Hypsignathus monstrosus, H. Allen, 1861
|
publication ID |
https://doi.org/10.5281/zenodo.6448815 |
|
DOI |
https://doi.org/10.5281/zenodo.6448953 |
|
persistent identifier |
https://treatment.plazi.org/id/03AD87FA-FFE0-F60F-8CBB-3763FF1AF8AD |
|
treatment provided by |
Conny |
|
scientific name |
Hypsignathus monstrosus |
| status |
|
58. View Plate 4: Pteropodidae
Hammer-headed Fruit Bat
Hypsignathus monstrosus View in CoL
French: Hypsignathe monstrueux / German: Hammerkopf / Spanish: Hypsignatus cabezudo
Taxonomy. Hypsignathus monstrosus H. Allen, 1861 View in CoL ,
“Western Africa.”
This species is monotypic.
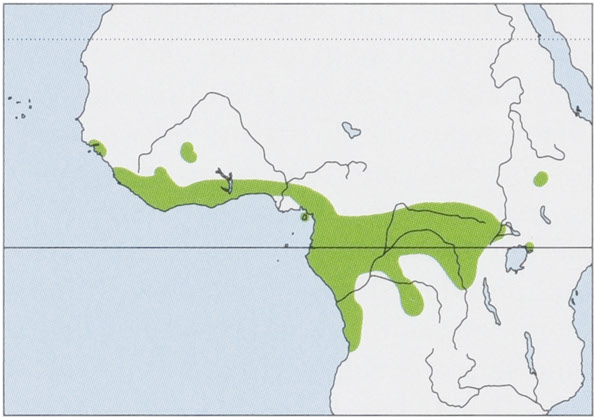
Distribution. W & C Africa from Guinea E to South Sudan, Ethiopia, Uganda, and W Kenya, S to DR Congo and NW Angola, also on Bioko I. View Figure
Descriptive notes. Head-body 160- 297 mm (males) and 165-255 mm (females), tailless, ear 30-41 mm (males) and 25-38 mm (females), hindfoot 36-40 mm (males) and 25-36 mm (females), forearm 120-139 mm (males) and 112-127 mm (females); weight 291-419 g (males) and 207-302 g (females). The Hammer-headed Fruit Bat is the largest and most sexually dimorphic (fruit) bat in Africa, with greatly enlarged muzzle. Remarkably, males are almost twice as heavy as females. Head is noticeably hammer-shaped in males, in which muzzle is greatly expanded and ends in flat, fleshy plate formed by both lips; muzzle of females is less developed; and tongue is large, with expanded tip and backward-pointing papillae, well adapted for rasping fruit. Eyes are large; irises are brown. Ears are blackish brown and triangular, with anterior and posterior basal white ear patches, which can be inconspicuous in some individuals. Adult males do not have epaulettes, but they do have mantle of long, woolly, pale grayish brown hairs; dorsum is generally sepia-brown, sometimes with grayish brown tinges, changing to rusty brown on rump and legs; pelage is soft and mid-dorsally 9-13 mm, extending along forearm dorsally and ventrally; hairs are unicolored or with pale grayish brown tips. Venteris slightly paler than dorsum, with whitish collarjoining mantle; pelage is 6-7 mm; and throat is thinly haired, appearing almost naked. Wings have claw on second digits; membranes are blackish brown; dorsal pelage is woolly brown along forearm, and strip of whitish pelage occurs on ventral side; wing membranes attach to second toes; and wingspan is almost 1 m. Skull is larger and more robust than in any other African bat; basicranial axis is deflected; rostrum is huge, relatively long, and laterally compressed, greatly elevated above dental line, especially in males; premaxilla and nasals are marked with prominent bumps; sagittal crest is moderately to well developed; zygomatic arches are moderate; and interdental palate is concave from left to right and from front to back. There are 10-11 palatal ridges; ridges 1-2 are very thick; ridge 3 is very thin; three interdental ridges are wide laterally and narrow medially; ridge 4 is partially post-dental, weakly serrated with wide cushion-like pad near each edge and shallow concave center; ridge 5 is similar to ridge 4 but more strongly serrated and less shallow in center; ridges 5-6 are thin and serrated; irregular post-dental ridges are also present that might or might not be divided; and post-dental palate lacks raised sides. Dental formulais12/2,C1/1,P 2/3, M 1/2 (x2) = 28. Supernumerary premolar can be present in upper tooth row; P* and molars are distinctly lobed. Larynx of males is extremely enlarged, up to one-half the vertebrate column and fills up most of thoracic cavity. Pair of inflatable pharyngealair sacs form resonating chambers that produces extremely loud honking calls. Chromosomal complement has 2n = 36 and FNa = 68, with 13 pairs of large to small metacentric or submetacentric and four pairs of medium-sized submetacentric or subtelocentric autosomes. X-chromosome is submetacentric or subtelocentric, and Y-chromosomeis possibly small subtelocentric.
Habitat. Lowland forests of wetter and drier types, swamp forests, mosaics of forest with grasslands, relict forests and Afromontane vegetation from sea level up to elevations of ¢. 1800 m. Hammer-headed Fruit Bats use riverine forests as corridors into woodland savannas.
Food and Feeding. The Hammer-headed Fruit Batis frugivorous and can commute up to 10 km between roosts and feeding areas in forests, second growth, or old plantations, where it primarily feeds on fruits in canopies. Females, non-adults, and nondisplaying males forage during the entire night along regular routes; they search for low-quality and continuously available fruits (e.g. Anthocleista , Gentianaceae ). Sexually active males start to forage late at night, feeding mainly on higher-quality fruits of Ficus (Moraceae) . Otherfruits are eaten from at least eleven plant genera and ten families, along with many cultivated fruits. Fruits are taken in the mouth and carried to a nearby tree, where they are squeezed for juice. Observations of Hammer-headed Fruit Bats attacking and eating chickens, scraps of meat, and skinned carcasses of small birds are questionable. The Hammer-headed Fruit Bat drinks by scooping water directly into his mouth while flying close to water or indirectly by wetting fur while skimming over water and licking water from fur.
Breeding. Litter size of the Hammer-headed Fruit Bat is one, and each female has two litters/year. Females are in local reproductive synchrony. In Gabon, reproductive chronology is continuous bimodal polyestry, with postpartum estrus. There are two breeding seasons per year, with matings during two periods of 1-3 months. In those periods, 20-135 males group together in trees along rivers in leks that are typically 40 m wide and 400-1600 m long. In these lek sites, males compete aggressively for display territories with radii of c. 5 m. A female selects a male and lands besides him, and they mate. Afterward, the female leaves, and the male resumes his display until 22:00-23:00 h. Up to 70% of males might not copulate during mating season, and only ¢.6% of males are involved in ¢.79% of copulations. Gestation lasts 5-6 months, and births take place in dry seasons: December-February and June-July. Sex ratio at birth is 1:1. Females are sexually mature at c.6 months old (even before reaching full size at ¢.9 months); males become sexually active at c.18 months old.
Activity patterns. During the day, males and females generally roost in large trees. Early in the morning, they defecate at their roost site and then rest or sleep until sundown. During the day, they do not make noises, groom, or squabble, and males, which generally rest on the outside of the group, do not approach females. In the evening, individuals wake, start grooming themselves, and leave individually to start foraging or fly to lek sites. Roostsites are 20-30 m aboveground, where Hammer-headed Fruit Bats hang on exposed branches beneath dense foliage. Other roost types exceptionally include undergrowth, rocks, and huts, and there is an unconfirmed record of a cave. Males compete in leks by beating their wings and especially by making loud honking sounds (c.2-3/second). These calls attract females.
Movements, Home range and Social organization. The Hammer-headed Fruit Bat roosts alone or in small groups of 4-25 individuals spread 10-15 cm apart. Roosts are changed every 5-9 days when they move to another roost that can be close by, 50-100 m away, or even as far as 1-10 km away. The Hammer-headed Fruit Bat apparently does not migrate; ripening of certain fruits influences its local occurrence and abundance.
Status and Conservation. Classified as Least Concern on The IUCN Red List. The Hammer-headed Fruit Bat has a wide distribution and presumably large population. It is unlikely to be declining fast enough to be listed in a higher category. It is threatened by hunting for food, and it is also persecuted for its noisiness at leks during mating seasons.
Bibliography. Bergmans (1978a, 1989), Bradbury (1977a, 1979), Gembu Tungaluna (2012), Haiduk et al. (1980), Happold, M. (2013)), Lang & Chapin (1917b), Langevin & Barclay (1990), Monadjem, Taylor et al. (2010), Tanshi (2016).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Hypsignathus monstrosus
| Don E. Wilson & Russell A. Mittermeier 2019 |
Hypsignathus monstrosus
| H. Allen 1861 |