Kyphopteryx yangi Du & Chen, 2019
|
publication ID |
https://doi.org/10.11646/zootaxa.4686.2.6 |
|
publication LSID |
lsid:zoobank.org:pub:84E4E15D-2F18-4DDE-AAF3-F467762D541C |
|
persistent identifier |
https://treatment.plazi.org/id/03AC87E1-FFFD-FF8B-FF70-F912FEBAFEBA |
|
treatment provided by |
Plazi |
|
scientific name |
Kyphopteryx yangi Du & Chen, 2019 |
| status |
|
Kyphopteryx yangi Du & Chen, 2019 View in CoL
( Figs. 1–5 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 )
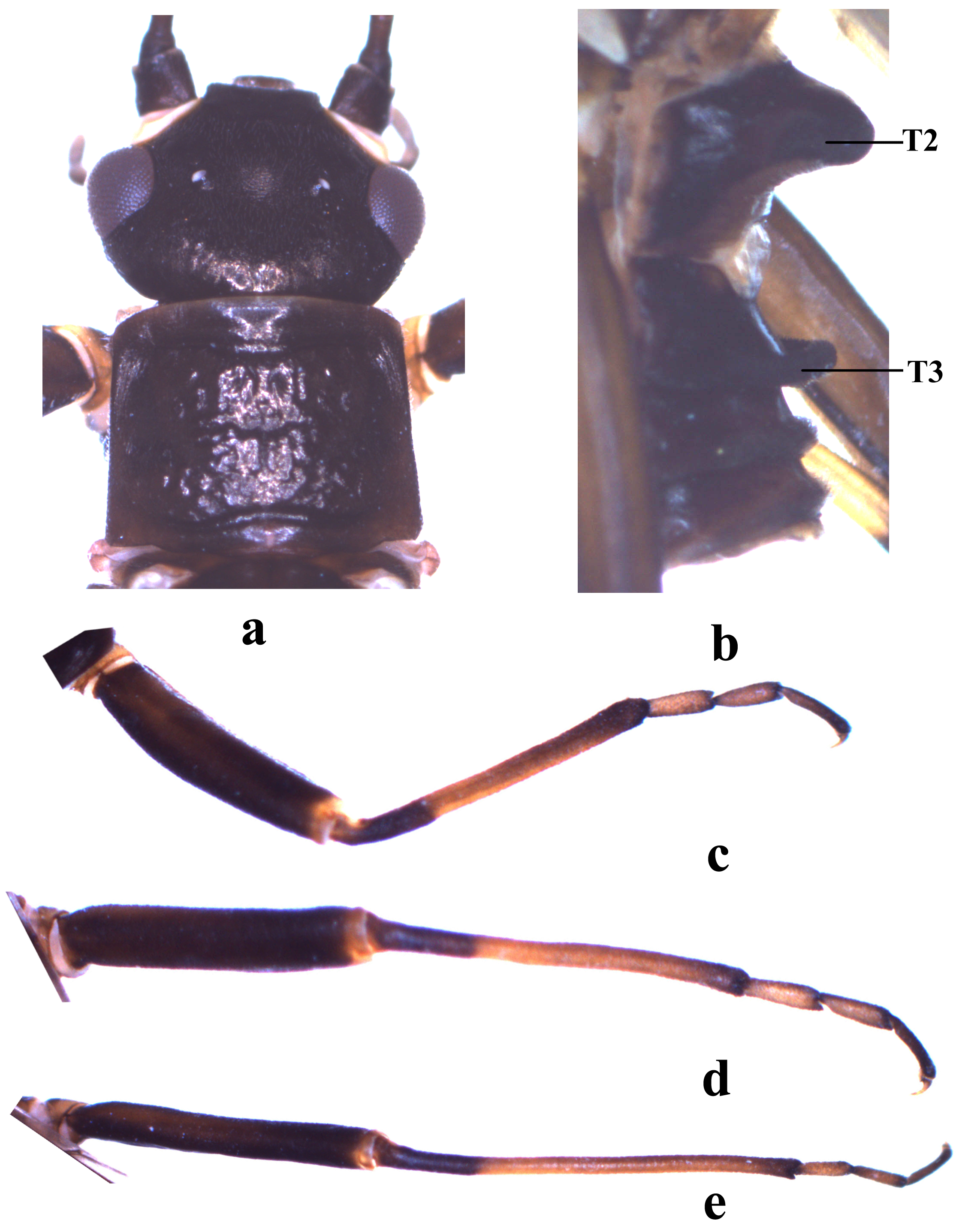
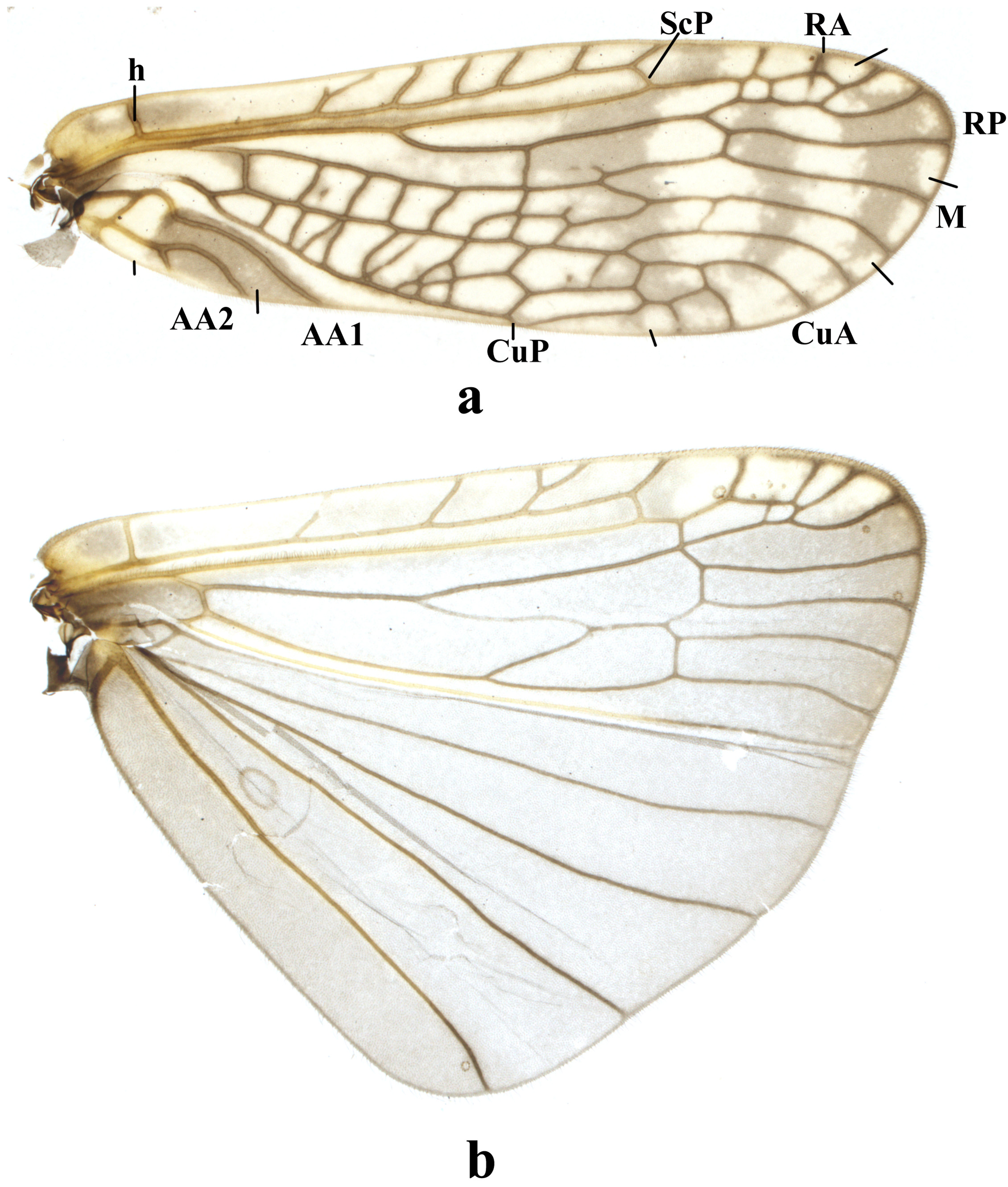
Adult habitus. Body length ca. 9.60 mm, forewing length ca. 8.28 mm, hindwing length ca. 7.16 mm. Slightly short winged. General color brownish to dark brown. Head black, slightly wider than pronotum; ocelli whitish and compound eyes blackish in ethanol; antennal scape and pedicel blackish, flagella brown. Pronotum nearly quadrate, posterior portion slightly wider, anterior margin slightly convex forward, generally blackish with obscure rugosities ( Fig. 1a View FIGURE 1 ). Legs patterned, femora and basal fourth of tibiae dark brown, coxae, distal portion of tibiae and the 3rd segment of tarsi brown, remainder brownish ( Figs. 1 View FIGURE 1 c–1e). Wings subhyaline, veins dark brown. Forewing membrane mottled, with five costal cross-veins between h and Sc and two cross-veins between ScP and RA; with three cross-veins between RA and RP; RP with three branches; M forked; CuA with four branches; CuP simple; AA1 with basal curve; AA2 biforked ( Fig. 4a View FIGURE 4 ). Hindwing subtriangular in shape, with three costal cross-veins between h and Sc, one cross-vein between ScP and RA, and three cross-veins between RA and RP; RP with four branches; anal veins six ( Fig. 4b View FIGURE 4 ).
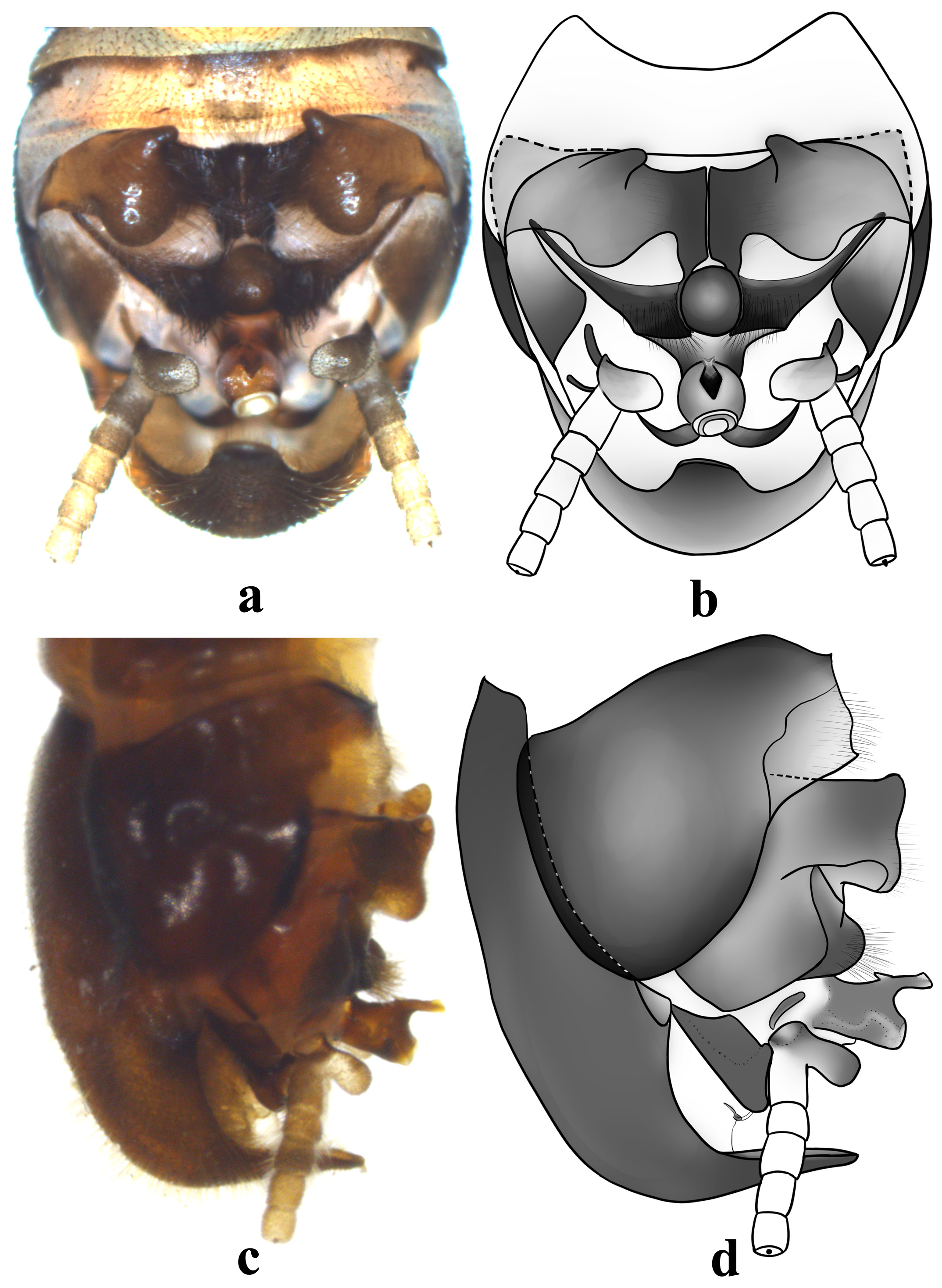
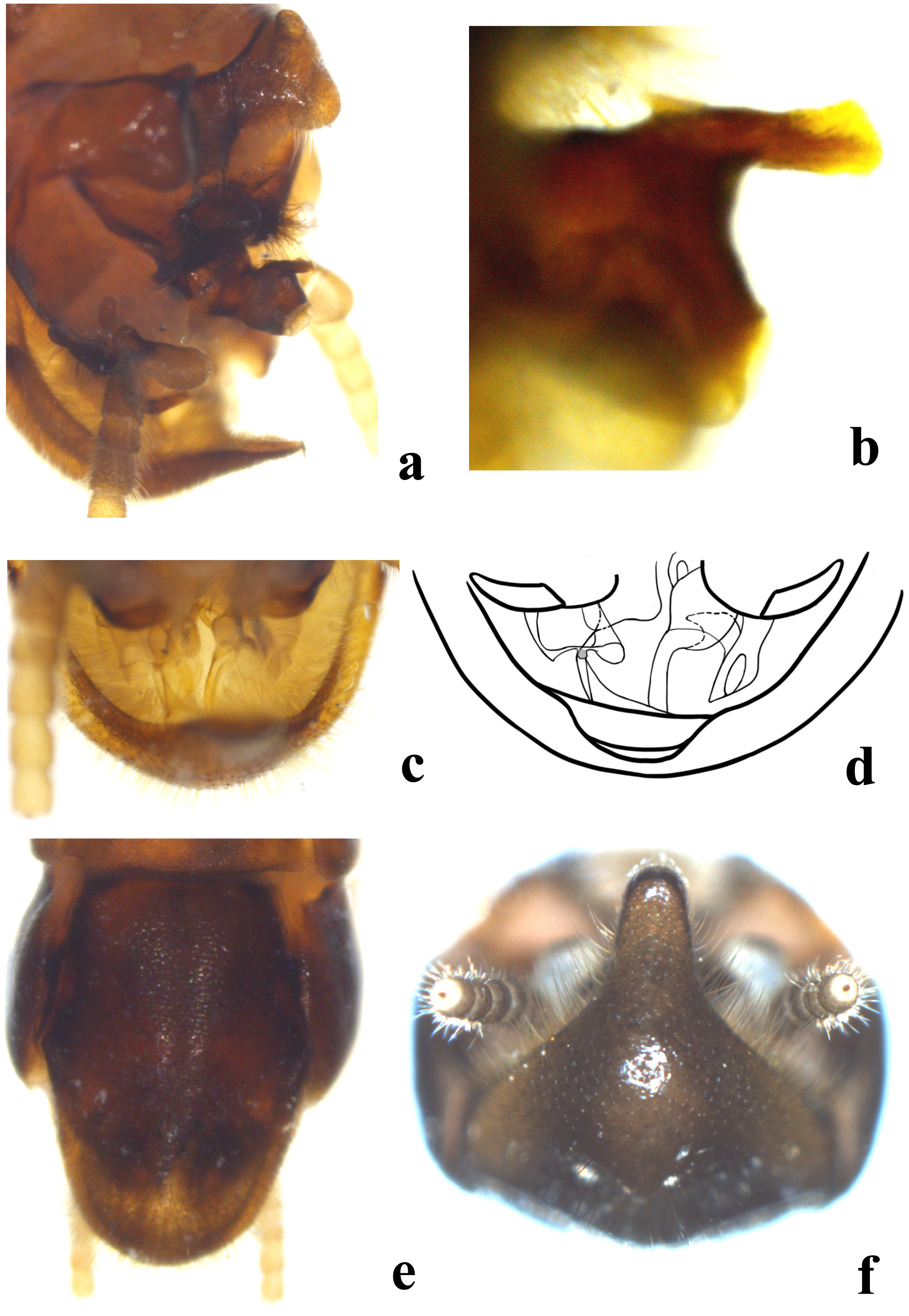
Male ( Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 ). Abdominal tergum 2 with a strongly sclerotized median process, elevated and conical in lateral view ( Fig. 1b View FIGURE 1 ). Posterior portion of abdominal tergum 3 also with a distinctly sclerotized median process, nearly erect, posterior portion of the process extended backward and becoming lower ( Fig. 1b View FIGURE 1 ). Posterior margin of abdominal terga 4–5 elevated in rounded humps covered by hairs ( Fig. 1b View FIGURE 1 ). Abdominal tergum 9 much extended laterally ( Figs. 2 View FIGURE 2 , 3e View FIGURE 3 ). Abdominal tergum 10 deeply cleft into a pair of hemiterga, hemitergal lobes greatly elevated and hairy, in dorsal view breast like with an anterior nipple and in lateral view resembling a hatchet ( Figs. 2 View FIGURE 2 , 3a View FIGURE 3 ). Basal plate of the epiproct covered with a cluster of strong hairs, medially with a sclerotized basal bulb ( Figs. 2 View FIGURE 2 , 3a View FIGURE 3 ). Epiproct upraised, with an oval apical bulb; anterior prong of the apex of epiproct strongly sclerotized, fingershaped with an enlarged extreme; in lateral aspect present a downcast basoventral triangular spine which in dorsal aspect is paired or bifurcate ( Figs. 2 View FIGURE 2 , 3 View FIGURE 3 a–3b). Outer member of paraprocts typically sclerotized, upcurved and recurved; lower member of paraprocts asymmetric, basally with subquadrate sclerite, distal part of sclerite of left paraproct with additional small sclerotized rounded lobe, membranous portion complex ( Figs. 2 View FIGURE 2 , 3 View FIGURE 3 c–3d). Cercus five-segmented: the first segment enlarged and elongate, basally with a subquadrate inner lobe; apical segment of cercus with a distal vestigial sclerite ( Fig. 2 View FIGURE 2 ). Subgenital plate sclerotized, generally scoop-shaped, distal portion upcurved and apically with a tongue-shaped process ( Figs. 2 View FIGURE 2 , 3 View FIGURE 3 e–3f).
Female. Unknown.
Examined Material. 1 male ( HIST), China: Yunnan Province, Deqin County, Baima Snow Mountain , 4290 m, 2016.III.22, W.H. Li .
Distribution. China ( Yunnan Province).
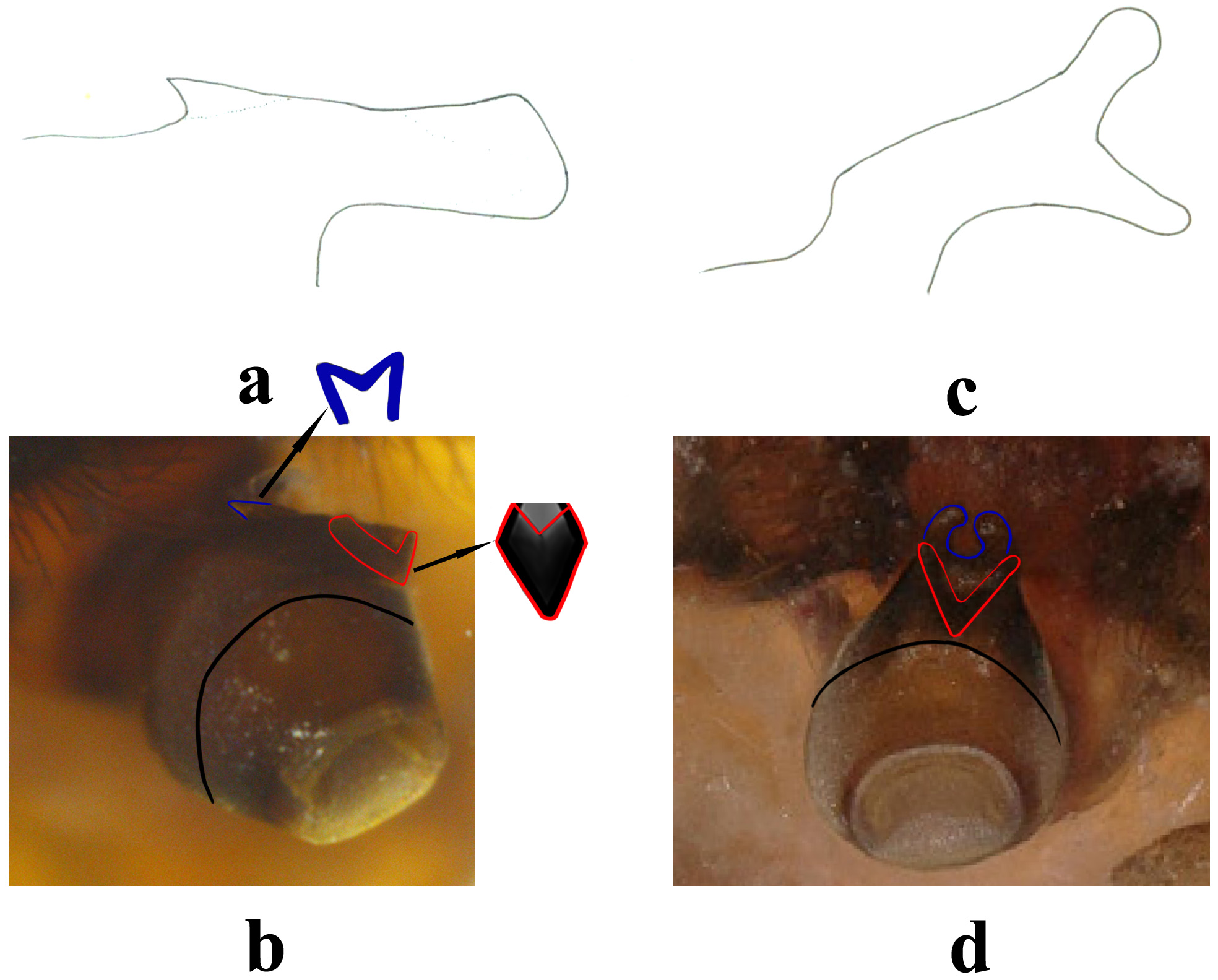
Remarks. The single male described above is identical in the dorsal processes of abdominal terga 2–5, the breast-shaped hemitergal processes of tergum 10, and the oval apical bulb of epiproct with the holotype of K. yangi . However, there are differences. In lateral view, the main portion of anterior prong of the apex of epiproct in our specimen is enlarged apically, distinctly narrower in holotype of K. yangi ( Fig. 5 a & c View FIGURE 5 ), and the ventral bifurcation of the epiproctal prong is located apically and forceps-shaped in the holotype, whereas the this bifurcation in the topotype is positioned at base of the prong, hardly observable in dorsal view ( Fig. 5 View FIGURE 5 a–d). However, similar in- traspecific variability of anterior prong of the epiproct, the only distinctive character between our male and the holotype of K. yangi , has been described and illustrated in the related genus Mesyatsia Ricker & Ross, 1975 from the Himalayan ranges by Zwick & Sivec (1980). The above differences between the holotype and our male of K. yangi is considered intraspecific variability. Studying larger series of this uncommon species will be required to test this hypothesis.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |