Loncophorus pustulatus (Champion, 1903)
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3636.3.4 |
|
publication LSID |
lsid:zoobank.org:pub:65578136-2BF2-4764-B430-75E991476E7A |
|
DOI |
https://doi.org/10.5281/zenodo.6147122 |
|
persistent identifier |
https://treatment.plazi.org/id/03AA87BD-FFDA-FFA7-FF28-FA1470423583 |
|
treatment provided by |
Plazi |
|
scientific name |
Loncophorus pustulatus (Champion, 1903) |
| status |
|
Loncophorus pustulatus (Champion, 1903)
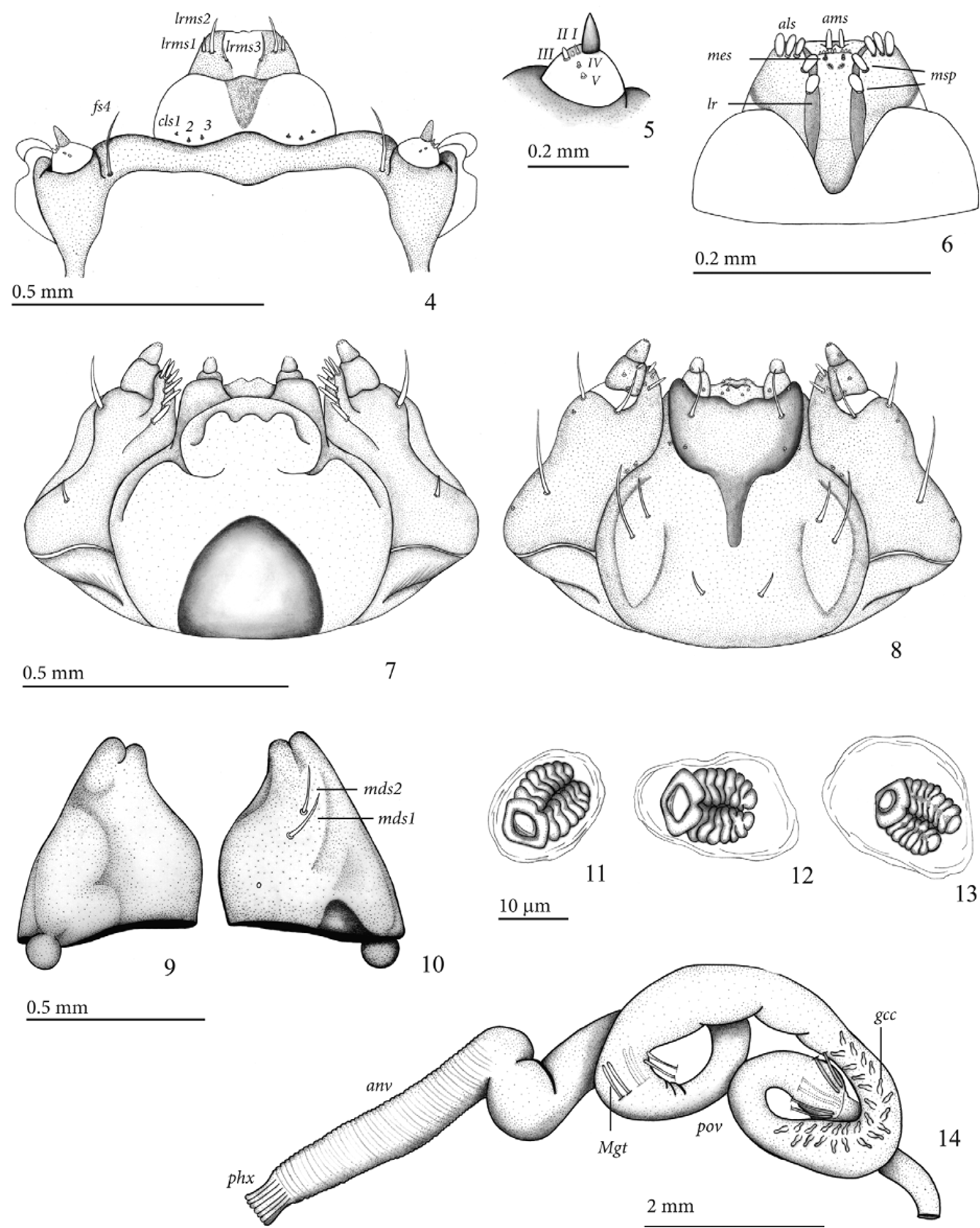
Mature larva description (third instar) ( Figs 1–22 View FIGURES 1 – 3 View FIGURES 4 – 14 View FIGURES 15 – 22 ). Length: 7.5–12.1 mm; prothorax width: 1.6–2.0 mm; head capsule width: 1.0– 1.25 mm; body sub-cylindrical, strongly curved dorso-ventrally, thoracic segments 2 and 3 subequal, segment 1 narrower, last four abdominal segments strongly convergent backwards ( Fig.1 View FIGURES 1 – 3 ). Head capsule light yellow to yellowish brown, mandibles darker; prothorax and abdomen creamy white, pronotal shields light yellow to yellowish brown; body setae short and fine.
Head ( Figs 2 and 3 View FIGURES 1 – 3 ). Hypognathous, sclerotized, free, head capsule slightly wider than long, sides rounded. Epicranial suture distinct, approximately 0.44 times as long as head capsule. Frontal suture distinct, complete, weakly sinuate, V-shaped. Median endocarina present, about 0.42 times as long as head capsule. One stemma present on each side. Antennae exposed ( Figs 4 and 5 View FIGURES 4 – 14 ), one-segmented, conical, accessory appendage elongate, about two times as long as basal width, and with five minute processes, one of them longer and stouter. Head capsule ( Fig. 2 View FIGURES 1 – 3 ) with four pairs of minute posterior epicranial setae (pes 1-4), more or less aligned and forming irregular oblique row; five pairs of dorsal epicranial setae, des1, des2, des4, and des5 long and similar in length, des 3 very short; four pairs of frontal setae, fs1 very small, fs2 and fs4 with median length, and fs3 much longer than other three, tip surpassing frontoclypeal suture; two pairs of lateral epicranial setae (les 1-2), les2 placed near anterior angle of head capsule; two pairs of ventral epicranial setae (ves), ves2 slightly longer than ves1. Epistoma and frontoclypeal sutures slightly arcuate. Clypeus ( Figs. 2 View FIGURES 1 – 3 and 4 View FIGURES 4 – 14 ) transverse, lateral margins rounded, anterior margin sinuate, with three pairs of very small clypeal setae (cls 1-3). Labrum ( Fig. 4 View FIGURES 4 – 14 ) transverse, anterior margin emarginated, with three pairs of labral setae (lrms 1-3), lrms3 slightly longer than lrms1 and lrms2. Epipharynx ( Fig. 6 View FIGURES 4 – 14 ) trapezoidal, with a transverse row of eight minute conical setae, parallel to anterior margin; one pair of anteromedian setae (ams), three pairs of anterolateral setae (als 1-3) on each side, one pair of median setae (mes) and 4 median spines (msp) placed between dark, thick, elongate, subparallel labral rods (lr), diverging anteriorly; six epipharyngeal sensory pores arranged in two clusters of three, located below mes. Maxillae ( Figs 7 and 8 View FIGURES 4 – 14 ) with cardo transverse, sub-triangular; stipes elongate with one short seta on basal third and one longer seta near anterior margin on dorsal side and with three setae on ventral side, basal one longest; mala rounded, with six marginal dorsal spatulate setae more or less aligned along outer margin ( Fig. 7 View FIGURES 4 – 14 ) and three smaller ventral setae; palpifer membranous; maxillary palpi two-segmented, proximal palpomere larger than distal one, with one seta and three sensilla on ventral side, distal palpomere with one sensillum on ventral side. Labium ( Fig. 8 View FIGURES 4 – 14 ): labial palpi twosegmented, basal palpomere elongate, longer and wider than distal palpomere, distal palpomere conical, about as long as wide, each palpomere with one ventral sensillum; prementum with three pairs of setae, median pair longest; premental sclerite with anterior margin strongly sinuate, posterior median extension very narrow; submentum with thre pairs of setae, median pair longest; ligula with five pairs of sensilla. Mandibles symmetrical ( Figs 9 and 10 View FIGURES 4 – 14 ), stout, apically bidentate, dorsally with two setae (mds) and one minute sensillum.
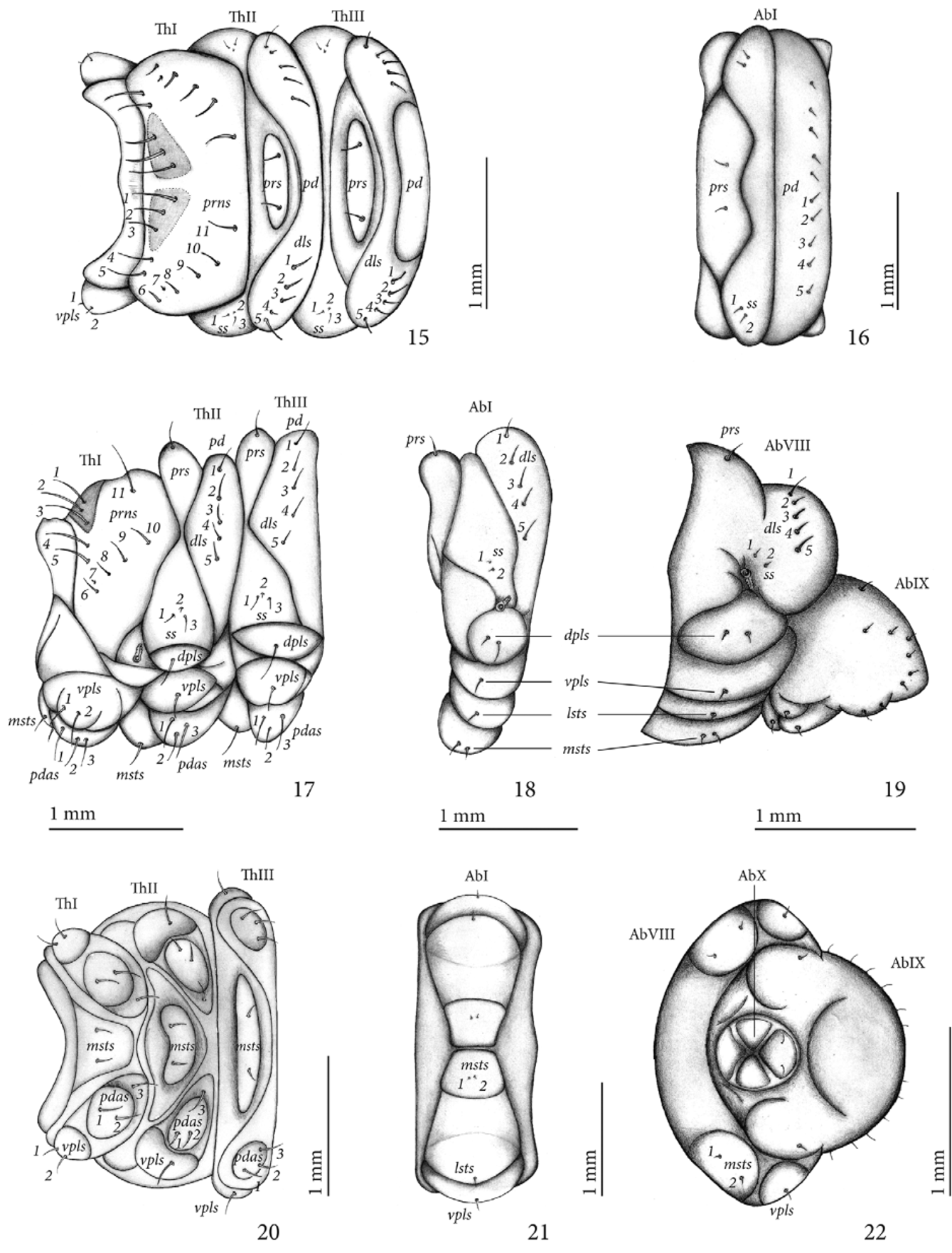
Thorax ( Figs 15, 17 and 20 View FIGURES 15 – 22 ). Pro-, meso- and metathorax transverse, each one narrower than abdomen. Prothorax with 11 pronotal setae (prns 1-11) on each side: pronotum with two small, triangular, sub-contiguous sclerites, each one with prns 1-3, anterior angle with prns 4-5, and an irregular curved row of six setae directed backwards (prns 6-11); pedal area with three pedal setae (pdas 1-3); ventropleural lobe with two setae (vpls 1-2); mediosternal fold with two mediosternal setae (msts 1-2). Meso- and metathorax: prodorsum of meso- and metathorax with one pair of prodorsal seta (prs), postdorsum of meso- and metathorax with five dorsolateral setae (dls 1–5); spiracular area of meso- and metathorax with three spiracular setae (ss 1-3), ss2 much smaller than ss1 and ss3; dorsopleural lobe of meso- and metathorax with one dorsopleural lobe seta (dpls); ventropleural lobe of meso- and metathorax with one ventropleural seta (vpls); mediosternal lobe with two minute setae (msts 1-2). Prothoracic spiracle ( Fig. 11 View FIGURES 4 – 14 ) anular, biforous, air tubes with seven annuli directed upwards, peritreme irregular oval.
Abdomen ( Figs 16, 18, 19, 21 and 22 View FIGURES 15 – 22 ) with 8 pairs of lateral spiracles ( Figs. 12 and 13 View FIGURES 4 – 14 ), bicameral, peritreme circular, air tubes with seven annuli; spiracles of abdominal segments I–VII similar, obliquely caudad, spiracles of segment VIII smaller, with air tubes directed downwards; segments I–VII each one with three folds; segment VIII two-folded; segment IX unfolded; segment X reduced, circular and ventral, anus subterminal, opening in middle of four prominent lobes; segments I–VII with similar chaetotaxy ( Figs 16, 18 and 21 View FIGURES 15 – 22 ): prodorsum with a pair of prodorsal setae (prs); spiracular area with two minute setae (ss 1-2); postdorsum with tranverse row of five pairs of dorsolateral setae (dls 1-5); ventropleural lobe and laterosternal lobe with one seta (vpls, lsts); mediosternal fold with two very thin and minute setae (msts 1-2); chaetotaxy of segment VIII ( Figs 19 and 22 View FIGURES 15 – 22 ) similar to segments I- VII, but pro-dorsal setae (prs) missing; segment X with two setae located on posterior anal lobe.
Alimentary canal ( Fig. 14 View FIGURES 4 – 14 ) lacking mycetomes; posterior ventriculus (pov) two coiled with 28 small gastric caeca (gcc) similar to bowling pins, axially aligned forming two lines of 14 caeca on lower ventricular coil. Malpighian tubules (Mgt) not thickened, arranged 2+2+2.
Pupa description ( Figs 23–27 View FIGURES 23 – 27 ). Length: male 6.1–7.8; female 5.3–9.1 mm. Adecticous and exarate. Coloration whitish-cream, with long stiff setae on pronotum, smaller setae on rostrum and very small setae placed on spiniform processes on dorsal side of abdomen. Head completely covered by pronotum in dorsal view; each side with one vertical setae (vs), one supraorbital setae (sos) and two minute orbital setae (os) located near eye margin; apex of rostrum in females reaching base of abdominal segment V, in males surpassing base of abdominal segment III; rostrum with a pair of short postantennal setae (pas) and two pairs of short rostral setae (rs 1-2). Pronotum transverse, conical, with one pair of suprapical setae (sas) and a pair of discal setae (ds) located on prominent spiniform processes, and with three lateral setae (ls1-3) and four posterolateral setae (pls1-4) on each side. Mesothorax (ThII) with three erect fine tergal setae on each side, located at base of a spiniform process; scutellum rounded. Metathorax longitudinally sulcated and divided into two distinct halfs, each one with three pairs of posterior tergal setae located on small spiniform processes and two minute spines inserted between the setae. Abdomen: segments I-VIII with four pairs of tergal setae located on small spiniform processes and three minute spines inserted between setae, forming one transverse row near posterior margin; III and IV slightly wider than others; VIII transverse with anterior margin rounded; IX with pseudocerci (pc) very acute, each one with one pair of small setae. Gonothaeca splitted in two in females ( Fig. 26 View FIGURES 23 – 27 ), not divided in males ( Fig. 27 View FIGURES 23 – 27 ). Seven annular spiracles placed at lateral sides of segments I to VII, well visible only on lateral view.
Remarks. The larva of Loncophorus pustulatus is very similar to the other two larvae described in the genus, L. fusiformis and L. santarosae . The main differences are in chaetotaxy. In L. pustulatus , fs3 is long and reaches the clypeus, while in L. fusiformis and L. santarosae fs3 is smaller and does not surpass the border of epistoma; on the other hand, L. fusiformis and L. santarosae have much longer fs1 and ds3 than L. pustulatus . Other outstanding differences are the following (characters of L. fusiformis and L. santarosae parenthetic): in L. pustulatus , the anterior margin of labrum is almost straight (rounded); the anterolateral setae (als) of epipharynx are stouter and broader (slender) and the labral rods are almost parallel-sided posteriorly (convergent); prothorax with pronotal setae 7 (prn7) much shorter (longer); ss2 of meso- and metathorax much smaller (larger); ss1 of abdomen smaller than ss2. The last instar larva of L. pustulatus has a transverse row of eight minute conical setae, located just behind the anteromedian setae (ams) of epypharynx. These structures are very small and could be present but were not represented in the illustrations of the last instar larvae of L. fusiformis and L. santarosae , described by Ahmad and Burke (1972), and Clark and Burke (1986), respectively.
The habitus of the pupa of L. pustulatus is very similar to that of L. fusiformis , described by Burke (1968), the only other species of the genus for which a pupal description is available. The observed differences are concerned with chaetotaxy. The pupae of the two species can be distinguished by the presence, in L. fusiformis , of two anteromedian setae located anteriad to the transversal rows of the tergal setae in the meso-, methatorax and abdomen, whilst the two anteromedian setae are absent in L. pustulatus ( Fig. 23 View FIGURES 23 – 27 ). The sexual dimorphism is similar in pupae of both species, the gonothecae IX is splitted in two in the female ( Fig. 26 View FIGURES 23 – 27 ) but not divided in the male ( Fig. 27 View FIGURES 23 – 27 ).
Host plant. Ceiba speciosa (A. St.-Hil.) Ravenna ( Malvaceae ) ( Fig. 41 View FIGURES 38 – 41. 38 — 40 ). According to Lorenzi (2000), the "paineira" reaches 30 m in height; the trunk is bottle-shaped and can attain 120 cm in girth. The leaves are palmate and deciduous. The flowers are large and colorful ( Fig. 28 View FIGURES 28 – 33. 28 ). Its blooming time is from mid-December to May. The large fruits are dehiscent, mature from August to September, and in this period the tree is completely devoid of leaves. The natural range is central (Goiás and Mato Grosso do Sul) and southeast Brazil (Minas Gerais, Rio de Janeiro, São Paulo and north of Paraná). As it is a startling tree, resistant to drought and moderate cold, it has been introduced in other Brazilian states and even other countries as an ornamental plant used for landscaping.
Biological notes on natural history. We observed the complete development of L. pustulatus inside the flowers of C. speciosa (A. St.-Hil.) Ravenna collected in the city of São Paulo. Oviposition occured in the "paineira" during the flowering period, between mid-December to May. In the same tree branch flowers were found in different stages of development, an event already reported by Donato (1991). Female weevils did not select a specific flower stage in which to oviposit, since weevil larvae of first instar were found in buds and open flowers as well. With the help of the rostrum, females drilled a hole into the apical region of the staminodium of buds of nonaborted flowers ( Fig. 29 View FIGURES 28 – 33. 28 ). Only one channel was drilled and a single egg was oviposited in each flower ( Fig. 30 View FIGURES 28 – 33. 28 ). As soon as the egg hatched, the first instar larva began feeding and burrowed a gallery through the staminodeum tissues in the direction of the central cavity ( Fig. 31 View FIGURES 28 – 33. 28 ). This movement was accomplished in two days. When the first instar larva reached the central cavity it moulted to the second instar larva.
The second instar larva moved in circular paths and enlarged the feeding area on the upper inner walls of the central cavity, for about three days. The bodies of the first and second instar larvae were covered by a sticky, mucilaginous substance. According to Donato (1991), the inner layer of epidermis that delimits the central cavity walls has groups of mucilaginous ducts. We observed that, as the larva burrows, the ducts are cut off and the mucilage is released. Part of the mucilage is eventually ingested during larval feeding, part remains free in the cavity and part gets stuck on the larval integument.
In the third instar, the larva also began to feed on the style tissues, rendering the flowers sterile. Next, the third instar larva, always feeding and moving in circular paths, went downward in the direction of the ovary ( Fig. 32 View FIGURES 28 – 33. 28 ). By the middle of the third instar, the ovary tissues, as well as the surrounding tissues near the base of petals, were completely eaten. The third instar lasted about seven days. After the larva stopped eating ( Fig. 34 View FIGURES 34 – 37 ), it took three and half days to construct the pupal chamber, which was covered with remains of the attacked tissues ( Fig. 33 View FIGURES 28 – 33. 28 ). The pre-pupa remained one and a half day in the pupal chamber before moulting to the pupa stage.
The pupal phase ( Figs 33 View FIGURES 28 – 33. 28 and 35 View FIGURES 34 – 37 ) lasted about six days. When disturbed, pupae rotated their abdominal segments in a circular motion. As soon as the teneral adults emerged ( Fig. 36 View FIGURES 34 – 37 ), they remained inside the pupal chamber for about two to four days. The length of this period may reach six days at lower temperatures.
On two occasions, we analyzed the frequency of aborted flowers infested by larvae of L. pustulatus found on the ground under C. speciosa (A. St.-Hil.) Ravenna. On 18.iv.2010, from 80 flowers collected, 26 (32.5%) harbored one larva of L. pustulatus ; on 3.v.2011, from 53 flowers collected, 18 (34%) harbored one larva.
Reared adults, mantained in laboratory, had been fed with petals of the "paineira" flowers and a complement of honey and water. In November, young leaves of sprouts of the host plant were offered to the adult weevils. After a day, the leaves had tiny circular holes. Very similar holes were observed in the field, in the leaves of the host plant where the adult weevils were collected. Whenever young and older leaves were offered at the same time, the adults of L. pustulatus showed a distinct fondness for the young leaves. Adults ( Fig. 37 View FIGURES 34 – 37 ) remained alive for up to nine months, from mid-May to mid-February, under uncontrolled laboratory conditions. The period of weevil death coincides with the beginning of the new blooming season. The entire cycle, from egg to adult, lasted 18 to 20 days. The larvae and adults were more active at night. As soon as it began to darken, adult weevils started moving.
One larva of L. pustulatus was parasitised by the wasp Catolaccus sp. ( Hymenoptera , Pteromalidae ) ( Figs. 38–40 View FIGURES 38 – 41. 38 — 40 ). Duration of the wasp pupal phase was eight days. Wasps of the same genus were reported as ectoparasitoids of other Anthonomini , as for instance Catolaccus grandis Burks, 1954 , as a parasitoid of the cotton weevil, Anthonomus grandis , in Campina Grande, State of Paraíba, Brasil (Araújo & Azevedo, 1997).
Discussion. Clark (1988) reported the following Bombacoidea (formerly Bombacaceae ) as hosts of L. pustulatus : Ceiba pentandra (L.) Gaertn, in Nicaragua; Pachira aquatica Aubl. , in Minas Gerais, Brazil; and, Pseudobombax longiflorum (Mart. & Zucc.) A. Robyns , in Minas Gerais, Brazil. The author (l.c.: 491) noted that weevil adults were reared from flower buds of P. aquatica Aubl. and P. longiflorum (Mart. & Zucc.) A. Robyns.
Fernández et al. (2008) determined the frequency of larvae of two species of Loncophorus on aborted and nonaborted flower buds ( L. fusiformis ) and developing fruits ( L. santarosae ) of C. pentandra (L.) Gaertn, in southeastern Costa Rica. The authors demonstrated that the incidence of flower buds with weevil larvae was lower in those attached to trees than in the aborted flowers that had fallen to the ground. They reported one similar and one higher frequency of flower buds infested by larvae than we did: 30.1% in 2003 and 40.4%. in 2005, while we obtained incidences of 32.5% in 2010 and 34 % in 2011. We have not observed the larvae of L. pustulatus attacking developing fruits of C. speciosa (A. St.-Hil.) Ravenna, in São Paulo.
The flowers of C. speciosa (A. St.-Hil.) Ravenna provides a shelter to all life phases of L. pustulatus : eggs, larvae, pupae and adults. As the larvae feeds on the reproductive structures, the flowers have their growth reduced and the ovaries do not produce fruits. Aborted flowers with weevil larvae inside show evident damage in shortened styles and darkened anthers. In all observations, larvae pupate inside the flower, although an earthy substrate was available as a pupation site.
Acknowledgments
We would like to thank Dr. Horace R. Burke (Department of Entomology, Texas A&M University, Texas, USA) for providing important bibliographic information not accessible to us; Dr. Sergio Ide ("Laboratório de Entomologia Geral", Instituto Biológico, São Paulo, SP), for permission to collect "paineira" flowers in the green areas of the institution; Dr. Valmir Antonio Costa (Instituto Biológico de Campinas, Campinas, SP) for his identification of the parasitoid wasp; M. Sc. Juares Fuhrmann (Museu de Zoologia, USP) for valuable suggestions; Ricardo Kawada (Museu de Zoologia, USP), for the stereomicroscope photo of adult wasp; and Mariana B. Vanin (Miami, Florida, USA) for the revision of English text.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.