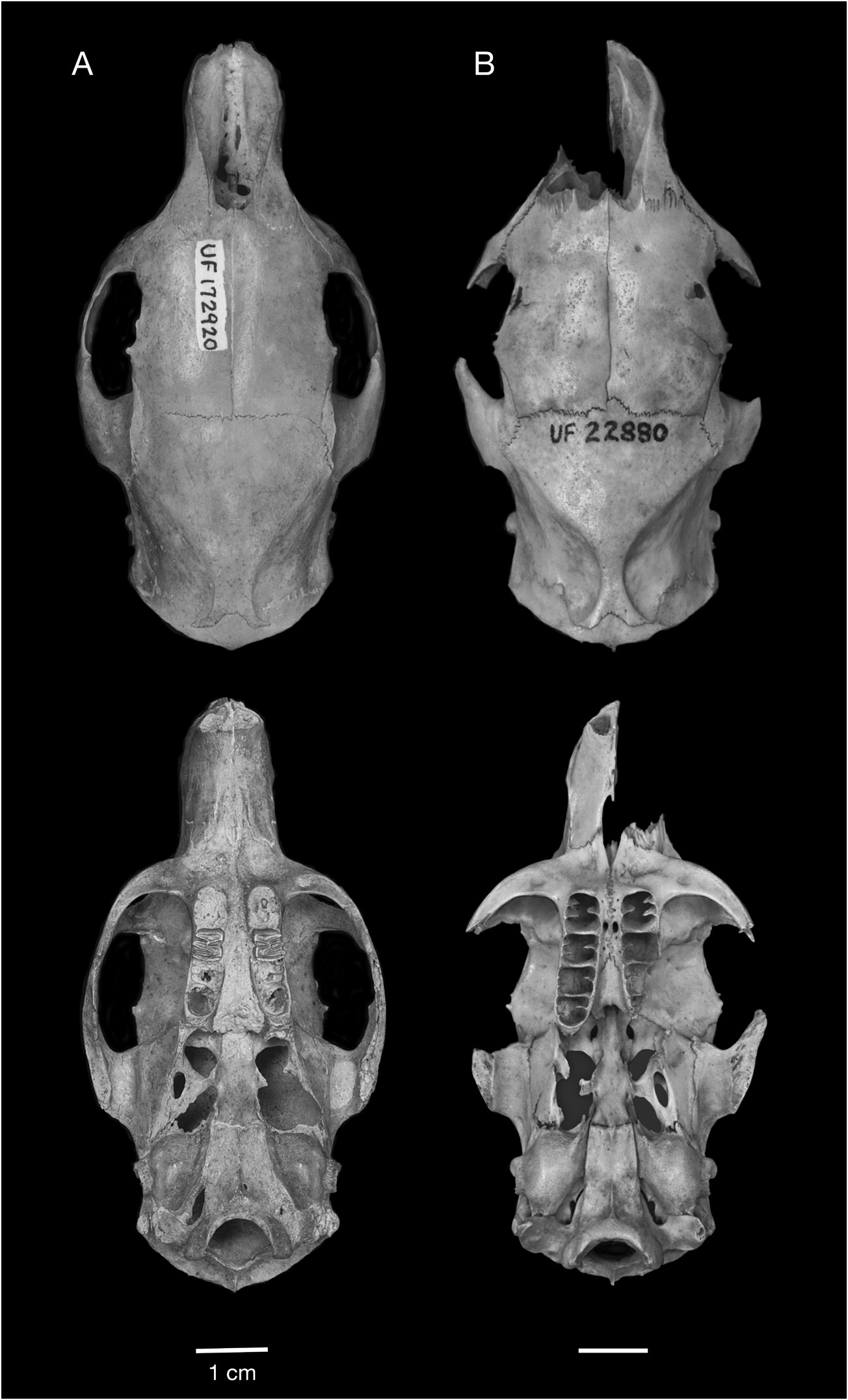
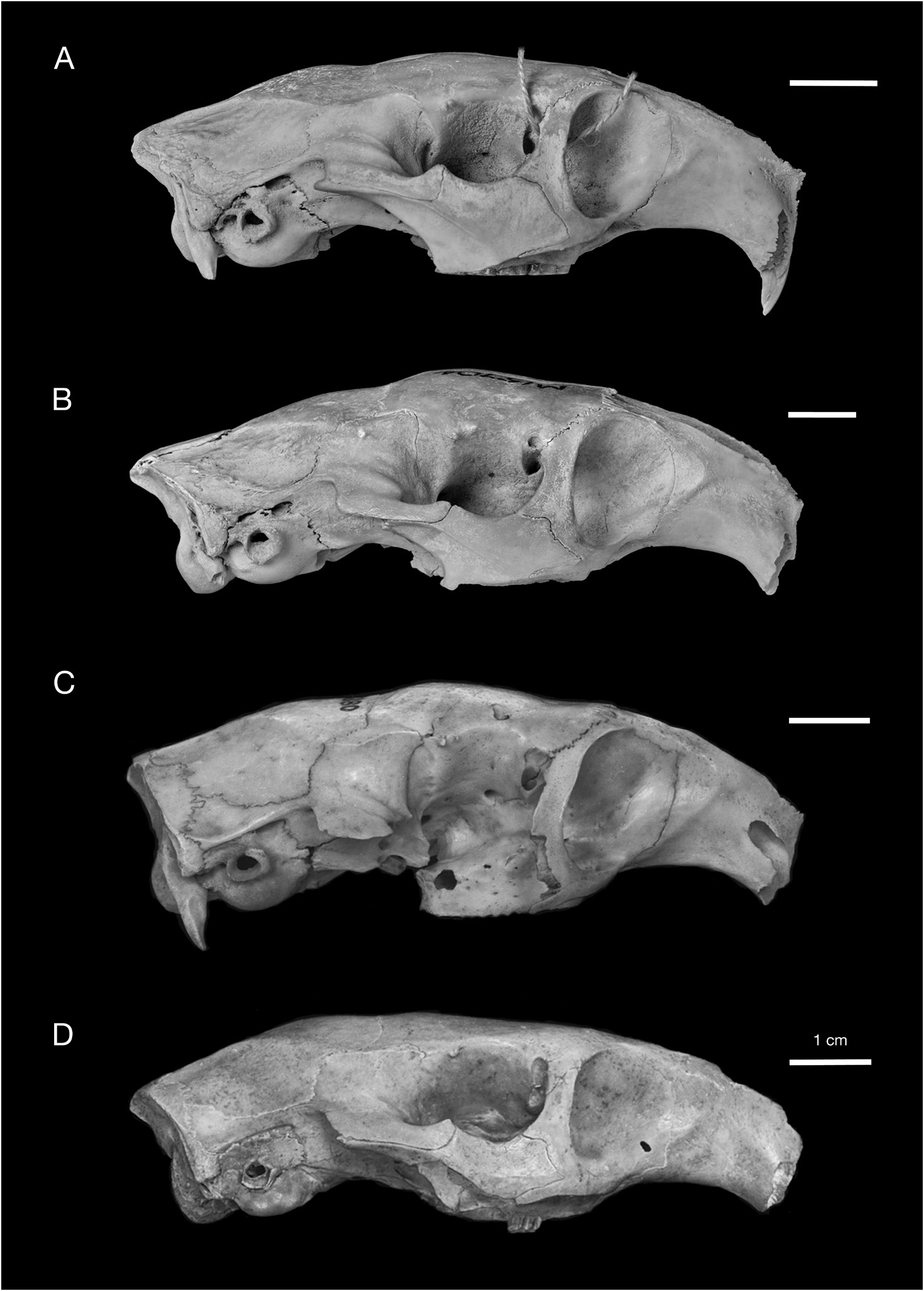
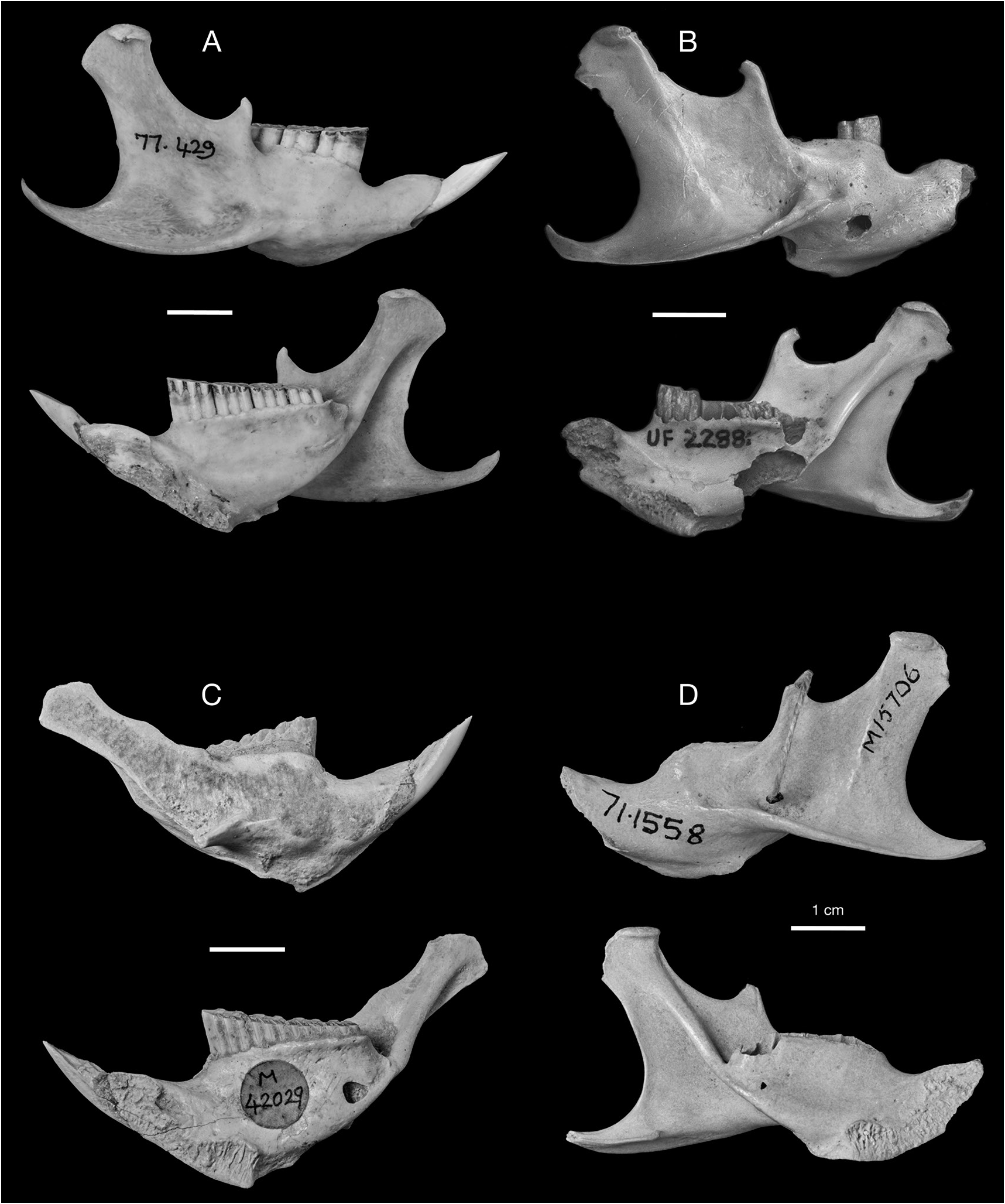
Figures 10–12 View FIG View FIG View FIG , 14–17 View FIG View FIG View FIG View FIG
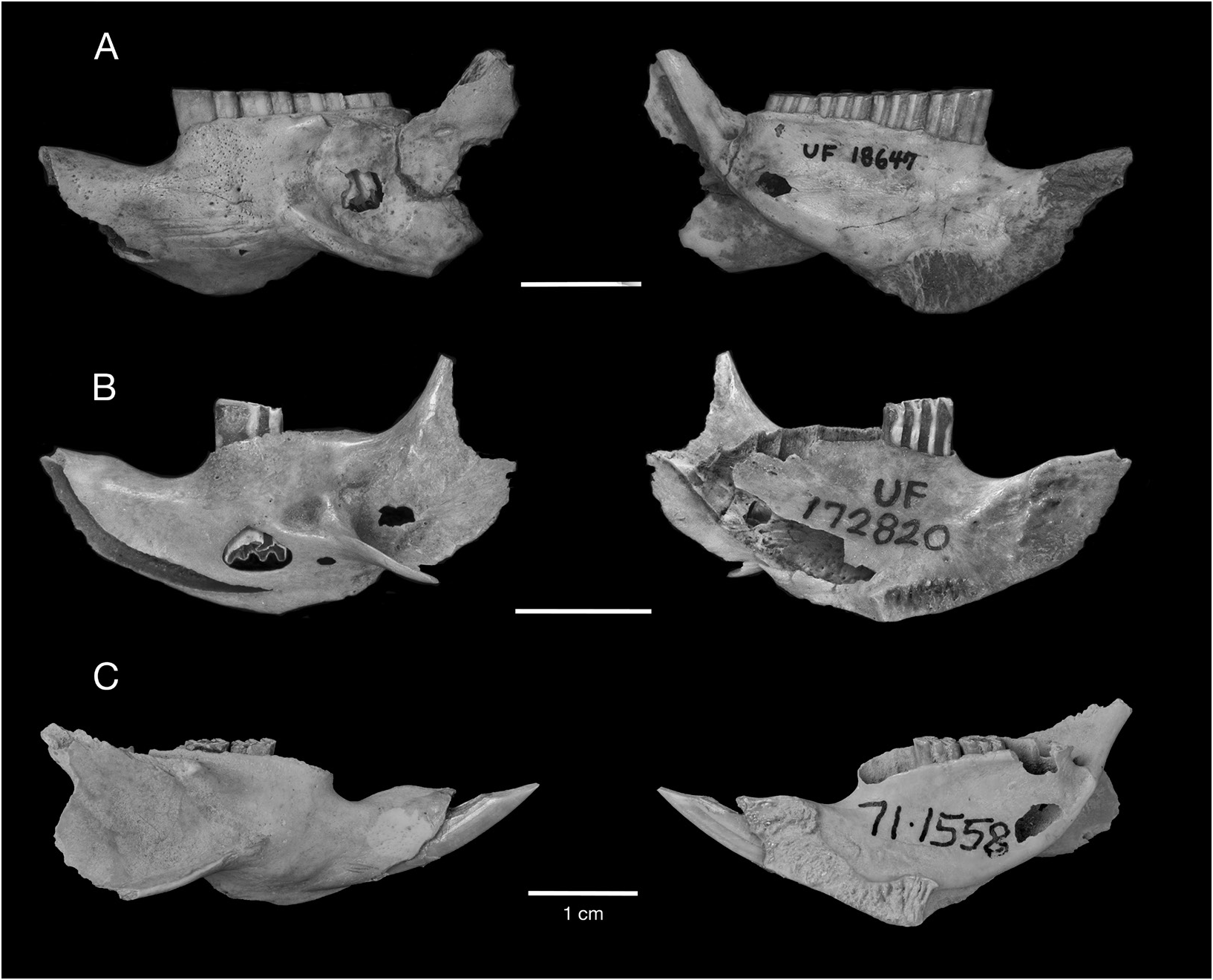
HOLOTYPE: NHM (Mammalogy) 71.1558/ M15705 View Materials , complete skull with left M1 and right PM4 –M3 (figs. 10A, 12A).
TYPE LOCALITY: Stake Bay Cave, 0.25 miles west of Stake Bay, Cayman Brac.
ETYMOLOGY: Named in honor of the late C. Bernard Lewis, former curator and director of the Institute of Jamaica and a member of the 1938 Oxford University Cayman Islands Biological Expedition, who collected the holotype skull of this new subspecies.
AGE: Late Pleistocene-Holocene (see Radiocarbon Dating).
DISTRIBUTION: Known only from Cayman Brac, Little Cayman, and Grand Cayman. The only representative of the genus Capromys known outside Cuba and its satellite islands.
REFERRED SPECIMENS: Cayman Brac: Stake Bay Cave: NHM (Mammalogy) 71.1558/ M15704, NHM (Palaeontology) M15733, M15734, M42027 View Materials , M42028 View Materials , skulls; UF 61291, nearly complete skull lacking nasals and right jugal, with left M1 and right M1–M2, and associated mandibles; NHM (Mammalogy) 71.1558/ M15706 View Materials , 71.1558/ M15707 View Materials , 71.1558/unnumbered, NHM (Palaeontology) M15735, M15736, M15737, M15738, M15739 View Materials , M15740, M42029 View Materials , mandibles. Blackie’s Cave: UF 172782, partial skull. Fig Tree Cave: UF 172759, 172760, edentulous mandibles. Hutia Cave: UF 172751, nearly complete edentulous skull lacking jugals, with associated edentulous mandibles, innominates, and tibia; UF 172752, 172753, 172758, partial skulls; UF 172755, 172763, edentulous mandibles. Patton’s Fissure: UF 18763, 21406, partial skulls; UF 21405, palate; UF 18647, 18650, 21385, mandibles with p4–m3; UF 18557, 18617, mandibles with p4–m2; UF 21395, mandible with p4; UF 21385, 22850, mandibles with m1–m3; UF 18542, 18588, 18597, 18618, 18619, 18670, 18671, 21351, 21355, 21395, 22853, edentulous mandibles. Peter Cave: UF 22855, partial skull; UF 61291, nearly complete skull with right M1–M2 and left M1; UF 22854, edentulous mandible. Pollard Bay Cave: UF 22886, palate; UF 18557, mandible with i, p4–m2. Shearwater Cave 2: UF 172792, 2 cervical vertebrae. Spot Bay Cave: UF 172764, skull; UF 172767, mandible with p4– m3; UF 172766, mandible with m2. Grand Cayman: Agouti Cave: UF 172870, partial skull; UF 172871, mandible with p4–m3; UF 172872, mandible with p4–m2; UF 172875, mandible with i, m2–m3; UF 172873, 172874, 172876– 172882, edentulous mandibles. Barn Owl Cave: UF 22875, 22877, 22878, palates; UF 22872, mandible with p4–m3; UF 22871, mandible with p4–m2; UF 22876, mandible with p4–m1; UF 22873, 22874, 22879, edentulous mandibles. Big Ear Cave: UF 162850, mandible with i1, m1. Chisholm Cow Well: UF 172821, premaxilla with I1; 172820, mandible with p4; UF 172836, edentulous mandible. Crab Cave: UF 22865, maxilla with P4; UF 22858, mandible with p4– m3; UF 22857, 22860, 22862, 22863, edentulous mandibles. Crocodile Canal: UF 61147, i1; UF 61149, 61150, 61154, femora. Dolphin Cave: UF 172920, nearly complete skull with left and right M1; UF 172921, edentulous partial skull; UF 172911, 172912, 172848, 172862, 172927– 172929, 411275–411277, edentulous mandibles. Furtherland Farms Cow Well: UF 172798, 172799, humeri; UF 172801, 172802, 172814, innominates; UF 172800, 172815, tibia. Miller’s Cave: UF 172089, partial skull; UF 172890, maxilla with P4–M1; UF 172896, mandible with i1, p4, m3; UF 172897, mandible with p4–m2; UF 172898, mandible with p4–m1; UF 172891, mandible with m1–m2; UF 172892–172895, 172899–172902, edentulous mandibles. Old Man Cave: UF 22880, nearly complete skull lacking left premaxilla, portion of left maxilla, both jugals, and all teeth; UF 22881, mandible with p4, m2. Queen Elizabeth II Botanic Park: UF 172947, mandible with p4–m3. Tadarida Cave: UF 61293, partial skull; UF 172837, mandible with m2; UF 172840, mandible with i1. Little Cayman: Agave Cave: UF 172794, humerus; UF 172793, femur. Franz’s Shelter: UF 172795, edentulous maxilla; UF 172796, 172797, edentulous mandibles. Weary Hill Cave: UF Environmental Archaeology Collection (uncat.), edentulous mandible. [This list of Referred Material does not include all Capromys fossils from the Cayman Islands, only the most complete cranial and mandibular specimens. Postcranial specimens are listed if they represent the only records of Capromys from a particular site. There is also a large sample of isolated teeth and postcranial elements not listed here.]
DIAGNOSIS: Differs from all described Cuban subspecies of Capromys pilorides in: smaller overall size; shorter maxillary and mandibular toothrows, resulting from smaller, lowercrowned cheekteeth with reduced amount of cement on anterior and posterior edges; maxillary toothrows strongly convergent anteriorly and nearly parallel from the anterior margin of P4 to the border between M1 and M2; narrow, anteriorly directed dorsal maxillary process; smaller orbit (about half the height of infraorbital foramen), owing to strong inflation of frontals posteriorly between orbits and parietal suture (frontals greater in height than parietals), and deeper zygomatic arch ventral to orbit; more constricted internal narial opening, which is rounded rather than triangular in ventral outline; shorter and less inflated auditory bullae; and shorter mandibular symphysis with reduced posterior margin.
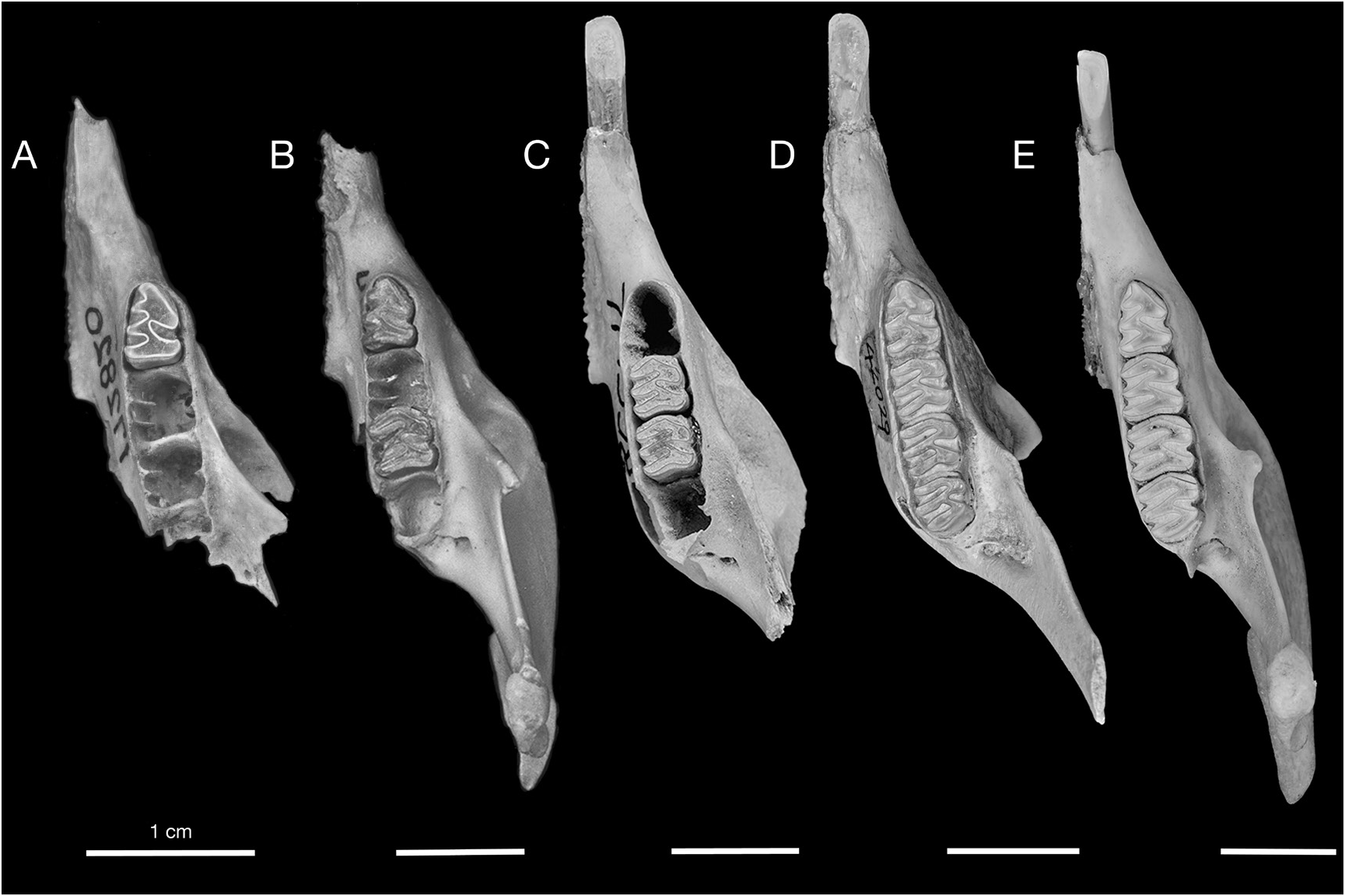
DESCRIPTION: One of the most characteristic features of the Cayman Capromys is the com- paratively short maxillary toothrows relative to the total length of the skull. The mandibular toothrows are also correspondingly short. The mean alveolar lengths of the upper toothrows and lower toothrows are very similar (tables 3, 4). Although the majority of partial skulls, isolated maxillae, and mandibles are edentulous, there are specimens that preserve partial or complete upper and lower dentitions (figs. 10–11, 16–17). In these specimens, the teeth are somewhat compressed anteroposteriorly, and they have a comparatively thin layer of cement on the anterior and posterior margins of the individual teeth. We were only able to take dental measurements of the upper cheekteeth (P4 and M1–M3) on a few specimens, including the holotype, but were able to measure a larger sample of lower cheekteeth (tables 3, 4).
The upper toothrows are strongly convergent anteriorly, with an average palatal width of only 3.3 mm opposite P4 (table 3). The convergence of the upper toothrows continues posteriorly to approximately the level of the border between M1 and M2, with the toothrows essentially parallel between P4 and M1. Posterior to M1, the toothrows begin to diverge laterally, reaching their greatest width at the posterior palatal margin. The convergence of the upper toothrows is also evident dorsal to the toothrows, as the internal narial opening is constricted both vertically and transversely, and is rounded rather than triangular in ventral outline. The root capsule for the incisor is barely visible on the external surface of the premaxilla and maxilla. The origin for the incisor root capsule is located immediately anterior to P4 and just dorsal to the maxillary root of the zygomatic. The root capsules of all four cheekteeth are visible as external swellings on the maxilla.
The ascending or dorsal process of the maxilla is relatively thin and oriented noticeably anteriorly. The dorsoventral height of the infraorbital foramen is almost twice that of the orbit. The small size of the orbit compared to other Capromys is a result of both the deep zygomatic arch ventral to the orbit and the inflated frontals dorsal to the orbit. The zygomatic arch, composed primarily of the jugal and also the dorsal maxillary process anteriorly, is vertically deep. The jugal fossa is rather large and oriented at approximately 45° to the ventral margin of the zygomatic arch. A well-developed jugal spine projects posteroventrally from the jugal. The frontals are noticeably inflated, from the anterior edge of the orbits posteriorly to the frontoparietal suture (fig. 12). This inflation is also evident on the portion of the frontals that comprises the internal wall of the orbits, and is especially prominent ventral to the postorbital processes. Only the anterior third of the frontals is not inflated, between the anterior edge of the orbits and the nasofrontal suture. In lateral view, the frontal inflation is emphasized by noticeable depressions both anteriorly dorsal to the anterior edge of the orbits and posteriorly along the frontoparietal suture. Because of this inflation, the width of the frontals posterior to the postorbital processes is greater than their width anterior to these pro- cesses (table 3). The temporal crests are well defined and nearly meet posteriorly along the midline of the skull, although they do not form a sagittal crest. The auditory bullae are comparatively short anteroposteriorly, giving them a somewhat circular to elliptical outline. The bullae are not inflated.
The posterior margin of the m3 alveolus in the mandible is oriented at a slight angle to the long axis of the toothrow. The mandibular symphysis is quite broad and well developed, but is truncated posteriorly with a reduced posterior margin. A thin ridge of bone extends posteriorly along the ventral margin of the mandible from the symphysis to approximately the level of m1 or m2. In side view, this thin elongate ridge gives the mandible the appearance of being deeper vertically. The mandible is relatively deep below the cheekteeth, indicating that the cheekteeth are quite hypsodont. The condyloid process is wide at its base, and the area for insertion of the masseter on the labial portion of the ramus ventral to the condyloid process is expanded in area, especially vertically.
The upper incisors are relatively broad and highly arched. As with most other capromyids, the cheekteeth are rootless and ever growing, but are relatively small compared to other Capromys . The p4 is longer and narrower than the other cheekteeth, and has a rounded anterior margin. The nature of the enamel band along the anterior lingual margin is quite variable: some specimens have no trace of a third reentrant in the anterior position, while other specimens have a notice- able infolding of the enamel band along the anterior margin of p4, and the average condition is a slight, medially directed infolding of the enamel band along the anterior lingual margin of p4. If this anterior infolding of the enamel band is not considered a true reentrant, then there are only two lingual reentrants on p4. The anterior lingual reentrant is the best developed of the two lingual reentrants. This anterior reentrant extends medially well beyond the median long axis of the tooth, and is anterior in position with respect to the single labial reentrant. The posterior reentrant is less well developed than the anterior reentrant, and does not extend medially past the median long axis of p4. The m3 is oriented at a slight angle to the other cheekteeth in the mandible, such that in an edentulous mandible the posterior margin of the m3 alveolus is also oriented at a slight angle to the long axis of the toothrow as opposed to the other three cheekteeth, which are perpendicular to this axis. The occlusal surfaces of the upper and lower dentition are flat and horizontal.
MORPHOMETRICS: The new subspecies displays some overlap in the observed range of most cranial measurements seen in a sample of 14 modern skulls of Cuban Capromys pilorides pilorides (tables 3, 4). The Cuban sample has a total skull length ranging from 85–106 mm (mean = 95 mm), whereas six complete or semicomplete skulls of C. pilorides lewisi range from 88–96 mm in total length (mean = 92 mm). However, there is no overlap in the alveolar length of the upper toothrows between these two samples ( C. pilorides pilorides : 21–24 mm, mean = 22 mm; C. pilorides lewisi : 18–20 mm, mean = 19 mm).
The largest available measurement series for the Cayman Capromys sample is mandibular alveolar toothrow length (Grand Cayman, n = 29; Cayman Brac, n = 13; Little Cayman, n = 2). Too few specimens are available from Little Cayman to permit statistical morphometric comparison with the other islands, but comparison between the Grand Cayman and Cayman Brac measurement series shows no statistically detectable size difference between samples from these two islands (Welch twosample t-test not assuming equal variance: Grand Cayman, mean = 19.81 mm; Cayman Brac, mean = 19.33 mm; p = 0.240). The entire available Cayman Capromys sample (mean = 19.70 mm) can therefore be pooled for analysis with Cuban Capromys samples.
The pooled Cayman Capromys sample has a significantly smaller mandibular alveolar toothrow length compared to a sample of C. pilorides pilorides from mainland Cuba measured from museum collections (n = 39; mean = 21.56 mm; p <0.001; appendix 2), to measurement data for the type series of C. pilorides doceleguas reported in Varona (1980) (n = 7; mean = 23.23 mm; p <0.001), and to measurement data for mandibular toothrow length (likely to be very close to alveolar toothrow length) for the type series of C. pilorides gundlachianus reported in Varona (1983) (n = 6; mean = 21.80 mm; p = 0.001). These represent the only unpooled measurement series available for direct statistical comparison with our Cayman Capromys sample. Borroto Páez et al. (1992) provided pooled measurement series and summary statistics for mandibular alveolar toothrow length for the other two described subspecies of C. pilorides , C. pilorides relictus (n = 18; mean = 22.30 mm) and C. pilorides ciprianoi (n = 19; mean = 22.63 mm), and reported that neither subspecies showed a statistically significant difference in this measurement from their sample of C. pilorides pilorides . We therefore interpret our Cayman Capromys sample as almost certainly also having a significantly smaller mandibular alveolar toothrow length than these two subspecies. The smaller toothrow lengths in Cayman Capromys appear to be a result of the more compressed molariform teeth (premolars and molars) with reduced cement on the anterior and posterior margins of the individual teeth.
Among the larger species of extinct Cuban capromyids in the general size range of C. pilorides, Silva Taboada et al. (2007) recognized two species in the genus Macrocapromys , M. acevedo and M. latus , both of which have since been placed in Capromys (Borroto-Páez et al., 2012) . Neither of these large species of Capromys is known from a complete skull, but measurements of the upper toothrow of C. acevedo and C. latus (range = 21–24 mm) are larger than the upper toothrow length of C. p. lewisi (range = 18–20 mm) ( Silva Taboada et al., 2007).
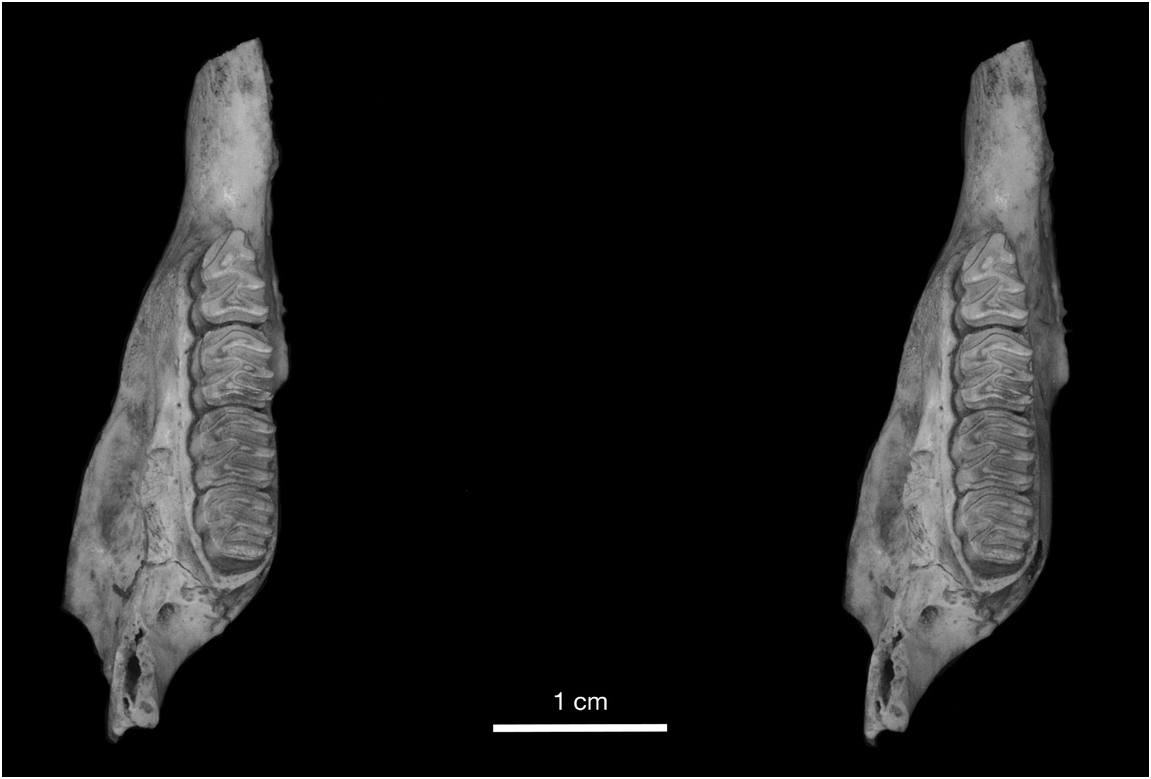
MORPHOLOGICAL COMPARISON WITH CUBAN CAPROMYS: In addition to size, Capromys pilorides lewisi differs from Cuban subspecies of C. pilorides in several cranial and dental characters. Cuban C. pilorides are characterized by having the infraorbital foramen considerably greater in height than the orbit compared to species of Mesocapromys and Mysateles , in which the infra- orbital foramen and orbit are similar in height. This character is even more extreme in the Cayman Capromys , in which the orbit is even smaller than in Cuban C. pilorides owing to the deeper zygomatic arch ventral to the orbit and the dorsal inflation of the frontals (figs. 12, 13).
One of the most diagnostic features of Capromys pilorides lewisi , and one in which this subspecies differs from Cuban C. pilorides , is the strong anterior convergence of the upper toothrows. The maxillary toothrows in C. pilorides lewisi are highly convergent anteriorly, and are essentially parallel between the P4s and M1s (figs. 10A, B, 11A, B). In contrast, the upper toothrows of Cuban C. pilorides are not as convergent anteriorly, diverging gradually from anterior to posterior (fig. 10C). Another characteristic feature of C. pilorides lewisi related to the convergence of the upper toothrows is the strongly constricted internal narial opening, which is smaller in both the vertical and transverse dimensions than in Cuban C. pilorides .
The frontals are noticeably inflated in Capromys pilorides lewisi from the anterior edge of the orbits posteriorly to the frontoparietal suture, and are particularly inflated dorsal to the orbits (fig. 12). In lateral view, this frontal inflation is delineated anteriorly by a depression dorsal to the anterior margin of the orbits and posteriorly by a depression along the frontoparietal suture. In contrast, the frontals of Cuban C. pilorides typically show no dorsal inflation and are essentially flat, with a smooth transition between the frontals and parietals and no depression along the frontoparietal suture. Because of this inflation, the width of the frontals is somewhat greater in C. pilorides lewisi than in Cuban C. pilorides , even though Cuban samples are larger in most other cranial measurements (tables 3, 4). However, some variation is observed in dorsal inflation of the frontals between Cuban Capromys individuals (fig. 13). The width of the frontals posterior to the postorbital processes is greater in C. pilorides lewisi than their width anterior to these processes, the opposite of the condition in Cuban C. pilorides in which the frontals are broader anterior to the postorbital processes. The auditory bullae also differ between Cayman and Cuban Capromys ; the auditory bullae in C. pilorides lewisi are rather small, anteroposteriorly short, with a rounded shape, and show no evidence of inflation, compared to Cuban C. pilorides in which the bullae are larger, longer, somewhat compressed laterally, and moderately inflated.
GENETICS: Extraction and amplification of UF 18588 from Patton’s Fissure on Cayman Brac successfully yielded the entire mitogenome (15,908 base pairs). Maximum likelihood and Bayesian analyses of hutia mitogenome data generated congruent phylogenetic trees, showing UF 18588 as the sister taxon to the Capromys pilorides mitogenome sample available on Gen- Bank (fig. 18). The monophyly of the Cayman + Cuban Capromys clade is supported by extremely strong Bayesian approximated posterior probability values (1) and bootstrap values for maximum-likelihood analysis (1).
Sequence divergence between UF 18588 and C. pilorides is low (table 5). Estimated divergence from the mainland Cuban C. pilorides sequence used in our analyses is only 0.5% across the entire mitogenome, and only 0.6% across the entire 1140 base pair cytochrome b (cyt b) gene (table 5).
REMARKS: The larger of the two hutia taxa present in the Quaternary record of the Cayman Islands is clearly referable to the genus Capromys on the basis of the considerably greater dorsoventral diameter of the infraorbital foramen relative to that of the orbit, the relatively thin and anteriorly oriented dorsal process of the maxilla, the presence of a small medial enamel infolding (anteroflexid) along the anterior lingual margin of p4, and its overall large size ( Kratochvil et al., 1978; Díaz-Franco, 2001; Silva Taboada et al., 2007; Borroto-Páez and Mancina, 2011). Our genetic analysis also confirms genus-level assignment of the extinct Cayman population to Capromys .
Capromys View in CoL is the most abundant and widespread fossil mammal in Quaternary deposits on the Cayman Islands, and the specimens of Capromys View in CoL reported from Little Cayman are the only fossil vertebrates recorded from that island ( Morgan, 1994a). It was the first fossil vertebrate reported from the Cayman Islands ( Moyne, 1938; Westermann, 1953), and the former occurrence of Capromys View in CoL on the Cayman Islands has been widely recognized by previous authors who have reviewed fossil material from the islands without providing a formal description of the taxon ( Varona, 1974; Morgan, 1977, 1994a; Steadman and Morgan, 1985; Morgan and Woods, 1986; Harvey et al., 2016). The only exception to this is Patton (1966), who mentioned only Geocapromys View in CoL as occurring on Cayman Brac, but investigation of material that he collected reveals that this sample also contains Capromys View in CoL .
Except for its presence in the Quaternary record of the Cayman Islands, Capromys View in CoL is restricted to Cuba and its satellite islands (Isla de la Juventud and offshore archipelagos), where only one extant described species, C. pilorides View in CoL , is currently assigned to the genus. Capromys garridoi , a second named species that was described by Varona (1970) from a single individual collected from Cayo Majá, Archipiélago de los Canarreos, has been reinterpreted as a misidentified specimen of C. pilorides View in CoL ( Silva Taboada et al., 2007; Borroto-Páez and Mancina, 2011). Based on information provided by C.B. Lewis, Westermann (1953) and Varona (1974) interpreted Capromys View in CoL fossils from Cayman Brac as conspecific with C. pilorides View in CoL ; other authors have conversely left the Cayman Capromys View in CoL in open
0.04
nomenclature, or have considered it to represent a distinct but undescribed species. Cayman Capromys specimens can be differentiated from Cuban Capromys according to both qualitative morphological characters and statistical morphometric size differences. Several of these differences may represent evolutionary adaptations to the distinct ecological conditions of the Cayman Islands. The reduction in body size may be explicable on biogeographic grounds, consistent with vertebrate body size decreasing with land area on other oceanic islands (Burness et al., 2001). Compared to Cuban Capromys , the Cayman Capromys has cheekteeth that are vertically shorter (even though both taxa have rootless teeth) and with a reduced occlusal surface area, and has a reduced mandibular symphysis possibly indicative of altered biomechanical bite forces, all of which might be associated with differences in dietary ecology caused by differing vegetation structure and diversity between the Cayman Islands and Cuba (Brunt, 1994; Proctor, 1994). Comparable differences in crown height and area of cheekteeth are also shown between other Quaternary hutia taxa ( Plagiodontia , Hyperplagiodontia ) that likely differed in their trophic ecology ( Hansford et al., 2012).
Considerable morphological variation has been documented between different mainland and insular Cuban Capromys populations, but the relationship between this morphological variation and genetic differentiation between populations is complicated and very poorly understood, leading to confusion over the taxonomic status and relationships between allopatric Capromys populations. Five Cuban subspecies of C. pilorides have been proposed, all of which have been diagnosed morphologically on the basis of craniodental and soft tissue characters: C. pilorides pilorides (Cuban mainland), C. pilorides relictus (northern Isla de la Juventud), C. pilorides ciprianoi (southern Isla de la Juventud), C. pilorides doceleguas (Archipiélago de las Doce Leguas), and C. pilorides gundlachianus (Archipiélago de Sabana) ( Varona, 1980, 1983; Borroto Páez et al., 1992; Silva Taboada et al., 2007; Borroto-Páez and Mancina, 2011). Based on analysis of the first 415 base pairs of cyt b for four of these five subspecies, however, the two putative subspecies from Isla de la Juventud ( C. pilorides ciprianoi and C. pilorides relictus ) show a low level of divergence (0.4%) similar to that observed within other subspecies of C. pilorides (0.0–0.5%) ( Woods et al., 2001; Borroto-Páez et al., 2005); C. pilorides ciprianoi has therefore been interpreted as a junior synonym of C. pilorides relictus by some authorities (e.g., Woods et al., 2001), but is retained as a valid taxon by others (e.g., Silva Taboada et al., 2007).
Cyt b divergence data have also been used to propose the existence of an undescribed subspecies from Cayo Campo, Archipiélago de los Canarreos ( Woods et al., 2001), and a further three offshore Cuban populations have been proposed to also represent distinct but unnamed subspecies (Borroto-Páez et al., 2012). A Capromys specimen studied by Borroto-Páez et al. (2005) from Cayo Ballenato del Medio, Archipiélago de Sabana-Camagüey, which was reportedly morphologically similar to individuals of C. pilorides , showed a markedly higher level of sequence divergence within the first 415 base pairs of cyt b (5.5%–6.4%) compared with levels of divergence seen between samples from the five named C. pilorides subspecies (0.4%–1.9%). This specimen has been variously interpreted as representing a previously unrecognized cryptic Capromys species (Borroto-Páez et al., 2005), or as representing the existing subspecies C. pilorides gundlachianus ( Kilpatrick et al., 2012) , potentially elevated to species level as C. gundlachianus ( Woods and Kilpatrick, 2005) ; however, it remains undescribed because the skull of the only available specimen is damaged. Most recently, analysis of three mitochondrial genes (cyt b, COI, 12s rRNA) by Upham and Borroto- Páez (2017) demonstrated a primary cyt b divergence of 5.2% between populations all previously considered to represent C. pilorides pilorides from eastern and western mainland Cuba, which is probably consistent with species-level divergence. These authors also demonstrated a further divergence of 2.0% within the western clade between populations from mainland Cuba and from Isla de la Juventud and nearby Cayo Cantiles. However, these patterns of genetic divergence have not yet been related to named taxonomic units within Capromys .
Although the morphological differentiation shown by the Cayman Capromys population led Morgan (1994a) to consider that it had likely diverged from the Cuban Capromys source population early in the Pleistocene, sequence divergence demonstrated in our ancient DNA analysis between UF 18588 and a C. pilorides sample from central mainland Cuba is surprisingly low in comparison to divergences between other hutia taxa (table 5). Insight into the taxonomic status of the extinct Cayman Capromys population is provided by consideration of sequence divergence within the cyt b region, for which previously published data are available across a wider sample of extant hutia taxa. Whereas estimated sequence divergence across the entire cyt b gene is only 0.6% between UF 18588 and the mainland Cuban C. pilorides sequence used in our analysis, cyt b sequence divergence between the two recognized extant Geocapromys species ( G. brownii and G. ingrahami ), which constitute the other congeneric hutia taxon pair included in our analysis, is almost an order of magnitude greater at 5.5%. Conversely, levels of estimated divergence for the first 415 base pairs of cyt b reported by Borroto-Páez et al. (2005) between the named allopatric subspecies of C. pilorides vary between 0.4%–1.9%, and are therefore much more comparable to the estimated divergence between UF 18588 and mainland Cuban C. pilorides in our analyses. Indeed, the values reported by Borroto-Páez et al. (2005) may represent underestimates of sequence divergence across the entire cyt b region, as the first half of cyt b evolves at a slower rate than the second half ( Irwin et al., 1991; Spotorno et al., 2004). Levels of divergence across the entire cyt b region between the three subspecies of Plagiodontia aedium (1.0%– 2.9%) are also greater than seen between UF 18588 and mainland Cuban C. pilorides (Brace et al., 2012) .
Available sequence divergence data therefore do not support recognition of the Cayman Capromys population as a distinct species, as although it is genetically distinct from mainland Cuban C. pilorides , it shows much lower divergence from this population compared with any interspecific divergence values between welldefined species seen across the Capromyinae , with genetic variation instead consistent with evolutionarily recent subspecies-level differentiation. As this extinct population is demonstrably morphologically distinct from all described Cuban subspecies of C. pilorides , we recognize it as a new subspecies, C. pilorides lewisi . Given the confusion over the taxonomic status of different allopatric Capromys populations, however, it is currently impossible to reconstruct the precise evolutionary affinities of C. pilorides lewisi either to other named subspecies of C. pilorides or to the various putative unnamed but apparently distinct taxa within the genus that have been identified by different authors, and this must await further sampling of the genus across its Quaternary distribution.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.