Brachypeplus apicalis Murray, 1864
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5103.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:9E1A72E7-3862-44F7-B69F-ECE64B239FF9 |
|
DOI |
https://doi.org/10.5281/zenodo.6839730 |
|
persistent identifier |
https://treatment.plazi.org/id/03AC7326-7656-D65E-75E0-FAC5FD92FD86 |
|
treatment provided by |
Plazi |
|
scientific name |
Brachypeplus apicalis Murray, 1864 |
| status |
|
13. Brachypeplus apicalis Murray, 1864
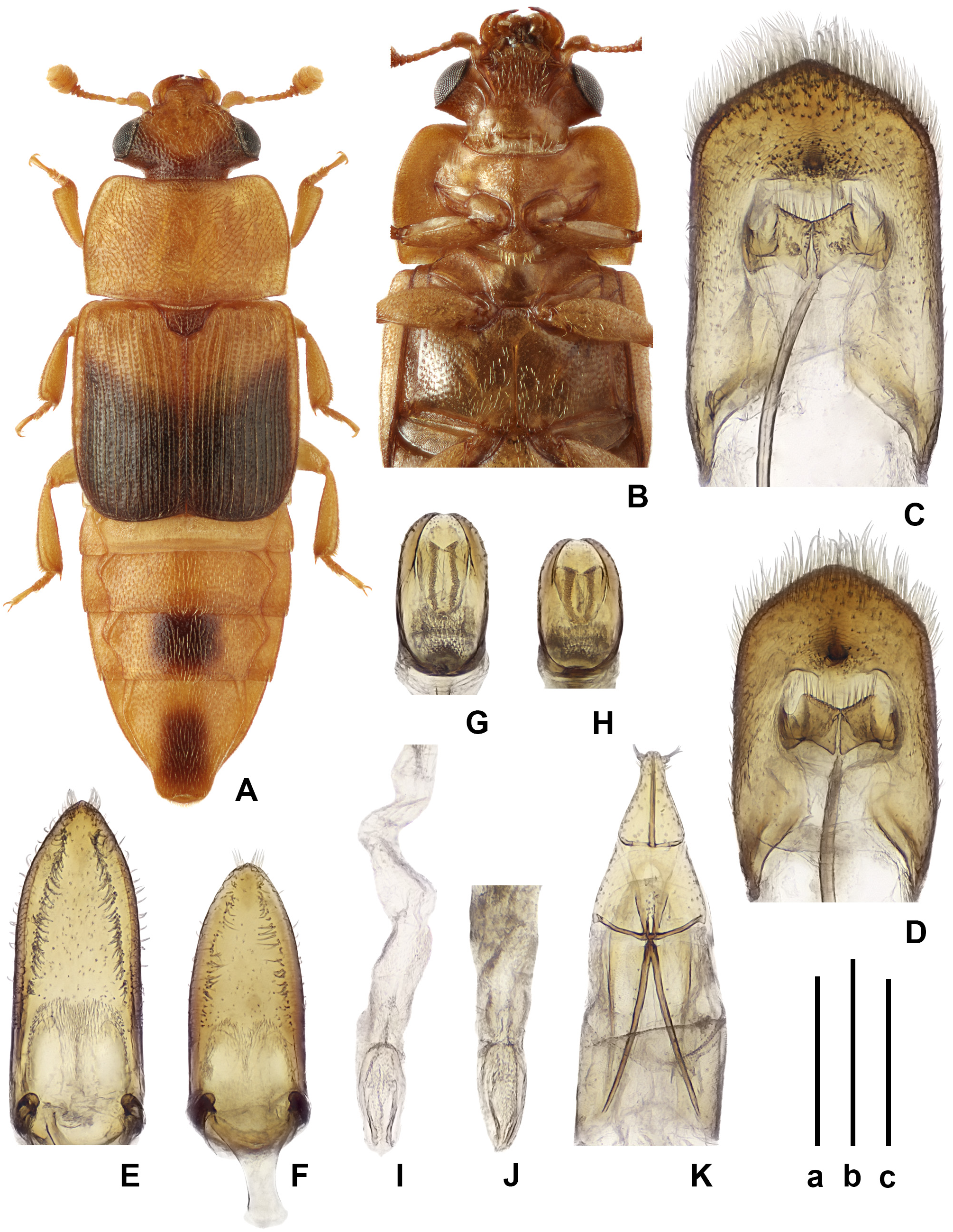
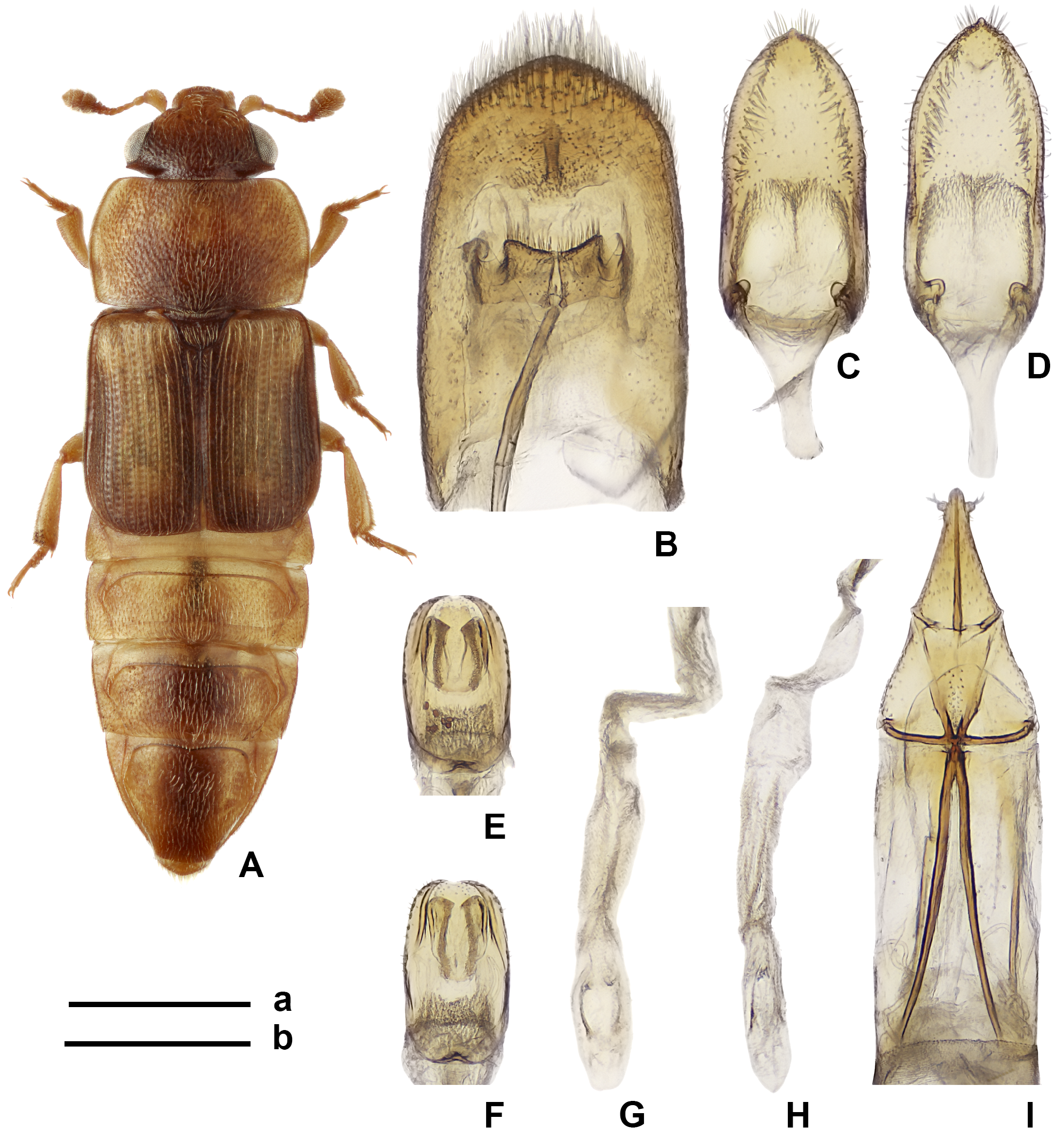
Figs 5 View FIGURE 5 , A–K; 11 View FIGURE 11 , A–C
Brachypeplus (Selis) apicalis Murray, 1864: 303 ; “Mysol”;
= Brachypeplus (Selis) caudalis Murray, 1864: 304 ; “Batchian”;
= Brachypeplus (Selis) fimbriatus Reitter, 1880a: 124 , syn. nov.; Ternate.
Specimens examined. Type specimens: Lectotype of Brachypeplus apicalis , female (BMNH), here designated— “ex Mus. Murray”, “ apicalis ”, “Moluccas, Mysol” (Misool), “Fry Coll.”; lectotype of Brachypeplus caudalis , female (NHML), here designated—“Bac, 103” ( Murray, 1864: 304: “Batchian” = Bacan), “[18]68.106”, “ caudalis ”; lectotype of Brachypeplus fimbriatus , male (MCNG), here designated—“Ternate, X.1875, Beccari” (Maluku Islands), “901”, “ Brachypeplus fimbriatus sp. n. Reitt.” and 17 paralectotypes of B. fimbriatus (MCNG, NRS) — with geographical labels as in lectotype (16 exx in MCNG) and “Ternate, X.1875, Beccari”, “280 82” (1 ex in NRS); Other specimens: Indonesia. West Papua: 1 ex (NHML)—green ellipse with unreadable note, “Pascoe Coll, 98–60”; 2 exx (NHML)—“Mysol” (in ring), “61.124” (named by J. Jelínek as B. caudalis ); 3 exx (SMNS, ZIN)—“ Irian Jaya, Jayapura, Sentani, Cyclops-Mts., 5.10. 1991, 400 m, A. Riedel”; 1 ex (SMNS)—“ Irian Jaya, Prov. Manokwari, Warmare, 22.8.1991, 200– 700 m, A. Riedel”; 3 exx (NME, ZIN)—“Halmahera, NW, 10 km S Jaillolo, 200 m, 26.1.2006, A. Weigel, plantage”; 6 exx (NME, ZIN)—“Halmahera, NW, 7 km N Jallolo, 10–100 m, 1˚04ˈN, 127˚24 ˈE, 24.1.2006, A. Skale, stream”; Papua New Guinea: 5 exx (TMB, ZIN)—“N. Guinea, Biró, 1899, Sattelberg” (named by O. Sjöberg as B. caudalis ); 1 ex (TMB)—“N. Guinea, Biró, 1897, Stephansort”, “Astrolabe B.” (named by O. Sjöberg as B. caudalis ); 1 (TMB)—“N. Guinea, Biró, 1898, Simbang, Huan Golf” (named by O. Sjöberg as B. caudalis ); 2 exx (TMB)—“N. Guinea, Biró, 1896, Friedrich-Wilh.-hafen” (named by O. Sjöberg as B. caudalis ); 1 ex (ANIC)—“Milna Bay Prov., Cape Vogel, Menafl Vuillage, 24–31 Oct. 1969, R. Wharton”; 1 ex (AMS)—“Murray Is., Torres Strait, 22 Jul. 1974, H. Heatwok & E. Cameron”, “attached to fruit fly bait forest”; Australia. QLD: 7 exx (SAM, ZIN)—“QLD, Cairns, 30 July 84, C. Rojewski”; 9 (ANIC, ZIN)—“Mt. Finnigan Sl., QLD, Cooktown, 400 m, 2 July 1982, S. & J. Peck”, “SBP 58, dung bait traps”; 5 (ANIC, ZIN)—“Inlatten, N. QLD, 10 Aug.—10 Sept. 1988, A. Walford-Huggins”, “edge of forest along creek ex intercept trap”; 1 (ANIC)—“Boulders Babings, N. QLD, 10 May 1967, D.H. Colless”; 10 (QMB, ZIN)—“Bellenden, Ker Range, N.Q, Cableway Base Stn., 100 m, 17 Oct.—9 Nov. 1981 ”, “Earthwatch/QLD Museum”, “Baited window trap” (ANIC Coleoptera Voucher N 83–0723); 7 (ANIC, ZIN)—“Kuranda, St. Forest, QLD, 3 km N Kuranda, 360 m, 25 June—3 Aug. 1982, S. & J. Peck”, “SBP 51, flight intercept trap, rainforest”; 1 (ANIC)—“Berlesate, ANIC 311, R.W. Teylor, J. Feeham”; 2 exx (ANIC)—“Laceys Creek, QLD, 10 km SE, El Arish, 40 m, 23 June—5 Aug. 1982, S. & J. Peck”, “SDBP 47, flight intercept trap, rainforest”; 2 exx (ANIC)—“Claudie R.nr. Iron Rg., QLD, 19–25 July 1978, J.F. Lawrence”, “ANIC Berlesate N 648”; 4 exx (ANIC, ZIN)—“N.Q, Iron Ra., rainforest, 143.14 x 12.45, 14 June 1971 ”, “Berlesate, ANIC 312, R.W. Teylor, J. Feeham”; 2 exx (ANIC)—“ 12.44S 143.14E, 3 km ENE of Mt. Tozer, QLD, 28 June—16 July 1986, T. Weir”, “flight intercept trap with through rainforest”; 1 ex (ANIC)—“QLD, Thorton Range, 100 m, 23.VI.1971, Teylor, Feeham”, “ANIC, 2 exx (ANIC) –“ 12.44S 143.14E, 3 km ENE of Mt. Tozer, QLD. 28 Jun.—4 Jul. 1986, T. Weir & A. Calder”; 1 ex (ANIC)—“ 12.43S 143.17E, QLD, 9 km ENE Mt. Tozer, 5–10 July 1986, J.C. Cardale”, “ex yellow traps”; Bedrlesate N 332, rainforest, 16.14S 145.26E ”; 45 exx (ANIC, ZIN)—“ 16.05S 145.28E, QLD, Cape Tribulation, Pilgrim Sands, 1 Feb.1989, R. Lott, S. McIntyre”, “ex fallen fruits of Normanbaya normanbyi” (“ 22 Feb.1989 ”); 14 exx (ANIC)—“ 16.03S to 16.05S 145.28E, Cape Tribulation area, QLD, 21–28 Mar. 1984, A. Calder & T. Weir”; 13 exx (ANIC, ZIN)—“Julatten N QLD, 10 Sept.—29 Sept. 1987, A. Walford-Huggins”, “edge of forest along creek, ex intercept trap” (“ 10 Aug.—10 Sept. 1987 ”); 44 exx (ANIC, ZIN)—“Mt. Finnigan Sl., QLD, 30 km S of Cocktown, 400 m, 2 July 1982, S. & J. Peck”, “SPB 58, dung bait traps”; 1 ex (ANIC)—“The Boulders Babinga, N QLD, 10 May 1967, D.H. Colless”; 1 ex (NMV)—“ Queensland, H.J. Carter Coll., P. 20.4.22”.
Diagnosis. This species differs from other congeners in the very characteristic coloration and in most cases it can be easily identified among Australian congeners (including Brachypeplus nypicola sp. nov. apparently closely related to it) after the above key to Australian and Tasmanian species. Most distinct characters of this species for discrimination of it from the most similar Brachypeplus dorsalis are present in the peculiarities of body coloration, as well as in the lack of one median tubercle at the apex of the longer tegmen, weakly sclerotized and markedly shorter ovipositor with subapical styli ( Brachypeplus dorsalis has the visible small median tubercle at the apex of the shorter tegmen, moderately sclerotized and longer ovipositor with the styli located at distance from the apex).
Notes. Brachypeplus apicalis together with B. dorsalis (see below) and B. nypicola sp. nov. (also one still undescribed Australian species and one undescribed New Guinean one also have such temples), in contrast to other Australian species, have the temples sharply projecting laterally behind eyes and considerably shorter labrum. Besides, the pregenal processes of this species and B. dorsalis are comparatively wider and with sharply acute outer apical angles (similar to those in Brachypeplus barronensis rather than those in other Australian species). The Australian and New Guinean populations (treated here as Brachypeplus apicalis ) are very similar to the north populations, for which two or three species names were proposed (see below: Brachypeplus dorsalis , B. decoratus and very probably B. ornatus ), but differ from the latter in the pattern of elytral coloration (the specimens from north territories have a light stripe between darkened adsutural portion and darkened sides, and also darkened epipleura— Fig. 10 View FIGURE 10 , A) and almost always lighter head and pronotum. Besides, the pair Brachypeplus apicalis and B. dorsalis seems to show small differences in structure of genitalia: the former with longer tegmen, peculiarities of the armature of the inner sac of penis and shorter ovipositor having the styli situated subapically; and the latter with shorter tegmen, peculiarities of armature of inner sac of penis and longer ovipositor with the styli situated at some distance from apex—see more in the below comments to B. dorsalis and Figs 10 View FIGURE 10 , A–I. Before, the names “ apicalis ” and “ dorsalis ” were synonymized ( Kirejtshuk 2005), although recent examination of new specimens and re-examination of other ones have shown that this joining could be problematic. At the moment it is difficult to decide if Brachypeplus dorsalis is enough isolated to consider it as a separate species or it is the north variety of B. apicalis isolated only as a subspecies. In addition, most Australian specimens have the antennal club somewhat shorter than that in representatives from the northern insular systems. Finally, Brachypeplus apicalis is somewhat similar to B. cuneatus , but the latter differs from it in the larger body, peculiarities of its coloration, coarser and deeper punctation of the dorsum and different male genitalia (comparatively longer tegmen). Brachypeplus nypicola sp. nov. is closely related to the considered pair of species ( B. apicalis and B. dorsalis ) and can be diagnosed after the above key to Australian species. See also the below Diagnosis of B. nypicola sp. nov.
Notes on synonymy. The type series for each of two names ( Brachypeplus apicalis and B. caudalis ) includes the alone type specimen here designated as the lectotype, because the describer did not indicate the number of specimens used for the proposal of these names. A. Murray (1864: 304) defined the following difference of Brachypeplus (Selis) caudalis from B. (S.) apicalis : “It has the thorax less convex and a little shorter and more transverse, the sides towards the posterior angles more explanate, the punctures deeper, and the pubescence less clear yellow, with the head a little darker, and the elytra wholly black, except for a triangular space from behind the shoulders to the suture, reaching to about the middle of its length; the edge also is pale to the very apex. It is as if the black apex in B. apicalis had extended obliquely up towards the shoulder; the transverse punctation in the interstices of the elytra is deeper than in that species.” All the mentioned features are still clearly present in both lectotypes, however they are in the scope of the variability demonstrated by the specimens listed above. The type specimens of Brachypeplus (Selis) fimbriatus matches its original description. According to the latter Brachypeplus (Selis) fimbriatus “Von Br. cuneatus Murray schon durch die Färbung, von den beiden andern Murray'schen Selis -Arten durch einfaches Halsschild abweichend.” ( Reitter 1880a: 125), i. e. it differs by the character rather variable in this common species.Also about relation between Brachypeplus apicalis and B. dorsalis — see above. The authors studied a paralectotype from NRS and also thanks to assistance from Roberto Poggi they examined after the high quality pictures of all specimens in the type series deposited in MCNG. One pin with two males has the labels “ lectotypus ” and “ paralectotypus Brachypeplus fimbriatus Reitter, 1880 .” The above specimen of this pair is recognosed as a lectotype and the below one as a paralectotype.
Addition to description. Body entire length 3.1–4.6 mm. Body (bright) reddish, distal two thirds of elytra darkened to blackish (each elytron with darkened spot having oblique anterior edge and leaving lateral explanate side light), middle of 2–3 uncovered tergites and head base usually infuscate; upper surface usually with moderately dense and moderately conspicuous yellowish pubescence forming on elytra longitudinal rows, pronotal and elytral lateral edges distinctly ciliate.
Head with punctures about 1.5 × as coarse as eye facets, interspaces between punctures somewhat greater than one puncture diameter and alutaceous. Pronotum with punctures about 2.0 × and more as coarse as eye facets, interspaces between punctures smaller than half of puncture diameter, alutaceous. Elytra with longitudinal rows of punctures: in bottom of very shallow striae about as coarse as those on pronotum and on very weakly elevated interstrial spaces markedly finer, interspaces between punctures alutaceous.Above sclerites of uncovered abdominal segments with finer and sparser punctation, and also with dense obliterated microreticulation; below thoracic sclerites and abdominal ventrite 1 with punctures nearly as coarse as those on head but sparser, interspaces between punctures with dense obliterated microreticulation.
Head with sharply projecting temples; labrum slightly exposed from under frons; oval antennal club about 1.3– 1.5 × as long as wide. Pregenal processes comparatively wide and with sharply acute outer apical angle. Pronotum with shallowly emarginate anterior edge and scarcely projecting anterior angles, moderately widely explanate sides (considerably less widely explanate than scape wide), with even lateral edges and clearly bi-emarginate base.Prosternal process with convex apex, almost 4.0 × as wide as distance between procoxae; distance between mesocoxae about 3.0 × greater and that between metacoxae about 2.0 × greater than that between procoxae. Abdominal ventrite 3 somewhat longer and ventrite 2 somewhat shorter than ventrites 1, ventrite 4 somewhat longer than each of ventrites 1–3. All tibiae triangular, their outer apical angle not projecting and spur moderately developed and rather thick; all tarsi 0.3 × as wide as protibia. Male pygidium shallowly emarginate to subtruncate or widely rounded at apex and widely rounded at apical angles. Male hypopygidium about 2.5 × as long as ventrite 1 and widely truncate at apex; female pygidium rounded at apex. Female hypopygidium nearly 3.0 × as long as ventrite 1 and rounded at apex.
Male anal sclerite dorsoventraly compressed, with rounded to subangulate and scarcely serrate apex. Aedeagus moderately sclerotized. Tegmen about 2.5 × as long as wide and narrowly rounded at apex. Penis trunk about 2.0 × as long as wide and widely rounded at apex. Armature of inner sac of penis consisting of two undefinite U-shaped sclerotizations located consistently closely to base of penis trunk and one paramedian pair more definitely outlined sclerites at proximal end of inner sac.
Ovipositor slightly to moderately sclerotized moderately wide; its gonocoxites about 1.3 × as long as wide with inner lobes transversely divided, lateral lobes slightly more than 0.5 × as long as gonocoxites in general; sides of gonocoxites subrectilinear before apex and styli disposed subapically.
Notes on variability. Except the mentioned variability in coloration and sculpture of the integument described in the above Diagnosis and Addition to description, it is necessary to note also some variable peculiarities in structure of genitalia. Aedeagus show some variations in the length of tegmen and penis trunk, and particularly in the expression of sclerotization and outlines of sclerites of the inner sac of penis.
Distribution. Indonesia, Maluku Islands (type locality of Brachypeplus apicalis: Misool , type locality of B. caudalis: Bacan , type locality of B. fimbriatus : Ternate), Indonesian New Guinea; Papua New Guinea; Australia: QLD, Torres Strait Islands).
Notes on bionomy. Adults of this species have a very wide range of habits and can use very various products of plant origin decaying with fungal participation (under bark, fermented tree juice, decaying fruits (rather frequently), etc.). Adults were collected also on dung.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Brachypeplus apicalis Murray, 1864
| Kirejtshuk, Alexander G. & Kovalev, Alexey V. 2022 |
Brachypeplus (Selis) fimbriatus
| Reitter, E. 1880: 124 |
Brachypeplus (Selis) apicalis
| Murray, A. 1864: 303 |
Brachypeplus (Selis) caudalis
| Murray, A. 1864: 304 |