Chrysopodes (Neosuarius) flavescens ( Blanchard, 1851 )
|
publication ID |
https://doi.org/ 10.3897/zookeys.44.387 |
|
DOI |
https://doi.org/10.5281/zenodo.3788342 |
|
persistent identifier |
https://treatment.plazi.org/id/03AC87E3-FFDA-5815-64F3-E4E1C365FD14 |
|
treatment provided by |
Plazi |
|
scientific name |
Chrysopodes (Neosuarius) flavescens ( Blanchard, 1851 ) |
| status |
|
Chrysopodes (Neosuarius) flavescens ( Blanchard, 1851) View in CoL
Figs 40b View Figure 40 , 41b, 48b, 49–55
Hemerobius flavescens Blanchard 1851 View in CoL , in Gay 1851: 123, Fig. 9 View Figure 9 [MNHN, Lectotype (by present designation): Chile].
Chrysopa flavescens ( Blanchard, 1851) View in CoL . Navás 1910c: 238 [transfer to Chrysopa View in CoL ; note stating that six types from Chile are in the MNHN] ; Penny 1977: 18 [species list].
Chrysopa nosina Navás, 1913: 85 View in CoL [MZB, Lectotype (by present designation): “ Nos ( Chile), Marzo 1910 (Col. M. Porter debit)”]. Navás 1918: 222 [redescription]; Navás 1921a: 259 [distribution]; Navás 1922b: 361; [distribution]; Navás 1923: 116 [distribution]; Navás 1925c: 307 [distribution]; Navás 1926: 326 [distribution]; Navás 1927: 324 [distribution]; Navás 1929b: 19 [distribution]; Navás 1932b: 80 [distribution]; Navás 1934: 14 [distribution]; Monserrat 1985: 238 [list of Navás types in MZB] ; Brooks and Barnard 1990: 272 [transfer to Chrysopodes (Neosuarius) View in CoL ]; Oswald 2007 [catalog listing as Chrysopodes (Neosuarius) nosinus View in CoL , nomenclature]; Legrand et al. 2008: 157 [Navás specimens]. New Synonym.
Chrysopa jaffuelina Navás, 1918: 222 View in CoL [MZB, Lectotype (by present designation): “ Chile: Los Perales, Marga-Marga, Enero de 1918, P. Jaffuel (Col. M.)”]. Navás 1921b: 443 [species list]; Navás 1921a: 259 [distribution]; Navás 1934: 14 [distribution]; Penny 1977: 19 [species list]; Monserrat 1985: 238 [list of Navás types in MZB]; Brooks and Barnard 1990: 272 [transfer to Chrysopodes (Neosuarius) View in CoL ]; Os- wald 2007 [catalog listing as Chrysopodes (Neosuarius) jaffuelinus View in CoL , nomenclature]. New Synonym.
Chrysopa bullocki Navás, 1933a: 230 View in CoL [MZB, Lectotype (by present designation): “ Chile: Angol, 10-I-1932 ”]. Penny 1977: 17 [species list]; Monserrat 1985: 237 [list of Navás types in MZB]; Brooks and Barnard 1990: 279 [listed as ‘Chrysopa’ incertae sedis]; Oswald 2007 [catalog listing]. New Synonym.
Chrysopodes (Neosuarius) flavescens ( Blanchard, 1851) View in CoL . Adams and Penny 1985 [1987]: 436 [transfer to Chrysopodes View in CoL ]; Brooks and Barnard 1990: 272 [species list]; Oswald 2007 [catalog listing, nomenclature].
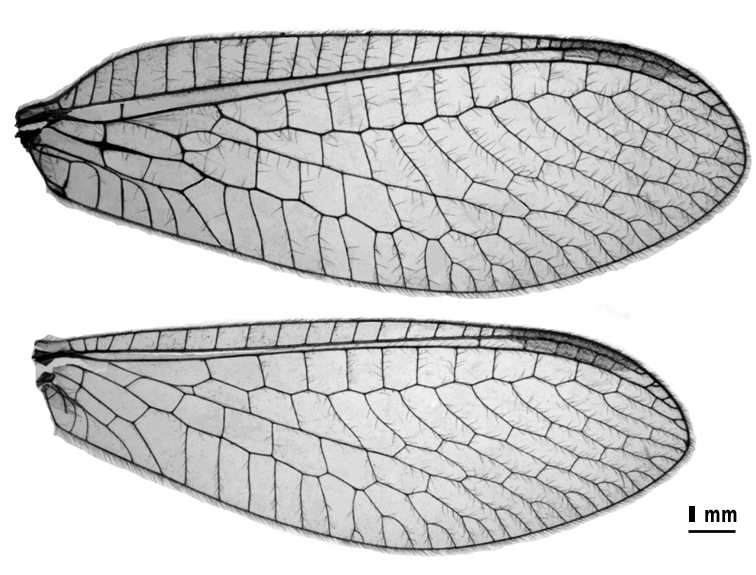
Diagnosis. Chrysopodes (N.) flavescens is one of several Andean species [including C. (N.) porterinus and C. (N.) escomeli ] that have robust, more or less darkly marked bodies (with variable coloration). Definitive differentiation of the three species requires examination of the genitalia. However, most specimens of C. (N.) flavescens can be distinguished by their slightly broad wings ( Fig. 50 View Figure 50 ), the longitudinal veins of which are green with only very small dark spots at the junctions with crossveins. The head and body markings, as well as the dark antennae resemble those of C. (N.) porterinus, but the C. (N.) flavescens body tends to be lighter colored; for example, the brown longitudinal stripe down the center of the pronotum (if present) is generally narrower and lighter than that in C. (N.) porterinus. C. (N.) flavescens generally have a single brown stripe (sometimes incomplete) on the dorsolateral margin of the scapes ( Figs 40b View Figure 40 , 41b). The stripe is usually absent from the C. (N.) porterinus scape, and the C. (N.) escomeli scape has two pairs of stripes.
The male C. (N.) flavescens is recognized by its broad gonarcus, the position and shape of the apodemes on T9+ectoproct, and the invaginated apodeme along the midline of S8+9 (Figs 51, 52). In mature female C. (N.) flavescens , the spermatheca has one sharp turn and a bean-shaped enlargement at the bursal end; the subgenitale has a distinct, rounded terminal process extending from a substantial neck; the length (ventral surface) of the neck is about equal to the height of the distal surface of the process ( Figs 48b View Figure 48 , 54 View Figure 54 , 55 View Figure 55 ). The subgenitale also has a relatively large, clear, smooth (or only lightly folded) area above S7; this area is absent from C. (N.) porterinus females, and it is transversely folded, not smooth, on C. (N.) escomeli females ( Fig. 48 View Figure 48 ).
Description. Head ( Figs 40b View Figure 40 , 41b, 49): Width (frontal, including eyes) 1.5 - 1.6 mm; ratio, head: eye width = 2.7–2.9:1; distance (straight-line) between tentorial pits 0.44–0.51 mm. Distance between antennae ̴ 0.10–0.14 mm; length of frons (mid-antenna – midway between tentorial pits) ̴ 0.41–0.48 mm; clypeus ̴ 0.20–0.28 mm long. Antenna ̴0.6× length of forewing (9.3–9.5 mm). Vertex slightly raised, flat throughout, with upward fold posteriorly; surface of vertex smooth, without setae. Frons relatively flat, smooth, shiny throughout. Clypeus mostly flat, very slightly raised in middle; surface smooth. Labrum flat, surface smooth; distal margin straight or with very small cleft.
Head coloration: Head cream with markings reddish brown to brown; vertex with reddish brown around entire edge of dorsal torulus, reddish brown slash within torulus, diffuse reddish brown mark outside margin of raised area; posterolateral region usually unmarked. Genae marked with brown to light brown distally; frons cream, with pair of brown marks on mesal margin of torulus; clypeus cream to amber, lateral edges emarginated with brown. Scapes cream with brown, dorsolateral stripe; pedicel
Figure 5 View Figure 5 ļ. Chrysopodes (Neosuarius) flavescens : Male terminus, lateral (gonarcal complex and membrane at tip of S8+9 inflated). c.a. caudal arm of apodeme on T9+ectoproct c.c. callus cerci d.a. dorsal arm of apodeme on T9+ectoproct g.c. gonarcal complex h.i. hypandrium internum inv sclerotized invagination along midline of S8+9 ev.m. eversible membrane s.a. submarginal apodeme of S8+9 s.p. setose subrectal plate S8+9 fused eighth and ninth sternites T8 eighth tergite T9+ect fused ninth tergite and ectoproct v.a. ventral arm of apodeme on T9+ectoproct.
cream, with brown mesal band; flagellum dark brown throughout. Maxillary palp with distal segment dark brown; penultimate, middle segments dark brown laterally, pale mesally; basal two segments pale. Labial palp with terminal segment brown, basal two pale. Venter cream, unmarked except base of mentum with small brown marks laterally.
Thorax ( Fig. 40b View Figure 40 ): Pronotum wider than long: ̴ 0.51–0.76 mm long; ̴ 1.17–1.33 mm wide; cream to light tan, with thin, crooked or broken, reddish brown stripe on midline; pair of reddish brown crooked sublateral stripes (0.09–0.22 mm wide), lateral margin brown throughout or cream-colored with small, brown marks anteriorly; numerous long, stout, brown setae (longest ̴ 0.21–0.25 mm long). Meso-, metanota cream-colored, usually with pair of reddish brown longitudinal bands laterally, sometimes entirely cream colored; setae sparse, generally brown on mesothorax, pale on metathorax. Pleural areas cream-colored, without markings. Legs cream-colored without markings, except coxae with brown mark posteriorly, small on procoxae, large on meso-, metacoxae; tarsi amber-tinged. Tarsal claws amber, recurved with broad cleft, quadrate base.
Wings ( Fig. 50 View Figure 50 ): Forewing 13.9–16.1 mm long, 4.9–5.7 mm wide; L:W ratio, 2.8–2.9; width greatest near midpoint, tapering at basal 1/4th and distal 3/4th of wing; costal margin fairly straight, sloping gradually at base; apex fairly broad, round- ed. Distal section of M (before furcation of M1 and M2), ma, m-cu1, base of Cu1 (above icu1) crassate. Costal area slightly enlarged; height of tallest costal cell (#5, 6) = 4.1–4.9× length of first costal vein, 0.18–0.19× width of wing. Subcosta, radius sinuate; most subcostal veinlets, radial crossveins straight. Eleven to twelve closed radial cells (between R and Rs), height of tallest radial cell 1.4–1.5× width; other than radial crossveins, only gradate veins in contact with PsM. Four b cells, four to five b’ cells. First intramedian cell ovate, 0.6× width of third medial cell; inner row of five to seven gradate veins; seven to eight outer gradate veins; both rows regularly stepped, very slightly convergent to each other distally. Second cell beneath Rs with i.g. at base = 1.8–2.2 mm tall, 2.7–3.4× width; third cell = 1.4–2.1 mm tall, 3.0–3.3× width. Second gradate cell 1.4–1.5 mm tall, 2.2–2.6× width; third gradate cell 1.4–1.6 mm tall, 2.6–3.0× width. Length of second cell beneath Rs with i.g. at base = 1.3–1.5× length of second gradate cell. Three intracubital cells; distal one open, icu1, icu2 each shorter than icu3; icu1, icu2 similar in length. Vein 1A forked. Hindwing narrow, with tip ovate; 12.7–14.4 mm long, 4.3–5.1 mm wide. Eleven to twelve radial crossveins; four to six inner gradates; seven to eight outer gradate veins; three b cells, plus small t cell; four 4 b’ cells; two intracubital cells, distal one open.
Wing coloration: Hyaline; stigma tinged with tan. Longitudinal veins largely green, with brown at intersections, forks; costal veinlets, crossveins mostly green or green with brown at intersections; gradates usually brown.
Abdomen: Dorsum, pleuron mostly cream-colored, with scattered brown spots; venter usually brown basally (S1-S4), cream-colored distally (S5-S7), variable from all cream-colored to all brown; terminal segment of male (S8+9) cream-colored, with margins, midline usually outlined in brown. Callus cerci pale; trichobothria pale. Tergites
T8 T9+ect
6, 7: roughly quadrate, with rounded margins; length ̴1.6–2.1 times greater than height (lateral view). Sternite 6: quadrate, dorsal margin straight; length ̴0.9–1.2× height. Spiracles oval, not enlarged; atria not enlarged.
Male (Figs 51, 52): T8 rounded anteriorly, posteriorly (lateral view), with setose ventrolateral extension reaching below spiracle. Tergite 9+ectoproct rounded, fused dorsally, indented distally; posteroventral region with tuft of long, robust setae; ventral margin with apodeme heavy, straight basally, dividing mesally, with dorsal arm curving behind callus cerci almost to top of T9 (in well sclerotized individuals), with ventral section curving around distal margin of callus cerci, also curving distoventrally as bifurcated, invaginated, ventral and caudal arms. Ectoproct of well-sclerotized individuals with ventral margin bending mesally, invaginating adjacent to invaginated apodemes. Callus cerci taller than wide (̴ 0.20 mm tall, ̴ 0.14 mm wide), with ̴31 trichobothria. S8+9: ratio, length: height (basal), 1.5: 1; with heavily sclerotized, sub-basal, transverse apodeme, longitudinal, invaginated, sinuous apodeme on midline; ventral margin straight throughout; basal margin straight, rounded dorsally; dorsal margin sclerotized, slightly convex, tapering to acute tip; terminus with robust membrane extending from distal margin, laterally bearing small, dense field of acute gonocristae laterally (only in heavily sclerotized individuals). Subanal plate small, lightly sclerotized, with sparse, short setae. Gonarcus robust, broad, almost transverse, tightly attached to T9+ectoproct via short subanal membrane; bridge well-sclerotized, but not heavy, with gonarcal apodemes extending broadly from distal margins of bridge; span of gonarcus near arch ̴ 0.29–0.33 mm, span between gonarcal apodemes distally ̴ 0.72–0.73 mm. Mediuncus broadly attached to distal edge of gonarcus, ex- tending perpendicularly from frontal edge of gonarcus, with pair of internal, elongate rods extending from slightly below lateral margins of bridge distally about midway to tip of mediuncus. Tip of mediuncus bent downward, tapering to rounded, beak-like apex; membrane immediately below beak with four raised membranous protuberances bearing ̴6 pairs of short gonosetae in deep sockets; gonosaccus membrane distal to gonarcus with fields of peg-like scales. Surface of mediuncus with dense, short, thin setae. Hypandrium internum roughly anchor-shaped, with relatively robust arms, Cshaped comes.
Female ( Figs 48b View Figure 48 , 53–55 View Figure 53 View Figure 54 View Figure 55 ): Tergite 8: length 1.3–1.4 x height (lateral view); ventral extension with medium length, slender setae. Tergite 9+ectoproct (lateral view) mostly vertical, not extending much beneath T8; proximal margin fairly straight throughout or with slight bulge mesally; distal margin extending well beneath ventral margin of gonapophyses laterales. Callus cerci taller than broad (0.19–0.20 mm tall, 0.13–0.15 mm wide), with ̴33 slender trichobothria. Gonapophyses laterales <0.4× height of T9+ectoproct; ̴3.1–3.9 times taller than wide; with fairly long, robust setae; rounded dorsally, ventrally; orientated posteroventrally (̴30° angle from midline). Sternite 7: with robust setae; lateral view: length ̴1.7–1.8× height of proximal margin, with distal 3/4 th gradually sloping slightly to terminus; terminus approximately same height as midsection. Subgenitale broad, with base rounded, clear, unfolded; attached to S7 via short, transversely folded, shallowly invaginated membrane; distal process knob-like, rounded, not noticeably bilobed distally, extending perpendicularly from neck; without digitiform process. Pair of large, bulbous bursal glands with elongate, narrow ducts opening on anterolateral margin of bursa, distally with long, narrow accessory ducts. Bursa long, narrow, extending well beyond spermatheca; dorsal surface with small longitudinal folds. Bursal duct enlarged, fluted, folded, flat, with most folds sharp-edged externally. Spermatheca elongate, tubular, with one sharp mesal bend, bean-shaped enlargement, open to bursa via elongate dorsal slit throughout; spermatheca ̴ 0.8 mm long, 0.12 mm in diameter; invagination elongate, narrow (̴ 0.4 mm long, ̴ 0.05 mm wide); velum not identified. Spermathecal duct short (̴ 0.44 mm long), with distal section (0.15 mm) brushy, arising from tip of spermatheca on dorsal, right side, extending into subgenitale, with one U-shaped curve. Colleterial gland smooth, extending beyond middle of seventh segment, with small, bulbous, smooth-textured reservoir; secondary glands not found. Transverse sclerotization well-formed, flat, ellipsoid, with elongate hair-like teeth, located mesally behind gonopophyses laterales.
Larvae. Unknown.
Eggs. Unknown
Biology. Unknown. Adults have been collected in all months from September through April, but not during late fall or winter months.
Type material. Hemerobius flavescens Blanchard. Four syntypes are in the MNHN; all are in poor condition. Here , the only specimen with an abdomen (a female, examined) is named as the Lectotype (present designation). Its label data are: – (1) “Museum Paris / Chili / Gay 15–43”; (2) “15 / 43” [circular]; (3) “ Chrysopa [Sic!] / flavescens / Blanch.” [hand-written] (4) “ LECTOTYPE / Hemerobius flavescens / Blanch. 1851; des. / C. A. Tauber ’08” [red]. Three wings, the antennae and many legs are missing. Two of the other three types (paralectotypes) carry identical labels as the Lectotype ; the third has label (1) the same as the Lectotype, and label (2) “19 / 43”; and label (3) “ Hemerobius / flavescens / Blanch”. All now carry paralectotype labels: “ PARALECTOTYPE / Hemerobius flavescens / Blanch. 1851; det. / C. A. Tauber ’08” [yellow]. All three specimens are without abdomens ; one without wings; one without hindwings; one lacking forewings and one hindwing.
Chrysopa nosina Navás. Although Navás (1913 r#569: 85) mentioned only one collection date in his original description, he did not state how many specimens he examined when he described C. nosina . Thus , the female in the MZB (examined) that Navás labeled as a type and that carries label data matching the original description is the Lectotype (present designation). Its labels read: (1) “Nos ( Chili), Marzo 1910 ” [handwritten (Navás)]; (2) “ Chrysopa nosina Nav. Navas S. J. det.” [hand-written (Navás)]; (3) “Typus” [pink; hand-written (Navás)]; (4) “Type Chrysopa (Suarius) nosina Nav. ♀ det. P. Adams 74” [hand-written (Adams)]; (5) “ LECTOTYPE, Chrysopa nosina Navás , des. C. A. Tauber ’08” [red]; (6) “ Chrysopodes flavescens (Blanchard) , det. C. A. Tauber 2008”; (7) “78-1689 MZB ”. The abdomen is dissected (in a microvial with glycerin).
A second specimen in the MZB (female, examined, not dissected) from the same locality, but collected 15-11-10 is also labeled “Typus” [Navás’ hand]; a fourth label in Adams’ hand states: “Not the type; only 1 specimen mentioned in orig. desc. ‘ Marzo 1910 ’.” It is not clear whether Navás examined this specimen when he prepared the description of Chrysopa nosina , so I did not label it as a paralectotype; it also is a C. (N.) flavescens (78-1690 MZB). A third specimen (examined) in the MZB, from Curacantín, Chile, was determined as Chrysopa nosina by Navás and is labeled as “Cotypus” in Navás’ hand; however, it was collected in 1919 (after the original description was published). It is a teneral male of C. (N.) porterinus (det. P. Adams ’74; confirmed C. A. Tauber, 2008), and it bears a label, written by P. Adams in 1974, stating that the specimen was reported by Navás (1921a: 259). Two additional (non-type) specimens (examined) that were identified as Chrysopa nosina by Navás are in the MNHN ( Legrand et al. 2008: 157); both are C. (N.) flavescens .
Chrysopa jaffuelina Navás. A specimen labeled by Navás as the type of C. jaffuelina (examined) is in the MZB. Because the original description did not state whether Navás had more than one specimen, this specimen (female; abdomen dissected, in glycerin, in microvial on pin) is here designated as the Lectotype (present designation). Its labels are as follows: (1) “Los Perales, Chile, I.1918 ”; (2) “ Chrysopa jaffuelina Nav. P. Navás S. J. det.”; (3) “Typus”; (4) “ LECTOTYPE, Chrysopa jaffuelina Navás , desig. C. A. Tauber, 2008” [red]; (5) “ Chrysopodes flavescens Blanch. , det. C. A. Tauber 2008”; (6) “78-1697 MZB ”. There is another specimen (examined), in the MNHN, that Navás studied; it was not mentioned in the original description and is not part of the type series ( Legrand et al. 2008 [2009]: 144).
Chrysopa bullocki Navás. There are two syntypes of C. bullocki in the MZB (examined). They each have labels that read: (1) “Angol / ( Chile) / 10.1.32” [hand-print- ed, Navás]; (2) “ Chrysopa / Bullocki Nav. / P. Navás S. J. det.” [hand-printed, Navás, printed]; (3) “Typus” [red]. One of the specimens, a slightly teneral female, is chosen as the Lectotype (present designation). It has three additional labels: (4) “78-1701 MZB ”; (5) “ LECTOTYPE / Chrysopa bullocki / Navás, desig. / C. A. Tauber 2008” [red]; (6) “ Chrysopodes flavescens / (Blanchard) / det. C. A. Tauber 2008”. The second specimen, a male dissected by Adams in 1974, has four labels that differ from those on the lectotype: (4) “abd. in glycerine, prep. P. Adams 1974”; (5) “78-1985 MZB ”; (6) “ PARALECTOTYPE, Chrysopa bullocki Navás , desig. C. A. Tauber 2008”; (7) “ Chrysopodes flavescens (Blanchard) , det. C. A. Tauber 2008”. Adams’ notes indicate that the genitalia of this specimen were teneral, but in reasonably good condition when he examined it in 1974. However, when I examined it in 2008, parts of the abdomen and the genitalia were missing.
Specimens examined (in addition to type material listed above). ARGENTINA. Neuquén: Ao Aucapan, 7 km. S. Pilolil, II/27/1978, C. M. & O. S. Flint, Jr. (1M, USNM); Ao. del Gato, 8 km. S. Rahue, III/2/1978, C. M. & O. S. Flint, Jr. (5M, 6F USNM); Pucara, XI/1957 (2M, CAS), I/1956, L. E. Pena (1F, CAS); II/1960, L. E. Pena (1M, CAS); San Martin de los Andes, XII/19/1954 (1M, CAS-PAA), 1/1958, H. J. Molinari (1M, CAS, determined as a new sp. by P. A. Adams). CHILE. Araucanía Region: Angol, II/1/1938, D. S. Bullock (1F, USNM); Angal [= Angol?], Cerda (coll.), II/11/1953 (1M, AMNH). Atacama Region: Chamonate, W. Copiapo, X/4/1980, L.E. Pena (1F, 1M, teneral, 1? CAS); Copiapo, 1600m, X/2/1980, L. E. Pena (1F, CAS); Juntas (Copiapo), 1600m, X/2/1980, L.E. Pena (1F, CAS); El Transito, X/25/1980, L. E. Pena (1M, 4F, CAS); W. Vallenar a. Maitencillo, X/11/1980, L. E. Pena (3M, 3F, 1?, CAS); Atacama, SE Vallenar Pinte, 1600 m, X/25/1980, L. E. Pena (2F, CAS); Quebrada, Algodon, Nr Carrisal Bajo Atacama, III/24/1954, L. E. Pena (2F, CAS). Bíobío Region: Arauco, 5 km. W. Tucapel, XII/23/1950, XII/28/1950, Ross & Michelbacher (1M, 3F, CAS); Arauco, Contulmo, II/1/1953, L. E. Pena (1M, CAS); Ñuble, Rio Teno, I/25–27/1968, L. E. Pena G. (1M, USNM); Rio Huequecura, 350 m, 37°42'S, 71°45'W, at light, XI/31/1998 - I/1/1999, DD Judd & AVZ Brower (1F, OSU); Ñuble, Alto Trequalemu, 500 mtrs, ca. 20km. SE. Chovellen, I/26–27/1979, D. & M. Davis & B. Akerbergs (2M, USNM); Ñuble, Recinto, I/1955, L. E. Pena, leg. (22M, 8F, CAS); Chillan, Recinto, I/1979, L. E. Pena (1M, CAS); Ñuble, Atacalco, nr. Diguillin R., I/22/1955, L. E. Pena, leg. (1M, 6F, CAS); Ñuble, Las [transos?], I/22/1955, L. E. Pena, leg. (1F, CAS); Sta. Barbara, II/4/1959, L. E. Pena (1F, teneral, CAS). Coquimbo Region: Elqui, 11 km S. Vicuña, X/26/1992, Rozen, Sharkov, Snyder (1F, AMNH); 12 mi. E. Vicuna, XII/4/1950, Ross & Michelbacher (1M, CAS); Rio Turbio, X/28/1957, L. E. Pena (1F, CAS); Rivadavia, X/24/1940, L. E. Pena (3F, CAS); Vicuna, nr. irrigat. ditch, XI/30/1976, at light, Gurney & Barria (1M, USNM); Elqui, 20 mi. E. of La Serena, XII/3/1950, Ross & Michelbacher (5F, 5M, CAS); 50 km. S Serena, XII/1/1950, Ross & Michelbacher (3M, 8F, CAS); Quero, Los Vilos, I/1984, L. E. Pena (1F, teneral, 1?, CAS, det. N. Penny); 15 mi. S Los Vilos, XII/13/1940, Ross & Michelbacher, (1M, teneral, 1F, teneral, CAS); Samo Alto, XI/2/1957, L. E. Pena (5M, 5F, 3?, CAS); Talcuna, X/15/1958, L. E. Pena (1F, CAS); 10 km. E. Fray Jorge Natl Pk, XII/28/2966, dry wash, M. E. Irwin (1M, CAS); Fray Jorge Natl Pk, 15 km. NW Pachingo, 100–200 m, X/20/1966, E. I. Schlinger (1M, CAS). Limari, 20 km N Combarbala, XI/20/1959, L. E. Pena (1M, CAS). Maule Region: Curicó, Rio Teno, 1300m, II/7–14/1965, L.E. Peña (25M, 23F, CAS); Curicó, El Buchen, Cord. II/12/1956, L. E. Pena (2F, CAS); Curico, Rio Tono, II/7–14/1965, L. Peña (1M, CAS); Linares, Fundo Malcho, XII/1952, L. E. Pena (3M, 9F, CAS); Linares, Tranque de Bullileo, 800m, I/10–12/1979, D. & M. Davis & B. Akerbergs (1M, USNM); Carrizalillo, 250m, I/30-II/5/1981, L. E. Peña (1F, USNM); Cayuranquil, 400m, W. Cauquenes, I/23–31/1981, L. Peña (1M, USNM); Linares, Hornitos, 750 m, I/5/1986, P. Mazry (1M, 5F, SDNHM); 20 km. S. Linares, XII/3/1990, P. Mazry (1M, 1F, SDNHM); Maule, 22 mi. N.Talca, XII/22/1950, Ross & Michelbacher (1M, 2F, CAS); Tranque de Bulleo, I/1–12/ 1979, 800 m, D. & M. Davis & B. Akerbergs (1F, USNM); Trequalemu, 600 m, I/27/1979, L. E. Pena (1F, 2?, CAS, det. M. Penny). O’Higgins Region: La Leonera, II/1/1953, XII/30/1954, L. E. Pena (2F, CAS); La Leonora, Graneros, II/12–13/1986, L. E. Pena (1M, teneral, 1F, CAS); El Manzano, 1000 m, IX/11/1951, I/9/1951, L. E. Pena (2F, CAS). Santiago Region: Conchali, XI/19/1978, L. E. Pena (1F, CAS); El Alfalfal, II/29/1968, Flint & Pena (10M, 3F, USNM); El Manzano, nr. San Jose de Maipo, XII/19/1976, Gurney & Barria (1M, USNM); El Peumo, nr. river, XII/18/1976, Gurney & Barria (1M, 1F, USNM); Guayacan, L. E. Pena, VI/2/1946 (1F, CAS), XII/1948 (1F, CAS); Guayacan, Santiago, VI/2/1946, III/1951, L. E. Pena (2F, CAS); Colina, III/1980, L. E. Pena (2M, 2F, 4? CAS); Portezuelo, 7 km N Santiago, 500 m, X/22–25/1981, D. & M. Davis (2F, USNM); Santiago, XI -XII/1952, M. Cerda (3M, 1?, AMNH), XII/19/1979, L. E. Pena (1?, CAS); Santiago Cordillera, Rio Colorado, III/2/1989, R. Miller & L Stange (1F, FSCA); Rio Colorado, Santiago, IV/10/1953, L. E. Pena (2M, CAS); nr. L. Rapel, XII/19/1976, Gurney (1M, 1F, USNM); La Reina, XIII/28/1947, L. E. Pena (1F, CAS); Cerro las Viscaches, 1250 m., XII/8/1951, P. C. Hutchison (1F, CAS); Santiago Prov., Queb de La Plata 510m, Fundo Rinconada, Maipú 33°31'S, 70°47'W, malaise, III/9/1966, M. E. Irwin (1F, CAS); Valle de Ramon, II/1955, L. E. Pena (8M, 4F, CAS); Valle Ramon, 1000 m, II/21/1955, L. E. Pena (1M, 3F, CAS). Valparaíso Region: Valparaiso, IV/7/12 (1F, MNHN, det. as Chrysopa nosina by Navás; det. as Chrysopodes (Neosuarius) flavescens by Adams ’84); La Cruz, IX/26/63, X/6–9/1964, X/28/1965, J. Aranda R (3M, 3F, CIS); Est. Marga-Marga, nr. Perales, III/9/1968, Flint & Pena (2F, USNM); Margo Margo, IV/14/1951, L. E. Pena (1M, CAS); Aconcaqua, El Tartaro, II/4–6/1984, L. E. Pena (2M, CAS); Aconcagua, Guardia Vieja, XII/26–18–1986, D. Pendleton (1F, SDNHM), XII/6/1961, Peña (1F, CAS); Acgua.: Rio Branco, III/10/1968, Flint & Pena (4 M, USNM). Chile, E. C. Reed (1M, 1F, USNM): 10 km. E. Zapudo, XI/28/1950, Ross & Michelbacher (3M, 1 teneral, 1F, CAS).
Known distribution. Argentina, Chile.
Variation. The degree of sclerotization and invagination of the ventral region of the ectoproct varies considerably among male specimens, In mature, well developed individuals, the invagination is relatively large, triangular, and heavily sclerotized; in
teneral and lightly sclerotized individuals, the invaginated apodeme is absent or small, and the posteroventral region of the ectoproct has a finger-like projection bearing a tuft of setae mesally on the ventral margin.
A small proportion of specimens with C. (N.) flavescens -like terminalia express varying degrees of C. (N.) porterina -like external characteristics (color and sclerotization). In one case, there are two such unusually dark, male specimens among a fairly large number of C. (N.) porterina from San Martín de los Andes, Neuquén, Argentina (CAS); Adams identified one as a “n. sp. nr. porterina ”. Similar specimens are a male from Pucara, Neuquén, Argentina (CAS), and a female from La Leonera, O’Higgins, Chile (CAS). The wing venation appears dark [as in C. (N.) porterinus ], the specimens are very oily and the body and wings appear to have been discolored. However, they have a dark brown stripe on the dorsal surface of the scape, the flagellum is dark brown throughout, and the brown stripe on the midline of the pronotum is narrow – all characteristics that are typical of C. (N.) flavescens . The genitalia are like those of some teneral or very lightly sclerotized C. (N.) flavescens ; e.g., in the males the apodeme along the ventral midline of S8+9 is absent and the dorsal arm of the apodeme on T9+ectoproct is weak, but the gonarcus is C. (N.) flavescens -like.
| MNHN |
Museum National d'Histoire Naturelle |
| MZB |
Museum Zoologicum Bogoriense |
| USNM |
Smithsonian Institution, National Museum of Natural History |
| CAS |
California Academy of Sciences |
| AMNH |
American Museum of Natural History |
| OSU |
Oklahoma State University, Collection of Vertebrates |
| SDNHM |
San Diego Natural History Museum |
| FSCA |
Florida State Collection of Arthropods, The Museum of Entomology |
| CIS |
Cranbrook Institute of Science |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Chrysopodes (Neosuarius) flavescens ( Blanchard, 1851 )
| Tauber, Catherine 2010 |
Chrysopodes (Neosuarius) flavescens ( Blanchard, 1851 )
| Brooks SJ & Barnard PC 1990: 272 |
Chrysopa bullocki Navás, 1933a: 230
| Brooks SJ & Barnard PC 1990: 279 |
| Monserrat VJ 1985: 237 |
| Penny ND 1977: 17 |
| Navas L 1933: 230 |
Chrysopa nosina Navás, 1913: 85
| Legrand J & Tauber CA & Albuquerque GS & Tauber MJ 2008: 157 |
| Brooks SJ & Barnard PC 1990: 272 |
| Monserrat VJ 1985: 238 |
| Navas L 1934: 14 |
| Navas L 1932: 80 |
| Navas L 1929: 19 |
| Navas L 1927: 324 |
| Navas L 1926: 326 |
| Navas L 1925: 307 |
| Navas L 1923: 116 |
| Navas L 1922: 361 |
| Navas L 1921: 259 |
| Navas L 1918: 222 |
Chrysopa jaffuelina Navás, 1918: 222
| Brooks SJ & Barnard PC 1990: 272 |
| Monserrat VJ 1985: 238 |
| Penny ND 1977: 19 |
| Navas L 1934: 14 |
| Navas L 1921: 443 |
| Navas L 1921: 259 |
| Navas L 1918: 222 |
Chrysopa flavescens ( Blanchard, 1851 )
| Penny ND 1977: 18 |
| Navas L 1910: 238 |