Stenichnaphes Franz
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3915.2.4 |
|
publication LSID |
lsid:zoobank.org:pub:C73C82EC-861F-40C9-B1C7-06F3279FF879 |
|
DOI |
https://doi.org/10.5281/zenodo.5680887 |
|
persistent identifier |
https://treatment.plazi.org/id/03AAAB48-FFC2-FFEE-D3D6-FB34FB92C134 |
|
treatment provided by |
Plazi |
|
scientific name |
Stenichnaphes Franz |
| status |
|
Stenichnaphes Franz View in CoL
Stenichnaphes Franz, 1980: 25 View in CoL . Type species: Stenichnaphes urbanus Franz, 1980 View in CoL (orig. des.).
Revised diagnosis. Head approximately subtriangular, with vertex not expanded dorsoposteriorly; occipital constriction only slightly narrower than vertex; thick bristles on head and prothorax absent; frontoclypeal groove absent; tempora short and posterior margins of eyes nearly adjacent to occipital constriction; submentum demarcated laterally by sutures; antennae gradually thickening distally; pronotum with lateral edges developed in posterior half or third; base of pronotum with transverse groove connecting two lateral pits, without sublateral carinae; basisternal part of prosternum shorter than procoxal cavities; prosternum with rudimentary carinate prosternal intercoxal process; prothoracic hypomeral ridges absent; pronotosternal sutures entire; mesoventral intercoxal process developed as short and broad carina only in anterior half of mesoventrite, area between mesocoxae broad and weakly convex; mesoventrite with asetose lateral impressions behind anterior ridge (functioning as procoxal rests); mesothorax without lateral foveae; mesocoxal projection with large posterior lobe; metacoxae contiguous; metaventral intercoxal process subtrapezoidal; each elytron with two asetose rudiments of basal foveae; aedeagus with symmetrical median lobe but strongly asymmetrical internal sclerites, parameres free (i.e., not fused with median lobe); spermatheca globular.
Redescription. Body of males ( Figs. 1–2 View FIGURES 1 – 6 ) moderately convex, elongate and slender, with long appendages, BL 0.87–1.15 mm; cuticle glossy, brown, covered with sparse setae.
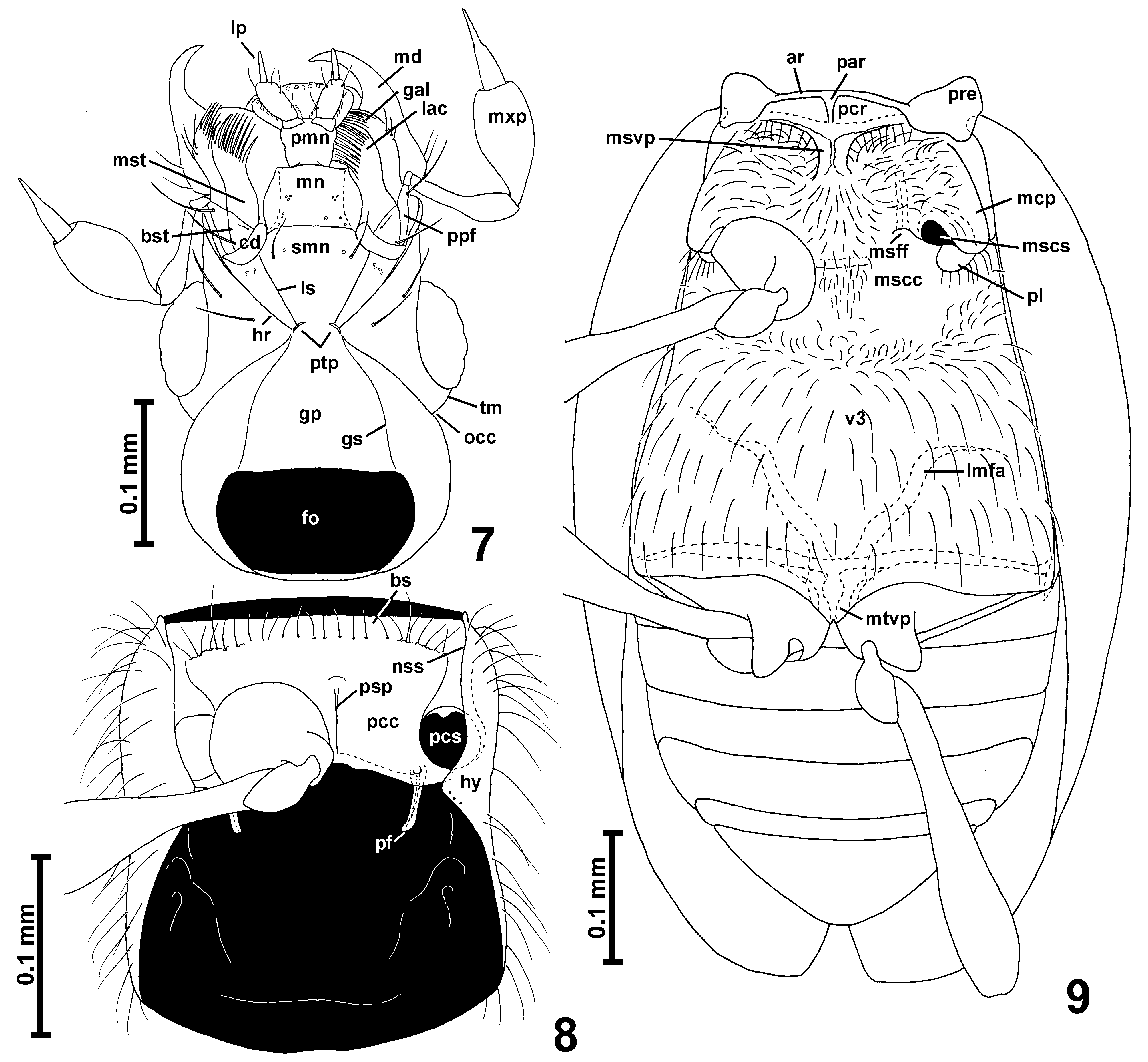
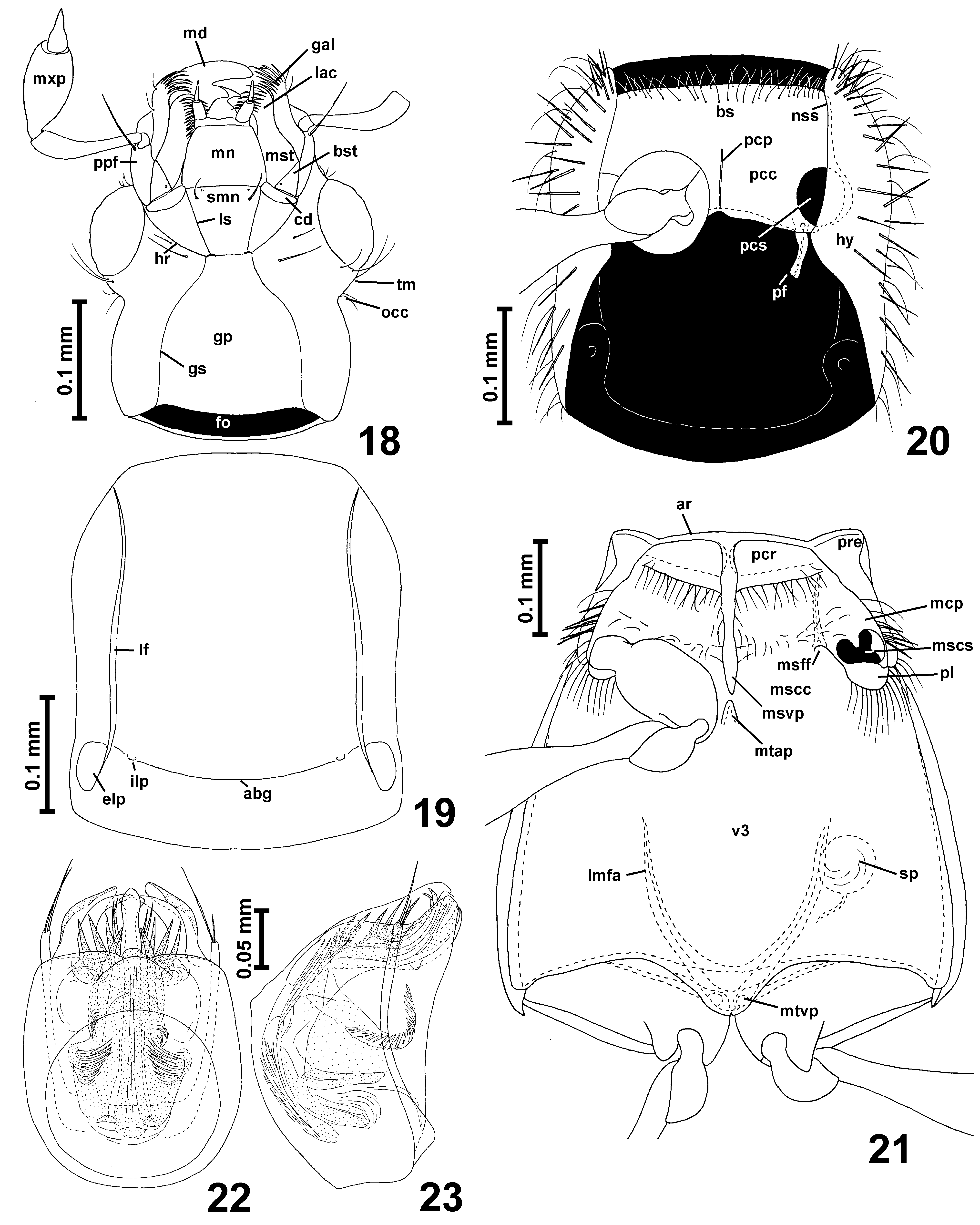
Head ( Figs. 1–2 View FIGURES 1 – 6 , 7 View FIGURES 7 – 9 ) with anterior part (in front of occipital constriction) subtriangular, with large eyes; occipital constriction ( Fig. 7 View FIGURES 7 – 9 ; occ) only slightly narrower than vertex; tempora ( Fig. 7 View FIGURES 7 – 9 ; tm) much shorter than eyes, without bristles; vertex broader than long, weakly convex; frons confluent with vertex, subtriangular; frontoclypeal groove absent; antennal insertions moderately broadly separated.
Labrum transverse with rounded anterior margin.
Mandibles ( Fig. 7 View FIGURES 7 – 9 ; md) symmetrical, subtriangular, each with broad base and slender, strongly curved apical tooth, without subapical teeth; basal part of mesal margin not visible in studied specimen.
Each maxilla with subtriangular basistipes ( Fig. 7 View FIGURES 7 – 9 ; bst), broad mediostipes ( Fig. 7 View FIGURES 7 – 9 ; mst), elongate galea ( Fig. 7 View FIGURES 7 – 9 ; gal) and lacinia ( Fig. 7 View FIGURES 7 – 9 ; lac) and strongly elongate and large maxillary palp ( Fig. 7 View FIGURES 7 – 9 ; mxp) composed of minute, elongate palpomere I, strongly elongate, pedunculate and slender palpomere II, large and strongly elongate palpomere III broadest near middle, and long, slender, subconical and pointed palpomere IV with indistinctly delimited apical part.
Labium with long subtriangular submentum ( Fig. 7 View FIGURES 7 – 9 ; smn) indistinctly demarcated posteriorly from gular plate by transverse impression and laterally demarcated from long postcardinal portions of hypostomae by lateral sutures ( Fig. 7 View FIGURES 7 – 9 ; ls); subtrapezoidal mentum ( Fig. 7 View FIGURES 7 – 9 ; mn); and long prementum ( Fig. 7 View FIGURES 7 – 9 ; pmn) bearing narrowly separated at bases long 3-segmented labial palps ( Figs. 14 View FIGURES 10 – 17 , 22 View FIGURES 18 – 23 ; lp), ligula not demarcated. Hypostomal ridges ( Fig. 7 View FIGURES 7 – 9 ; hr) distinct and extending to posterior tentorial pits.
Gular plate ( Fig. 7 View FIGURES 7 – 9 ; gp) large and distinctly narrowing anteriorly; gular sutures ( Fig. 7 View FIGURES 7 – 9 ; gs) distinct; posterior tentorial pits ( Fig. 7 View FIGURES 7 – 9 ; ptp) barely discernible in ventral view, hidden in transverse groove demarcating base of submentum from gular plate.
Antennae ( Figs. 1–2 View FIGURES 1 – 6 ) slender, gradually thickening distally.
Pronotum ( Figs. 1–2 View FIGURES 1 – 6 ) in dorsal view approximately bell-shaped, with rounded anterior margin and anterior parts of lateral margins, without anterior corners, with shallow constriction in posterior half; hind corners obtuseangled and blunt; posterior margin arcuate; lateral marginal carinae visible in posterior half or third as rather blunt edges; base of pronotum with distinct arcuate transverse groove connecting one lateral pair of shallow pits; sublateral carinae absent; pronotum only with thin setae, without thick bristles.
Prosternum ( Fig. 8 View FIGURES 7 – 9 ) with short basisternal part ( Fig. 8 View FIGURES 7 – 9 ; bs) not demarcated from procoxal cavities ( Fig. 8 View FIGURES 7 – 9 ; pcc); median part of sternum with narrow and weakly protruding ventrally prosternal intercoxal process ( Fig. 8 View FIGURES 7 – 9 ; psp); procoxal sockets ( Fig. 8 View FIGURES 7 – 9 ; pcs) closed by narrow posterolateral lobes of prosternum; hypomera ( Fig. 8 View FIGURES 7 – 9 ; hy) elongate, without hypomeral ridges; pronotosternal sutures ( Fig. 8 View FIGURES 7 – 9 ; nss) entire.
Mesonotum with small, subtriangular meoscutellum visible between bases of elytra in intact specimens, mesoscutoscutellar suture present.
Mesoventrite ( Fig. 9 View FIGURES 7 – 9 ) with narrow anterior ridge ( Fig. 9 View FIGURES 7 – 9 ; ar); mesoventral intercoxal process ( Fig. 9 View FIGURES 7 – 9 ; msvp) developed as moderately broad, short and weakly expanding ventrally carina in anterior half of mesoventrite, anteriorly not connected to median projection of anterior ridge ( Fig. 9 View FIGURES 7 – 9 ; par) and posteriorly not reaching intercoxal area, which is broad and weakly convex (although in intact specimens mesocoxae may touch each other); lateral asetose impressions functioning as procoxal rests ( Fig. 9 View FIGURES 7 – 9 ; pcr) present, subtriangular; mesanepisternum with moderately long prepectus ( Fig. 9 View FIGURES 7 – 9 ; pre) and posterior portion only partly visible in ventral view; mesepimeron not visible in ventral view; sides of mesothorax without foveae; mesocoxal projections ( Fig. 9 View FIGURES 7 – 9 ; mcp) prominent but weakly expanding laterally, with mesocoxal sockets ( Fig. 9 View FIGURES 7 – 9 ; mscs) located on their mesoventral surface and with exposed large posterior lobes ( Fig. 9 View FIGURES 7 – 9 ; pl) devoid of thick bristles, and only with sparse thin setae.
Metaventrite ( Fig. 9 View FIGURES 7 – 9 ; v3) subtrapezoidal, anteriorly fused with mesoventrite, without anterior metaventral process; posteriorly metaventrite shallowly bisinuate and with subtriangular median metaventral intercoxal process ( Fig. 9 View FIGURES 7 – 9 ; mtvp) slightly notched in middle. Metanepisterna and metepimera narrow.
Metendosternite (metafurca) ( Fig. 9 View FIGURES 7 – 9 ) with short stalk and divergent lateral metafurcal arms ( Fig. 9 View FIGURES 7 – 9 ; lmfa).
Elytra ( Figs. 1–2 View FIGURES 1 – 6 ) oval, each with two asetose rudiments of basal foveae, of which external one is more distinct, with short basal impression; humeral calli distinct and developed as longitudinal protuberances; subhumeral lines absent.
Hind wings well-developed, about twice as long as elytra.
Legs ( Figs. 1–2 View FIGURES 1 – 6 , 8–9 View FIGURES 7 – 9 ) moderately long and slender; procoxae subglobose, mesocoxae oval, metacoxae strongly transverse; all trochanters short; all femora weakly clavate; tibiae long and straight; tarsi slender.
Abdominal sternites ( Fig. 9 View FIGURES 7 – 9 ) unmodified, suture between VII and VIII distinct.
Aedeagus ( Figs. 10–17 View FIGURES 10 – 17 ) with symmetrical and thin-walled median lobe, paired apical projections and a system of strongly asymmetrical endophallic sclerites; parameres free (i.e., not fused with median lobe) and with apical setae.
Spermatheca sclerotized and globular.
Distribution and composition. Nominal species of Stenichnaphes were reported to occur in several disjunct southern hemisphere localities: New Zealand, Madagascar and Brazil. The type species, St. urbanus , is known to occur in New Zealand, and St. peloriensis , previously placed in synonymy with St. urbanus , is here reinstated as a separate species. Moreover, St. newtoni is here transferred to another genus. Therefore, two confirmed species of Stenichnaphes inhabit North Island and the northernmost tip of South Island of New Zealand ( Figs. 24–25 View FIGURES 24 – 26 ), and this is all that is known for sure about the distribution of this genus. Generic placement of all other species requires verification.
The only Brazilian species was originally described in Scydmoraphes Reitter, 1891 , later transferred to Alloraphes ( Franz 1980b) , and then to Stenichnaphes (Franz 1989) . Alloraphes myrmecophilus Franz, 1980b , another species treated by Franz in the same series of papers, traveled the opposite direction―from Alloraphes to Parastenichnaphes and back to Alloraphes ( Jałoszyński 2013a) . This shows that the generic placement of all yet unrevised species of the Stenichnaphes - Alloraphes - Parastenichnaphes complex should be treated as unclear. Perfectly symmetrical endophalli of Madagascan ' Stenichnaphes ', illustrated by Franz (1986b) also suggest that these species may not be congeneric with those living in New Zealand.
Remarks. Stenichnaphes was originally diagnosed as a genus similar to Neuraphes and Stenichnus ( Franz 1980a) . New Zealand species of Stenichnaphes externally seem to resemble more northern hemisphere Scydmoraphes in the general body form and the antebasal transverse pronotal groove. Also the mandibles without mesal teeth are similar to those of Scydmoraphes . However, the latter genus has complete prothoracic hypomeral ridges (absent in Stenichnaphes ), single deep basal elytral fovea (two indistinct rudiments in Stenichnaphes ), a long and narrowly carinate mesoventral intercoxal process extending from anterior mesoventral ridge to middle of mesocoxae (in Stenichnaphes process not connected to anterior ridge and not reaching anterior margins of coxae), the anterior metaventral process (absent in Stenichnaphes ), and the aedeagus with symmetrical endophallus (strongly asymmetrical in Stenichnaphes ). Neuraphes has a small subapical tooth on each mandible, procoxal cavities distinctly demarcated from the basisternal part of prosternum, the mesoventral intercoxal process and the anterior metaventral process as those in Scydmoraphes , single, deep basal elytral fovea filled with setae, and a peculiar structure of the aedeagus, clearly different than that in Stenichnaphes . Stenichnus , in turn, has slender, falciform mandibles, the pronotum without lateral edges and without transverse antebasal groove, each elytron with single and deep basal fovea, the mesoventral intercoxal process and the anterior metaventral process as those in Scydmoraphes , and the aedeagus with thick walls and symmetrical endophallus.
Within the Stenichnaphes - Alloraphes - Parastenichnaphes complex, each genus can be easily distinguished on the basis of the aedeagal structures. Stenichnaphes has the aedeagus with parameres and asymmetrical internal sclerites, but without a basal lentiform sclerotization extending into the inside of the median lobe as a longitudinal (axial) apodeme to which muscles are attached. Such a lentiform structure is characteristic of the aedeagi of Alloraphes and Parastenichnaphes , and the former genus has parameres, while in the latter parameres are entirely absent ( Jałoszyński 2005, 2013a). Alloraphes and Parastenichnaphes have also the mesoventral intercoxal process long and carinate, extending from anterior mesoventral ridge to at least middle of mesocoxae, a character clearly different in Stenichnaphes .
All the genera listed in previous paragraphs ( Neuraphes , Stenichnus , Scydmoraphes, Stenichnaphes , Alloraphes and Parastenichnaphes ) share peculiar ventral structures of the head―a pair of distinct, sharp sutures demarcating the prementum laterally, extending from posteromesal margins of cardines to posterior tentorial pits. Among Cyrtoscydmini , such sutures can be found also in the following genera, whose structures have been adequately studied: Palaeoscydmaenus from Australia ( Jałoszyński 2012a), Mexiconnus Jałoszyński, 2013c from Mexico, Siamites Franz, 1989 from Thailand ( Jałoszyński 2013d), Zeanichnus Jałoszyński, 2013b from New Zealand, Austrostenichnus Franz, 1971 from New Caledonia ( Jałoszyński 2013b), Scydmaenilla King, 1864 from Australia and New Zealand ( Jałoszyński 2013b and present paper) and Obesoconnus Jałoszyński, 2014 from Mexico and French Guyana. Obesoconnus can be easily distinguished from Stenichnaphes on the basis of the aedeagus, which is devoid of parameres and has the basal lentiform sclerotization (similar to that in Alloraphes and Parastenichnaphes ). Palaeoscydmaenus differs from Stenichnaphes in open procoxal sockets, lack of prosternal intercoxal process, lack of pronotal antebasal pits and lack of even traces of basal elytral pits. Mexiconnus and Siamites differ from Stenichnaphes in the long and carinate mesoventral intercoxal process reaching posterior margins of mesocoxal cavities and there fused with metaventrite. Zeanichnus and Scydmaenilla differ from Stenichnaphes in the mesoventral intercoxal process carinate and extending from the anterior ridge of mesoventrite to middle of mesocoxal cavities, where it meets the anterior metaventral process, with its tip directed anteriorly. Finally, Austrostenichnus differs from Stenichnaphes in lacking even a trace of mesoventral intercoxal process. These are only key differences, there are other structures that differentiate all these genera; they can be found in Jałoszyński (2012a, 2013b, c, d, 2014). It must be keep in mind that there are still unrevised genera distributed mostly in the southern hemisphere, which may also have the lateral sutures of submentum, and a useful identification key can be given only when they all have been properly studied.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Stenichnaphes Franz
| Jałoszyński, Paweł 2015 |
Stenichnaphes
| Franz 1980: 25 |
Stenichnaphes urbanus
| Franz 1980 |