Platymantis quezoni, Brown, Rafe M., Layola, Louise Abigail De, Ii, Antonio Lorenzo, Diesmos, Mae Lowe L. & Diesmos, Arvin C., 2015
|
publication ID |
https://doi.org/10.11646/zootaxa.4048.2.3 |
|
publication LSID |
lsid:zoobank.org:pub:0964C238-A12F-4334-B42A-26726A66996F |
|
DOI |
https://doi.org/10.5281/zenodo.5681237 |
|
persistent identifier |
https://treatment.plazi.org/id/03AC7634-5E09-6B46-3BF4-F8E4FB1FFB44 |
|
treatment provided by |
Plazi |
|
scientific name |
Platymantis quezoni |
| status |
sp. nov. |
Platymantis quezoni View in CoL , sp. nov.
“ Platymantis sp. 27” Brown et al. 2015 Figs. 2–4 View FIGURE 2 View FIGURE 3 View FIGURE 4
Holotype. PNM 9817 (RMB 4067; formerly KU 339542), adult male, collected on limestone outcrop in original forest (19:00 hr) on 27 November 2001 in Barangay Malinao Ilaya, Municipality of Atimonan, Quezon Province, Luzon Island, Philippines ( N 13.989°, E 121.818°; 275 m elevation; datum: WGS 85), by MLLD and G. V. A. Gee.
Paratypes (paratopotypes). Sixteen males: TNHC 61989–94, bearing the same data as the holotype; PNM 9910–12, collected on 19 October 2013 by LADL, AL, MLLD, and Jason Fernandez; PNM 9913–9916, collected on 15 December 2014 by LADL and AL; KU 328735 and 328736, bearing the same data as the holotype; KU 339543, collected 9 January 2011; three females ( PNM 9907–09, collected on 19 October 2013 by LADL, AL, MLLD and Jason Fernandez; one juvenile (KU 339544, bearing the same data as the holotype), all collected at the type locality.
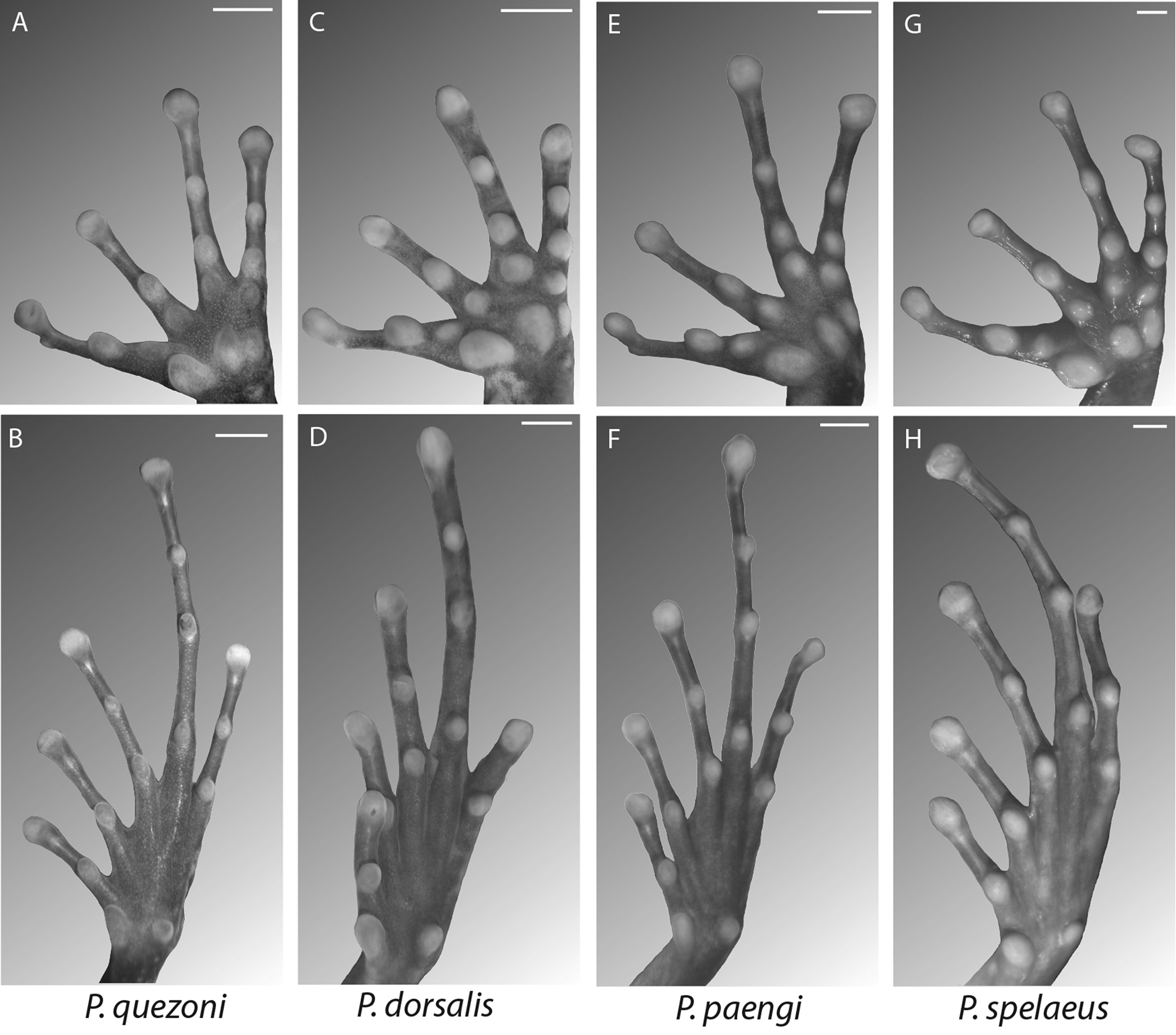
Diagnosis. Platymantis quezoni , sp. nov., can be distinguished from other Philippine congeners by the following suite of characters: body size 22.1–33.9 mm SVL for males, 33.8–39.7 mm for females; terminal discs of fingers and toes slightly expanded ( Fig. 2 View FIGURE 2 A,B); Finger I longer than Finger II; fingers and toes lacking dermal flanges along sides of digits, subarticular tubercles large and highly protuberant ( Fig. 2 View FIGURE 2 A,B); skin smooth, but with scattered and raised dermal tubercles ( Figs. 3 View FIGURE 3 , 4 View FIGURE 4 ); dorsolateral folds, mid-dorsal ridges, and other cephalic protuberances absent; color pattern medium to dark brown with black spots ( Fig. 3 View FIGURE 3 ); advertisement call a series of invariant low-frequency stridulated one-syllable barks: “Iyohk, iyohk, iyohk…” ( Fig. 6 View FIGURE 6 ) and its restriction to terrestrial microhabitats in forest covering limestone karst formations ( Fig. 5 View FIGURE 5 ). Diagnostic character state differences are summarized in Table 1 and we provide detailed comparisons, emphasizing diagnostic differences between the new species and its close relatives below.
Comparisons. We assign the new species to the subgenus Lupacolus Brown et al. 2015 on the basis of its placement (as “ Platymantis sp. 27”) in Clade R of the genus Platymantis (Brown et al. 2015: Fig. 2 View FIGURE 2 ). The subgenus Lupacolus is largely equivalent of the former P. dorsalis Group of Brown et al. (1997a) and Alcala & Brown (1999), and contains the named taxa P. cagayensis , P. dorsalis , P. indeprensus , P. mimulus , P. naomiae , P. paengi , P. pseudodorsalis , P. spelaeus , and P. t a y l o r i but does not include P. biak , P. levigatus , P. pygmaeus (placed in subgenus Lahatnanguri; Clade O of Brown et al. 2015: Fig. 2 View FIGURE 2 ) or P. corrugatus (subgenus Platymantis , clade name Tagomukhus; Clade N of Brown et al. 2015: Fig. 2 View FIGURE 2 ). Members of the genus Platymantis , subgenus Lupacolus, all possess the following combination of characters: body size moderate (~ 25–45 mm SVL in males); digital discs non-expanded to minimally expanded; first finger longer than second; fingers and toes lacking dermal flanges along sides of digits; subarticular tubercles large and highly protuberant ( Brown et al. 1997 a, c; Alcala & Brown 1999; Fig. 3 View FIGURE 3 ).
In overall phenotypic similarity Platymantis quezoni most resembles the related species P. paengi , P. spelaeus , (all three belong to the subgenus Lupacolus), the unrelated P. biak , P. insulatus (both of the subgenus Lahatnanguri), and P. bayani (subgenus Tahananpuno). All six taxa share the general mottled limestonecamouflaged color pattern and all exclusively inhabit limestone karst microhabitats. Platymantis quezoni shares with the other members of the subgenus Lupacolus ( P. paengi , P. spelaeus ) minimally expanded terminal digital discs, whereas these two species of subgenus Lahatnanguri ( P. biak , P. insulatus ), and all members of the subgenus Tahananpuno (including P. bayani ) have widely expanded terminal discs of the fingers and toes ( Brown & Alcala, 1970; Brown et al. 1997a, c; Siler et al. 2009, 2010). Additionally, P. bayani , P. insulatus , and P. spel aeus further differ by their much larger body sizes, which do not overlap the size range of P. quezoni ( Table 1). Finally, the advertisement call of the new species (a rapid series of loud, harsh, single-syllable barks) differs from that of P. biak (a much slower, low amplitude series of tonal notes), P. insulatus (a long clicking train of brief atonal pulses), P. spelaeus (a four-syllable, multinote complex call) and P. paengi (tonal, frequency sweep, amplitude modulated call).
Platymantis quezoni is distinguished from the remaining members of the subgenus Tahananpuno ( P. diesmosi , P. luzonensis , P. negrosensis , P. guentheri , and P. rabori ) by the presence of minimally expanded (vs. widely expanded) terminal digital discs of the fingers and toes. The terrestrial limestone karst microhabitat of P. quezoni further distinguishes the new species from all of these arboreal tree frog taxa except P. d i e s m o s i (a terrestrial species calling exclusively from cliff ledges on Mt. Malino; Brown & Gonzales 2007) and its advertisement call likewise distinguishes P. quezoni from the tree frogs with amplitude modulated calls ( P. luzonensis , P. negrosensis , and P. rabori ) and P. guentheri , an arboreal species with a tonal, frequency sweep call.
The new species is distinguished from all species of Philippine cloud frogs (formerly the P. ha z e l a e Group species) of the subgenus Tirahanulap ( P. hazelae , P. isarog , P. lawtoni , P. montanus , P. panayensis , P. polillensis , P.
sierramadrensis , and P. subterrestris ) by its larger body size (vs. much smaller in these species, typically ~ 20–28 mm in males), by unadorned digits (vs. digits with wide dermal fringes), by strongly protuberant (vs. ventrally flattened) subarticular tubercles, by possessing a longer first finger than the second (vs. second longest) and its terrestrial limestone karst microhabitat (vs. shrub-layer vegetation of montane forests). Species of the subgenus Tirahanulap all possess tonal, constant frequency calls with complex harmonic structure, contrasting sharply with the loud, harsh barking calls of P. quezoni .
From remaining members of the subgenus Lupacolus (previously referred to as the Platymantis dorsalis Group) ( P. cagayensis , P. dorsalis , P. indeprensus , P. mimulus , P. naomiae , P. pseudodorsalis , and P. tayl ori ), P. quezoni is distinguished by from all species except P. levigatus by the absence of dorsal folds and/or ridges, and limitation of dermal tubercles to its eyelids (vs. presence dermal folds/ridges and widely distributed tubercles over most dorsal body surfaces), and from all Lupacolus except P. paengi and P. s p e l a e us, by its terrestrial limestone forest microhabitat (vs. much more generalist inhabitant of leaf litter of forest floor and/or herb-layer vegetation in P. cagayensis , P. dorsalis , P. indeprensus , P. mimulus , P. naomiae , P. pseudodorsalis , and P. t a yl or i). Other than P. dorsalis and P. pseudodorsalis (which possess frequency sweep calls), these species possess complex, multisyllable call structure, which contrasts markedly with the simpler, single-syllable barking calls of P. quezoni .
The new species differs from Platymantis corrugatus (subgenus Platymantis , clade name Tagomukhus) by its smooth to shagreened skin texture (vs. dorsal skin with elongate dermal folds), by its lateral head coloration similar to other dorsolateral surfaces (vs. presence of a dark “mask” coloration on the lateral side of the head of P. corrugatus ) and by its exclusive limestone karst microhabitat (vs. a variety of terrestrial forest types). The advertisement call of P. quezoni is surprisingly quite similar to that of P. corrugatus (a loud series of single “quacks”) except that P. corrugatus is generally crepuscular whereas P. qu e z o ni calls beyond sunset and well into night-time hours if precipitation is sufficient.
The final set of comparisons for the recognition of P. quezoni is to the group of species comprising a recently discovered clade for which the subgenus Lahatnanguri was established (Brown et al. 2015). The new species differs from tree frogs of this clade by its terrestrial limestone karst microhabitat and possession of minimallyexpanded terminal discs of the fingers and toes (vs. widely expanded discs in the arboreal species P. banahao , P. cornutus ), from the forest floor leaf litter-inhabiting, miniature species P. pygmaeus (SVL 13–16 mm in males) by its much larger body size ( 22–34 mm) and limestone karst microhabitat, and from the semi-aquatic species P. levigatus by its light brown, mottled dorsal coloration (vs. dark, more uniformly colored dorsum), by its drier, limestone karst microhabitats (vs. moist, riparian environments). Finally, P. quezoni differs from the limestone karst frogs of the subgenus Lahatnanguri by its minimally-expanded terminal discs of the fingers and toes (vs. markedly expanded in P. biak , and widely expanded in P. insulatus ), the presence of distinct transverse banding patterns of the thigh and tibia (vs. absent or vague in P. insulatus ), and its simple, single-syllable barking calls advertisement call (vs. atonal clicking pulses in P. insulatus and the slow series of low, tonal notes in P. biak .)
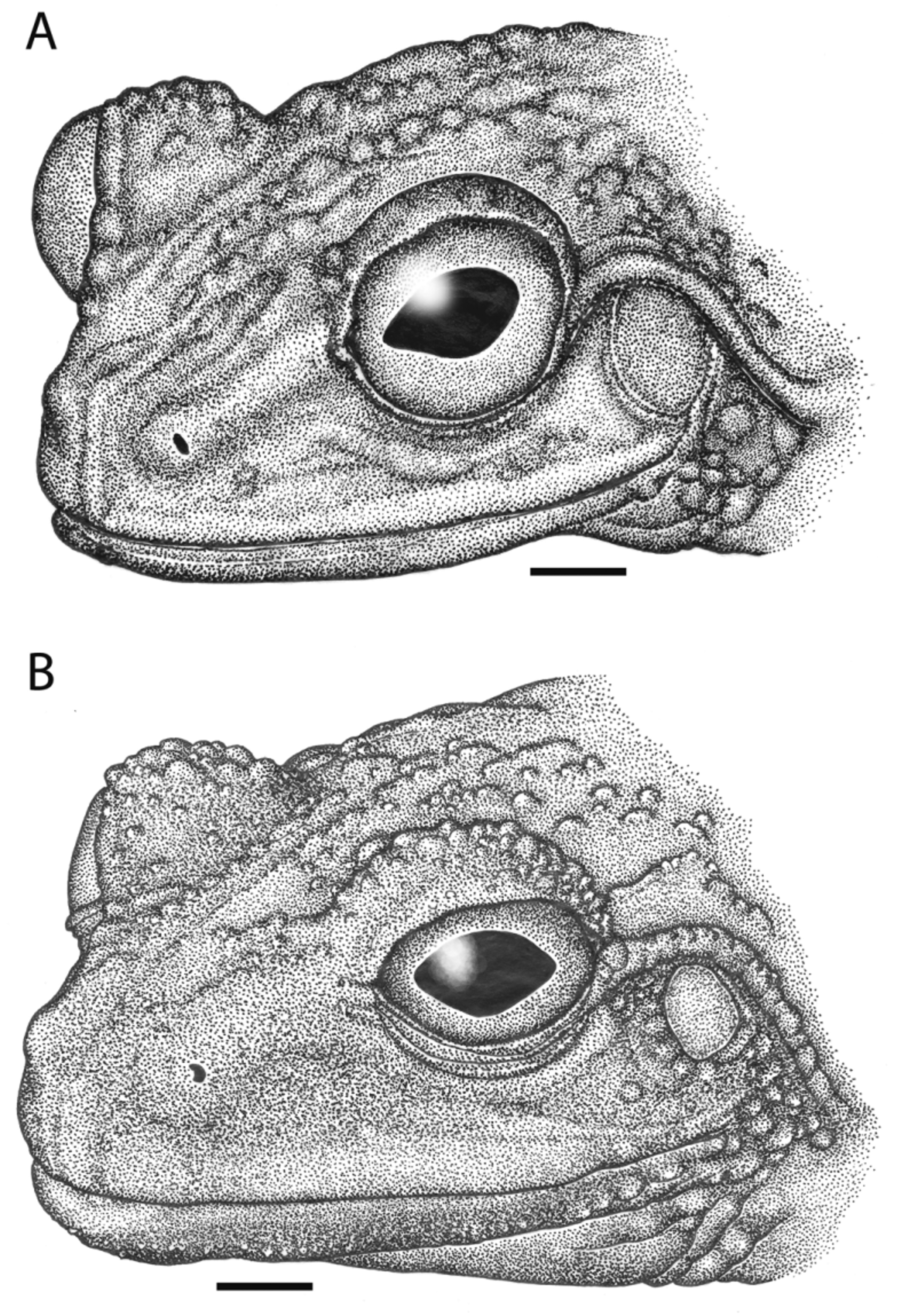
Description of holotype. A mature male ( Fig. 3 View FIGURE 3 ); habitus moderate; head as wide as body, head length 43.9% snout–vent length; snout protruding moderately beyond lower jaw, snout tip moderately pointed in dorsal and lateral aspects; snout 39.1% head length; eyes moderate and anterolaterally protruding, beyond width of head in dorsal aspect; eye diameter 15.1% snout–vent length, 34.4% head length; lips flared, labial region slightly swollen ( Fig. 4 View FIGURE 4 A); interorbital region flat; eye diameter 87.9% snout length, eye–naris distance 74.5% eye diameter; pupil horizontally elliptical; canthus rostralis strait, only sightly medially bowed; loreal region moderately concave, sloping ventrolaterally to labia; nostrils oriented laterally; internarial region slightly concave; tympanic annulus distinct, 60.1% eye diameter; supratympanic fold moderately protuberant, swollen and thick, extending from posterior corner of eye across entire dorsal margin of tympanum, along postero-dorsal edge of annulus, and to supra-axillary region.
Tongue elongate, wider posteriorly, with shallow posterior notch, a single small anterior lingual papilla, and narrow anterior attachment; choanae situated at anterolateral edge of palate, irregularly ovoid, separated by distance six greater than diameter of single choana, partially concealed in ventral aspect by maxillary shelf; dentigerous process of vomer distinct, with four teeth visible on left process, six on right; dentigerous process angled slightly anterolaterally, approximately at 20–30º incline with closest (posterior) points separated by distance equal to 2X diameter of single choana, their most distant (anterior) ends separated adjacent choana by a distance equivalent to one choana.
Hands and feet large; manus length 52.6% pes length; limbs well developed; tibia length 63.4% snout–vent length, 111.9% pes length; fingers slender, long; terminal discs moderately expanded ( Fig. 2 View FIGURE 2 A) with circummarginal grooves evident around distal edge of terminal discs; dorsal surfaces of terminal phalanges with small, cutaneous supra-articular flaps between ultimate and penultimate phalanges; relative lengths of fingers II <I <IV <III; subarticular tubercles prominent, convex, smooth; one subarticular tubercle below Finger I and II, two tubercles under Finger III and IV; supernumerary tubercles present at bases of all digits, distinct, round, prominent for Finger II–IV but elongate and modified into tubercular ridge under Finger I; inner metacarpal tubercle moderately enlarged, irregularly ovoid, with raised nearly spade-like anteromedial edge ( Fig. 2 View FIGURE 2 A); outer metacarpal tubercle consists of two substructures: an inner (medial) tubercle, slightly smaller and less pronounces than inner metacarpal tubercle; and outter postaxial tubercle, equal in size to a supernumerary tubercle; forearms slender, not hypertrophied; nuptial pads absent.
Skin of dorsal surfaces of head, trunk, and limbs smooth to slightly shagreened; dermal ornamentation minimal, consisting of one enlarged tubercle per eyelid, surrounded by a few much smaller palpebral tubercles, and a cluster of low, swollen post-symphysial tubercles, in a line from the posterior edge of the tympanic annulus to the posterior end of the supratympanic tubercular ridge ( Fig. 4 View FIGURE 4 A); skin of dorsal surfaces of limbs, hands and feet smooth with the exception of a shagreened texture at the knee; skin of chin, throat, and undersurfaces of limbs smooth; skin becoming increasing granular from sternal to inguinal regions; ventral surfaces of thighs granular.
Toes long, narrow ( Fig. 2 View FIGURE 2 B); terminal discs of toes moderately expanded, with circum-marginal grooves evident along distal margin of terminal pad; supra-articular cutaneous flaps present above ultimate-penultimate phalangeal articulations; plantar surface of foot smooth, with well-developed, prominently protuberant subarticular tubercles ( Fig. 2 View FIGURE 2 B); supernumerary tubercles absent; relative lengths of toes I <II <V <III <IV; outer metatarsal tubercle round, small, but moderately prominent; inner metatarsal tubercle elongate, prominent, length 2´diameter of outter metatarsal tubercle.
Measurements of holotype (in mm). Snout–vent length 33.7; head length 14.8; head width 13.9; snout length 5.8; upper arm length 5.9; forearm length 7.5; femur length 18.1; tibia length 21.7; tarsus length 10.9; pes length 19.4; manus length 10.2; Finger I length 5.9; Finger III length 7.7; Toe IV length 12.6; eye–narial distance 3.8; interorbital distance 3.2; internarial distance 3.0; eye diameter 5.1; horizontal tympanic annulus diameter 3.1; Finger I disc width 1.0; Finger III disc width 1.2; Toe IV disc width 1.3; Finger III penultimate phalange width 0.8; Toe IV penultimate phalange width 0.7.
Coloration of holotype in preservative. Dorsal surfaces of head, body, and limbs dark brown with black blotches; anterior portions of head darker greenish brown; limbs banded with black bars; interorbital bar present but faint: a thin, irregular, black line; lateral surfaces of head darker than dorsum, nearly greenish brown with black markings; black bar extends across dorsal half of canthus rostralis to anterior edge of eye, around margin of the palpebrum, and across suptratympanic fold; tympanum medium brownish-green with black covering dorsal-most half; lips with alternating dark green and black vertical labial bars, bordered anteriorly by black lower edge of canthal and subocular regions; three bold black bars radiate out of orbit dorsally, and extend ventrally to labial region to form the most conspicuous labial bars; transverse limb bars of irregular widths, with an overall darker coloration (compared to dorsum), due to a greater proportion of surface covered with black markings; upper arm almost devoid of black blotches, forearms each with two transverse black bars, thighs with five, tibia with three, tarsus with two; anterior flank coloration similar to dorsum on left, but predominantly black on right due to aggregation of black blotches, both sides progressively fading to less strongly patterned ventral color: dark brown blotches on throat, sternal region tan, inguinal region tan with steel bluish markings; ventral surfaces of limbs solid dark reddish brown; ventral surfaces of hands and feed dark reddish brown with light cream to tan subarticular tubercles; dorsal surfaces of hands are light brown, with alternating bands black bars on Fingers II–IV, and light tan on Finger I; dorsal surfaces of feet with solid redish brown above Toes I–III, boldly alternating light brown and black on Fingers IV and V.
Coloration in life. Dorsal surfaces of head, body, and limbs variably mottled shades of dark brown and greenish-gray, a few scattered bright gold spots, and with bold black markings scattered throughout but congregating irregularly into the following characteristics: distinct black suprascapular bar, interorbital bar, transverse limb bars (on thigh, tibia, shank, and upper arm and forearm), a dark bar from the nares, along the canthus, aligned with the enlarged pupil at night ( Fig. 3 View FIGURE 3 ), and posteriorly across the dorsal-most half of the tympanum; upper lips with distinct black labial bars; pupil bordered by bright yellow ciliary ring, iris silver; flanks with enlarged black blotches and interrupted by reduced dark brown of dorsal surfaces, grading ventrally across flank to cream ground color with black blotches on anterior portion of flanks; dorsal surfaces of digits mostly black, with small brown spots. Ventrum very dark gray with black blotches on throat and sternum; liver nearly bluish gray through translucent ventral skin of chest and groin; ventral surfaces of legs nearly solid black, fading to dark purplish gray on ventral tibia and shank; ventral surfaces of hands and feet dark purplish gray with lighter gray subarticular tubercles and distal digital discs.
Variation. Overall color pattern consistently mottled across the type series, typical of limestone karst inhabiting Platymantis ( Brown & Alcala, 1970, 1982a; Siler et al., 2009, 2010), with dorsal ground color light tannish yellow or mustard (e.g., KU 328735; Fig. 3 View FIGURE 3 ) to dark greenish-gray with black blotches (the holotype: PNM 9817; Fig. 3 View FIGURE 3 ). The degree and concentration of dorsal black markings ranges, from specimens nearly covered dorsally in black markings (KU 339543, TNHC 61993), to individuals predominantly light gray to tan, with few black markings (KU 61989, 61991, 61992; Fig. 3 View FIGURE 3 B). The suprascapular bar ranges from a distinct w-shaped pattern (the holotype: PNM 9817; TNHC 61990; KU 328736) to a few irregular, disconnected blotches (TNHC 61991) to an inverted v-shaped formation (KU 339543). Ventral surfaces less variable, predominantly as described for the holotype, but a few paratypes (KU 328735, 328736, TNHC 61990, 61994) possess darker throats, owing to aggregation of black blotches as in the holotype.
Summaries of univariate morphological variation in the series are presented in Table 2 View TABLE 2 . Females ( PNM 9907– 0 9, 9914) are considerably larger and more robust ( Table 2 View TABLE 2 ), and the single juvenile (KU 339544; SVL 24.3 mm) was more brightly patterned in life, with bolder and contrasting light tan versus black blotches.
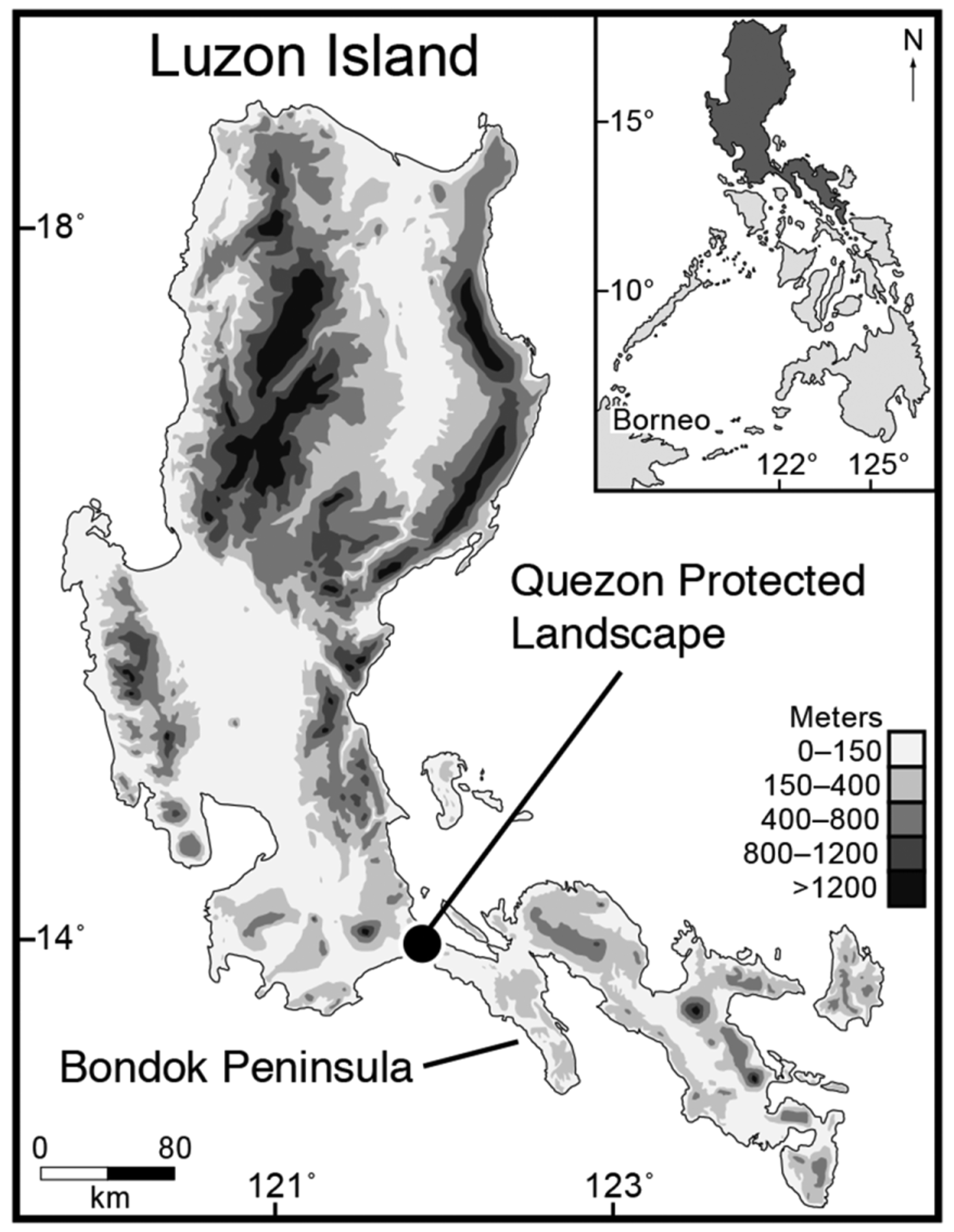
Distribution. Platymantis quezoni is known only from the type locality but may be more wide spread in scattered karst outcrops of northern portions of the Bicol Peninsula ( Restificar et al. 2006; MGB 2010), especially on limestone karst formations in the nearby Bondoc Peninsula ( Fig. 1 View FIGURE 1 ).
Ecology and natural history. Platymantis quezoni occurs in mature secondary-growth and mixed secondary growth combined with original forest, on the limestone karst substrates of Quezon Protected Landscape and most likely in other surrounding areas ( Fig. 1 View FIGURE 1 ). Both males and females were frequently encountered climbing vertical limestone rock faces, on narrow shelves of limestone at the base of karst cliffs, perched on top of large limestone boulders, in cracks and crevices, and around the entrances to small caves ( Fig. 5 View FIGURE 5 B). No specimens were observed climbing on vegetation growing from limestone, in contrast to the common scansorial habits of species of the other members of the subgenus Lupacolus ( Brown et al. 1997 a, b; Alcala & Brown, 1999; Brown & Gonzales, 2007).
Sympatric but non-syntopic anuran species include Rhinella marina ( Bufonidae , introduced), Hoplobatrachus rugulosus ( Dicroglossidae , introduced), Kaloula pulchra ( Microhylidae , introduced), K. picta , and K. conjuncta ( Microhylidae , native & endemic to the Philippines), Limnonectes macrocephalus , L. woodworthi , Occidozyga laevis ( Dicroglossidae , native & endemic), Sanguirana luzonensis ( Ranidae , native & endemic), Rhacophorus pardalis and Polypedates leucomystax ( Rhacophoridae , native, non-endemic). Syntopic native endemic species (collected in the immediate vicinity or adjacent microhabitats) include P. ( Lupacolus) dorsalis , P. ( Platymantis) corrugatus , P. ( Tahananpuno) luzonensis , Platymantis ( Lupalolus) n. sp. 14 (Brown et al. 2015) ( Ceratobatrachidae ), Kaloula picta , and K. cf. kalingensis (Microhylidae) .
Advertisement call. At the type locality, when visited in dry-season months (December and January), calling activity was heard during and following rainfall; in the rainy months of July through September, the new species called continuously in the afternoon and early evenings. Advertisement calls were recorded on two visits to the type locality, at ambient temperatures of 22.5–22.6°C on the first occasion ( 27–28 November 2001), and 23.1°C on our second visit. ( 9 January 2011). The following description is based on vouchered calls of seven collected males ( PNM 9817, holotype; TNHC 61989–92, KU 328735, 328736, paratypes). Neither ambient noises of wind in the canopy of mature forest in the area, nor advertisement calls of congeners ( P. dorsalis calls frequently at this locality) disrupted the activity of calling male P. quezoni . Calling activity begins in the late afternoon (16:30–17:30 hr) and typically lasts 3–5 hr. In the late afternoon, frogs call intermittently from relatively deep inside limestone crevices, or from within small caves. As the forest becomes darker, additional males begin calling and eventually can be seen on the surface of limestone, or calling from just inside crevices. As more individuals move to exposed perches, calling around limestone aggregations reaches maximal intensity. Periods of intense calling energy are separated by 30–120 s of silence, before males call again. Because all our recordings were made at the same locality, at very similar temperatures, and in almost identical atmospheric conditions (immediately following a heavy late afternoon rain), no temperature corrections were undertaken for this description.
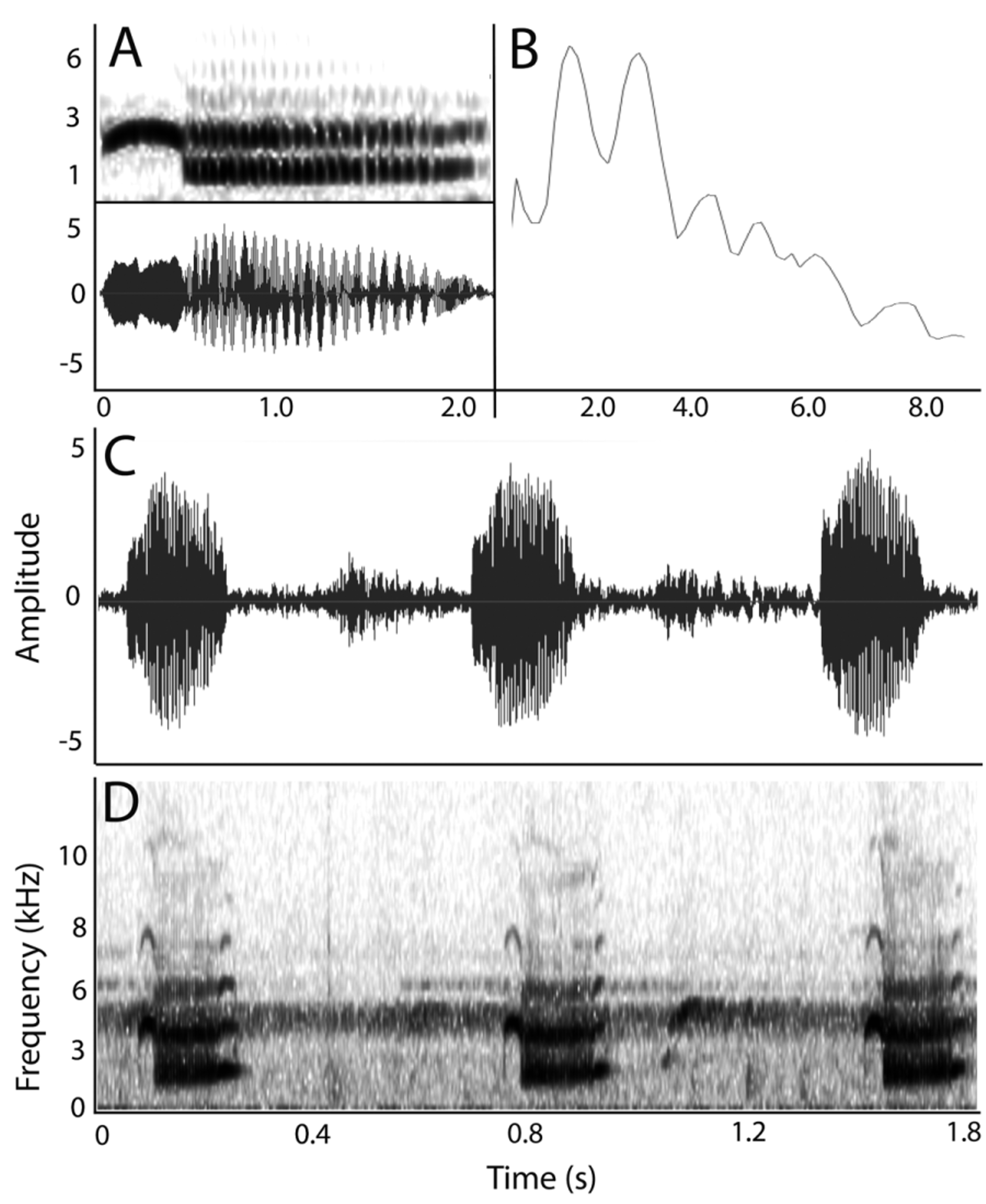
Males produce a largely invariant and unique advertisement call, sounding to the human ear like a long continuous series of low-frequency one-syllable barking notes ranging from: “yox, yox, yox…” to “iyohk, iyohk, iyohk …” Male calling behavior continues for up to several minutes of uninterrupted, steadily paced vocalizations. Calling begins when a previously resting male takes a more erect posture, raises the head, and takes several inhalations, buccal pumping while filling the lungs. The male then calls with a single note (described below) on expiration by contraction of body wall musculature, which fills the vocal sac. The elastic vocal sac then recoils, forcing air back into the lungs, with the process repeats over a series of up to a few minutes, with air being shuttled back and forth between the vocal sac and the lungs, and the multi-note call lasting a minute or more. Calls were separated by intervals of 12–78 ( x = 31.3 ± 22.7 SD; n = 13 x) s. Note repetition rate ([total number of notes – 1]/ time from beginning of first note to beginning of last) ranged from 0.37–1.17 ( x = 0.80 ± 0.28 SD; n = 7) notes/s. Apart from a slightly slower initial “warm-up” of a few seconds, no modulation of note repetition rate was detected across the call, and inter-note intervals were slightly variable, but not predictably so.
Individual notes have a complex structure of three main elements ( Fig. 6 View FIGURE 6 A,D), some of which are so brief that the call’s complex structure is nearly indiscernible to the human ear. Each note consist of a brief prefix, a second, croaking, main syllable, and a brief suffix differing from the prefix ( Fig. 6 View FIGURE 6 A). In the majority of calls, the prefix takes the form of a tonal frequency arc, which rapidly climbs from an initial frequency of approximately 2.2–2.6 ( x = 2.4 ± 0.08 SD; n = 7) kHz, reaches a maximum at 2.8–3.2 ( x = 2.9 ± 0.11 SD; n = 7) kHz, and then declines in a mirror-image fashion over a period of 30–41 ( x = 34 ± 4.3 SD; n = 7) ms. The final frequency of this variable tonal prefix (in most instances a full tonal arc, in others more of a decreasing frequency sweep) is identical in apparent range and peak frequency to the upper frequency of the call’s second syllable ( Fig. 6 View FIGURE 6 D). The second element, approximately 3–3.5 x duration of the first, is a harsh croaking syllable composed of two nearly equal intensity non-modulated frequency bands ( Fig. 6 View FIGURE 6 A,D). The two constant-frequency components consist of a lower fundamental frequency band spanning 0.9–1.7 kHz and an upper (dominant) frequency ranging across 2.2–2.8 kHz. Peak frequencies of these components varied from 2.25 to 1.4 ( x = 1.3 ± 0.05 SD; n = 7) kHz for the first band and 2.5 to 2.8 ( x = 2.6 ± 0.09 SD; n = 7) kHz in the second. The third note element is a highly variable tonal suffix, again typically of the same frequency as the upper frequency band of the main, second croaking syllable, and is a variable tonal ascending sweep which, like the call’s prefix, typically sweeps from ~2.4 to 2.9 kHz ( Fig. 6 View FIGURE 6 D). The spectral structure of notes ( Fig. 6 View FIGURE 6 A,D) consists of a pair of nearly equally emphasized frequencies (the lower, fundamental, and the upper, which is the first multiple of the fundamental). The FFT (power spectrum) demonstrates that the majority of energy in each call is found in the second frequency component (the dominant frequency, in contrast to the lower, fundamental frequency). Finally, the calls of P. quezoni have complex spectral structure ( Fig. 6 View FIGURE 6 D), with two detectable harmonics in most recordings and three in at least one call (TNHC 61990).
Etymology. The specific epithet is a patronym in the genitive singular, honoring Manuel Luis Molina Quezon. Quezon served as president of the Commonwealth of the Philippines during the American colonial period from 1935 through the conclusion of the Second World War. An exemplary statesman, he led the struggle for Philippine independence from American rule. Suggested common name: Quezon Limestone Forest Frog.
TABLE 2. — Summary of univariate morphological variation (Range, above; mean ± 1 S. D., below) among mensural characters in the type series of Platymantis (Lupacolus) quezoni, sp. nov.
| Female | Male | |
|---|---|---|
| n = 4 | n = 16 | |
| Snout–Vent Length | 32.4–39.7 (37.8 ± 2.7) | 22.1–33.9 (29.9 ± 4.9) |
| Head Length | 14.3–14.3 (15.8 ± 1.0) | 9.4–15.1 (12.4 ± 2.6) |
| Snout Length | 5.3–7.3 (6.5 ± 0.9) | 4.7–6.6 (5.7 ± 0.7) |
| Interorbital Distance | 3.0–3.5 (3.4 ± 0.2) | 1.9–3.0 (2.6 ± 0.4) |
| Internarial Distance | 2.4–3.5 (3.0 ± 0.5) | 1.9–3.7 (2.3 ± 0.4) |
| Eye Diameter | 5.0–6.0 (5.5 ± 0.4) | 3.5–5.2 (4.4 ± 0.7) |
| Tympanic Annulus Diameter | 3.1–3.6 (3.3 ± 0.2) | 1.9–3.1 (2.6 ± 0.5) |
| Eye–Tympanum Distance | 0.5–1.5 (0.8 ± 0.3) | 0.3–1.4 (0.7 ± 0.2) |
| Head Width | 14.8–17.2 (16.4 ± 1.1) | 9.2–14.3 (11.9 ± 2.4) |
| Upper Arm Length | 6.2–8.4 (7.6 ± 0.9) | 4.3–6.8 (5.2 ± 0.9) |
| Forearm Length | 8.9–10.9 (10.2 ± 0.9) | 5.5–9.4 (7.7 ± 1.4) |
| Femur Length | 15.4–21.7 (19.4 ± 2.7) | 10.5–17.4 (14.7 ± 2.9) |
| Tibia Length | 18.3–23.4 (21.3 ± 2.4) | 13.0–19.1 (16.8 ± 2.6) |
| Tarsus Length | 10.2–13.0 (12.0 ± 1.2) | 7.4–11.4 (9.6 ± 1.7) |
| Foot Length | 18.2–23.9 (22.0 ± 2.6) | 12.8–19.4 (16.6 ± 2.8) |
| Hand Length | 9.8–12.8 (11.5 ± 1.2) | 6.7–10.0 (8.6 ± 1.4) |
| Toe 4 Length | 11.8–15.0 (14.2 ± 1.6) | 8.8–12.7 (11.3 ± 1.5) |
| Finger 1 Length | 4.4–6.3 (5.6 ± 0.8) | 2.8–5.2 (4.0 ± 1.1) |
| Finger 3 Length | 6.4–8.4 (7.7 ± 0.9) | 4.1–6.6 (5.5 ± 1.1) |
| Finger 1 Disc Width | 0.6–1.2 (0.9 ± 0.3) | 0.5–1.2 (0.8 ± 0.3) |
| Finger 3 Disk Width | 0.8–1.6 (1.2 ± 0.3) | 0.1–1.2 (1.3 ± 0.2) |
| Toe 4 Disc Width | 1.0–1.6 (1.3 ± 0.2) | 0.6–1.3 (0.9 ± 0.2) |
| Penultimate Phalanx Width, Finger 3 | 0.4–0.6 (0.5 ± 0.1) | 0.3–0.5 (0.4 ± 0.1) |
| Penultimate Phalanx Width, Toe 4 | 0.5–0.8 (0.6 ± 0.1) | 0.4–0.6 (0.5 ± 0.1) |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |