Rosa gallica, L.
|
publication ID |
https://doi.org/10.1016/j.phytochem.2014.08.013 |
|
DOI |
https://doi.org/10.5281/zenodo.10568686 |
|
persistent identifier |
https://treatment.plazi.org/id/03AB6A4A-7017-AE2E-FFF1-AC2EFC0A285B |
|
treatment provided by |
Felipe |
|
scientific name |
Rosa gallica |
| status |
|
2.1. Isolation of flavonol glycosides from R. gallica View in CoL
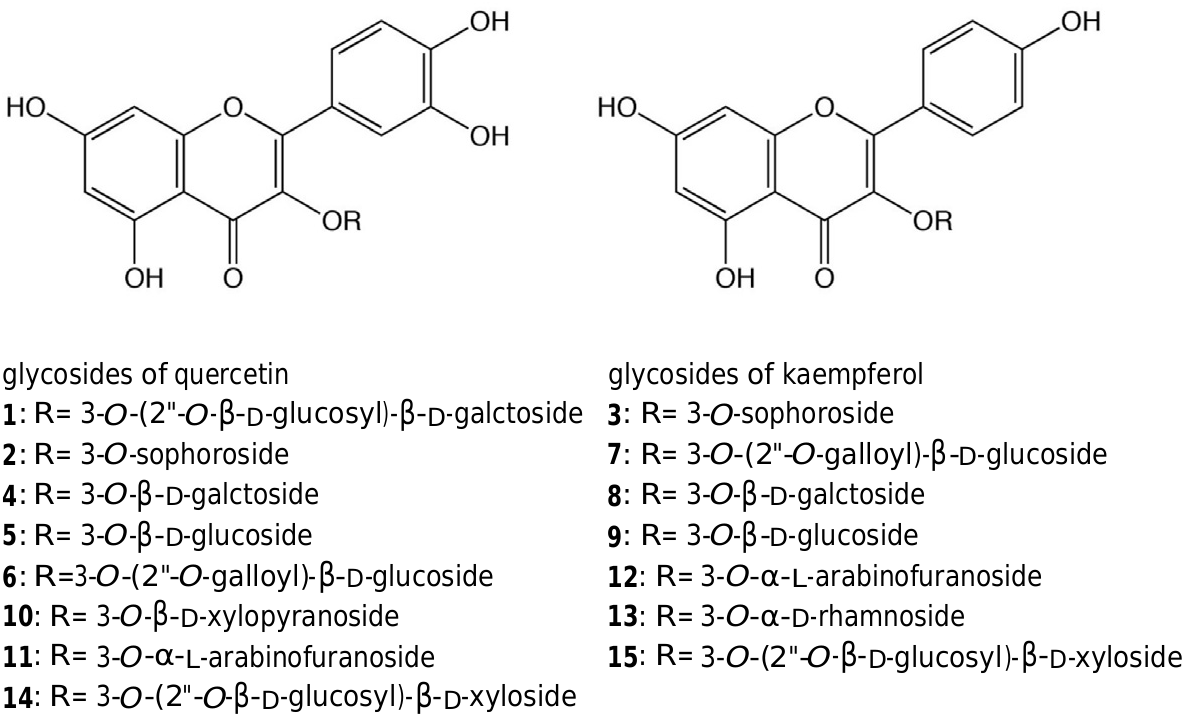
The 50% EtOH extracts of the petals of a hybrid of R. gallica collected in the Xinjiang province in China were continuously partitioned with a H 2 O–MeOH–CHCl 3 system, and the upper layer was fractionated by column chromatography on MCI gel CHP-20P followed by chromatography on Sephadex LH-20. The fractions containing flavonol glycosides were further purified using a reversed phase gel (Wakosil 40 C18) to give compounds 1–13. Compounds 1, 2, 3, 5 and 9 were isolated as the major flavonol glycosides in the petals of R. gallica , and were identified as quercetin 3- O -( 200 - O - β -D- glucosyl)- β -D- galactoside ( 1), quercetin 3- O -sophoroside ( 2), kaempferol 3- O -sophoroside ( 3), quercetin 3- O - β -D- glucoside ( 5) and kaempferol 3- O - β -D- glucoside ( 9), respectively, as previously described ( Sarangowa et al., 2010) and by comparison of their 1 H and 13 C NMR data and MS data with literature values ( Iwashina et al., 2005; Schliemann et al., 2006; Ross et al., 2005).
The 1 H NMR spectra of 4, 10 and 11 indicated the presence of quercetin as an aglycone. The 13 C NMR spectrum of compound 4 showed the presence of a hexose, and the spectra of 10 and 11 showed the presence of a pentose. Comparing the 1 H and 13 C NMR data and MS data with literature data ( Lin and Lin, 1999; Nowak and Wolbis, 2002; Park et al., 2012; Tanaka et al., 1981; Zhu et al., 2013), the structures of 4, 10 and 11 were identified as quercetin 3- O - β -D- galactoside ( 4), quercetin 3- O - β -D- xylopyranoside ( 10) and quercetin 3- O - α -L- arabinofuranoside ( 11), respectively. The positions of the sugar moiety on quercetin were confirmed by analysis of HMBC spectra. The C 1 –H of the sugar moiety gave a cross peak with the C 3 position of quercetin.
The 1 H NMR spectra of compounds 8, 12 and 13 indicated presence of kaempferol as the aglycone ( Xiao et al., 2006; Zhu et al., 2013). The 13 C NMR spectrum of 8 showed the presence of a hexose, and the spectra of 12 and 13 showed the presence of a pentose. Comparing the 1 H and 13 C NMR data and MS data of these compounds with literature values ( Nowak and Wolbis, 2002; Alaniya et al., 2012; Park et al., 2012), the structures of 8, 12 and 13 were characterized as kaempferol 3- O - β -D- galactoside ( 8), kaempferol 3- O - α -L- arabinofuranoside ( 12), and kaempferol 3- O - α -D- rhamnoside ( 13).
Quercetin 3- O -( 200 -O -galloyl)- β -D- glucoside ( 6) and kaempferol 3- O -( 200 -O -galloyl)- β -D- glucoside ( 7) were also isolated from R. gallica as detailed in our previous report ( Sarangowa et al., 2013) ( Fig. 1 View Fig ).
2.2. Quantitative determination of flavonol glycosides in sections
Gallicanae , Cinnamomeae , Caninae and Synstylae
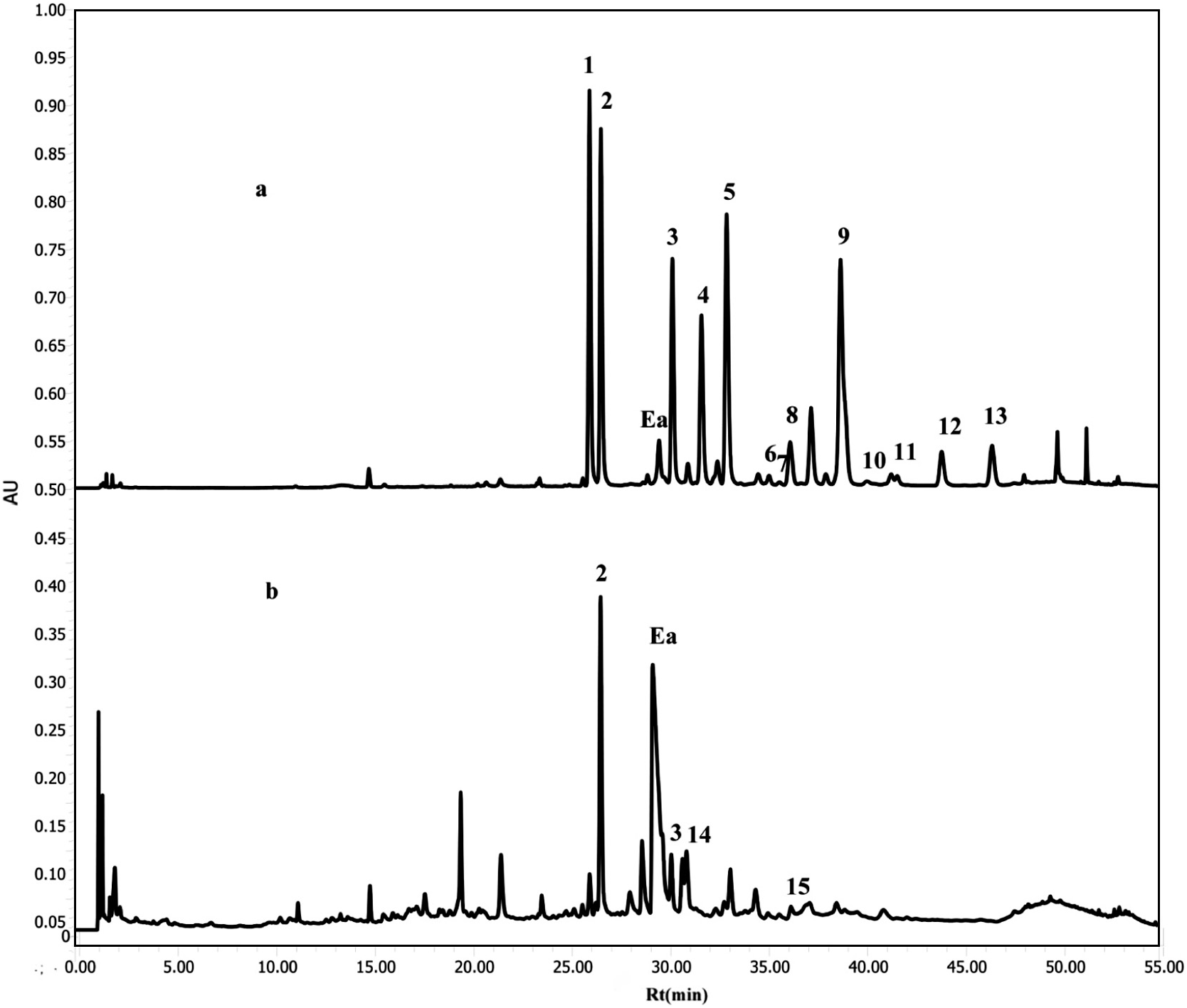
A method using ultra-performance liquid chromatography (UPLC) equipped with a photodiode array (PDA) detector was optimized for quantitative determination of flavonol glycosides in the 50% EtOH extract of the petal of 31 Rosa species ( Table 1 View Table 1 ). The monitoring wavelength was set at 350 nm, which was the absorption maximum for glycosides of quercetin and kaempferol. Chromatography peaks of quercetin and kaempferol glycosides were identified from their retention times and UV spectra monitored by PDA detector. The content of flavonol glycosides was quantitatively analyzed by UPLC.
Typical chromatograms of hybrids of R. gallica and R. rugosa are shown in Fig. 2 View Fig . Seven glycosides of quercetin ( 1, 2, 4, 5, 6, 10 and 11) and six glycosides of kaempferol ( 3, 7, 8, 9, 12 and 13) were detected in the chromatogram of the hybrid of R. gallica . However, in the chromatogram of R. rugosa , 3- O -( 200 -O - β -D- glycosyl)- β -D- xylosides of quercetin ( 14) and kaempferol ( 15) were detected along with 2 and 3, while other glycosides were not detected. The content of fifteen glycosides of quercetin and kaempferol are shown in Table 2 View Table 2 .
Fifteen flavonol glycosides were identified in this experiment; however, all of the flavonol glycosides were not detected in all samples. The total content of these glycosides was highest in the petals of Rosa species in section Gallicanae ( 28.68–119.10 mg /g), followed by section Caninae (30.78 and 41.52 mg /g) and section Synstylae (30.49 and 48.90 mg /g). The glycoside content in the petals of Rosa species in Section Cinnamomeae was lower than the other sections ( 0.48–7.5 mg /g).
Flavonol glycosides including a hexose ( 4, 5, 8, 9 and 13) and their galloyl derivatives ( 6 and 7), and those with a pentose ( 10, 11 and 12) were detected only in section Gallicanae , except for Rosa acicularis (Ci05), Rosa davurica (Ci08) and R. rugosa × Rosa rubrifoia (Ci02). On the other hand, the Rosa species in section Cinnamomeae mainly contained glycosides with a disaccharide ( 1, 2, 3, 14 and 15). Sophorosides of quercetin and/or kaempferol ( 2 and 3, respectively) were detected in almost all of the samples, and two patterns involving the presence and content of these glycosides along with the content of 1 were recognized. Samples belong to pattern 1 contained relatively large amount of 1, 2 and 3, and those belonging to pattern 2 contained lower amounts of these compounds (or were not detected). Compounds 14 and 15 were detected only in section Cinnamomeae .
glycosides of quercetin glycosides of kaempferol
1: R= 3- O -(2"- O -β- D- glucosyl)-β- D- galctoside 3: R= 3- O -sophoroside
2: R= 3- O -sophoroside 7: R= 3- O -(2"- O -galloyl)-β- D- glucoside
4: R= 3- O -β- D- galctoside 8: R= 3- O -β- D- galctoside
5: R= 3- O -β- D- glucoside 9: R= 3- O -β- D- glucoside
6: R=3- O -(2"- O -galloyl)-β- D- glucoside 12: R= 3- O -α- L- arabinofuranoside
10: R= 3- O -β- D- xylopyranoside 13: R= 3- O -α- D- rhamnoside
11: R= 3- O -α- L- arabinofuranoside 15: R= 3- O -(2"- O -β- D- glucosyl)-β- D- xyloside 14: R= 3- O -(2"- O -β- D- glucosyl)-β- D- xyloside
3. Discussion
3.1. Flavonol glycosides in R. gallica View in CoL
Xiao et al. (2006) reported the isolation of glycosides of kaempferol and quercetin such as 1, 3, 4, 9, 11 and 12 from ‘‘ R. rugosa ’’ collected in the Xinjiang province, China. In the present study, samples of Rosa plants were collected at the same location for analysis. As reported previously, the roses used as ‘‘Mei-gui’’ or ‘‘Kizil gul’’ in Xinjiang province are not R. rugosa , but are R. gallica and its hybrids. In this paper, glucosides 4, 9, 11 and 12 were not detected in 6 samples of R. rugosa collected in Japan, Korea and China, and 1, 3, 4, 9, 11 and 12 were detected in all of the samples collected in Xinjiang province. Therefore, the plants collected by Xiao et al. (2006) were supposed to be hybrids of R. gallica . In this study, additional glycosides of quercetin and kaempferol including 2, 5, 8, 10 and 13 were isolated for the first time from hybrids of R. gallica .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |