Microprosthema semilaeve (von Martens, 1872), Von Martens, 1872
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3630.3.4 |
|
publication LSID |
lsid:zoobank.org:pub:E69F5E49-1949-4224-BDA3-9F507248BE1F |
|
DOI |
https://doi.org/10.5281/zenodo.6146016 |
|
persistent identifier |
https://treatment.plazi.org/id/03AE8790-FFFC-475F-FF6D-FD01FE53FC50 |
|
treatment provided by |
Plazi |
|
scientific name |
Microprosthema semilaeve (von Martens, 1872) |
| status |
|
Microprosthema semilaeve (von Martens, 1872) View in CoL
( Figs. 1–8 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 )
Stenopus semilaevis von Martens, 1872: 144.—Herrick, 1893: 352.—Rankin, 1898: 241–242, pl. 29, fig. 2.—A. Milne- Edwards & Bouvier, 1909: 263.—Schmitt, 1924: 86.—Schmitt, 1936: 373.—Schmitt, 1939: 28.
Stenopusculus spinosus Pocock, 1890: 523 –524.
Microprosthema spinosum —Balss, 1915: 33.
Microprosthema semilaeve —Holthuis, 1946: 54–57, pl. III, fig. i.—Manning, 1961: 81.—Coelho, 1967/69: 252.—Williams et al., 1969: 11.—Chace, 1972: 144.—Coelho & Ramos, 1972: 158.—Fausto-Filho, 1974: 6.—Fausto-Filho, 1975: 80.—Zeiller, 1974: 74, fig.—Fausto-Filho, 1980: 113, 117.—Rodriguez, 1980: 175, 179, fig. 51d.—Voss, 1980: 84–85, fig.—Felder et al., 1985: 174.—Abele & Kim, 1986: 8, 282, 283, fig. c. —Markham et. al., 1990: 418.—Ramos-Porto, Austregésilo Filho & Lacerda, 1992: 35.—Alves & Ramos-Porto, 1994: 24.—Camp et al., 1998: 139.—Coelho & Ramos- Porto, 1998: 323.—Ramos-Porto & Coelho, 1998: 109.—Álvarez et al., 1999: 6.—Martinez Iglesias & Garcia Raso 1999: 544.—Boschii, 2000: 96.—Martin & Goy, 2004: 19–25, fig. 1–2.—Goy, 2005: 204.—McLaughlin et al., 2005: 212, 270. —Tagliafico et al., 2005: 91.—Coelho et al., 2006: 47.—Alves, Ramos-Porto & Viana, 2008: 46.—Felder et al., 2009: 1051. —Goy, 2010: 252, 256, fig. 65.21C.—Ortiz & Lalana, 2010: 22.— De Grave & Fransen, 2011: 250.
non Microprosthema semilaeve— Mahadevan, Rangarajan & Sankarankutty, 1962: 235–238, figs. 1–5.—Ranade, 1973: 570–572.—Raje & Ranade, 1978: 213–222, figs. 1–6.
Material examined. (1) female lectotype, cl 5.2, male paralectotype, cl 5.0, Antillen, leg. Werfel, ZMB 3006; (2) 1 female,cl 6.2. Andros, Island, Bahamas, March-April 1908, AMNH 4231; (3) 1 male, cl 4.1, north of sand pit, Piedra Priata Reef, Santo Domingo, 9 July 1933, AMNH 8757; (4) 1 male, cl 3.3, north end of El Cayo, Santo Domingo, 8 July 1933, AMNH 8758; (5) 1 female, cl 4.2, Piedra Priata Reef, Santo Domingo, 14 July 1933, AMNH 8759; (6) 1 ov. female, cl 4.6, Piedra Priata Reef, San Domingo, 10 July 1933, AMNH 8760; (7) 2 males, 7 females, 1 ov. female, cls 4.8, 6.1, 5.4, 5.7, 6.3, 6.8, 6.8, 7.0, 6.4, Havana, Cuba, leg. L. Howell Rivero, April 1937, det. F.A. Chace, Jr., MCZH 9746; (8) 1 male, 1 female, cls 5.8, 7.6, top of reef, Conch Bay, Andros, Bahamas, EJ- 71-16, depth 0.6–6 m; leg. W.G. Lyons, 27 August 1971, det. S. Cobb, FSBCI 8082; (9) 1 female, cl 6.3, intertidal, found on artificial hard substrate, Seminole Shores seawall at point of subdivision, Indian River, Martin County, Florida, hand, rotenone, leg. L.E. Scotto, L.B. & G.R.K., 20 June 1974, det. R.H. Gore, IRCZM – 089:01251; (10) 1 male, cl 5.2, intertidal, worm reef, Walton Rocks, St. Lucie County, Florida, night, hand, leg. R.H. Gore & L. E. Scotto, 27 June 1975; det. R.H. Gore, IRCZM – 089: 02626; (11) 1 male, cl 3.0, reef 2 miles S of St. Lucie Inlet, ~ 1 mile offshore, Atlantic Ocean, Martin County, Florida, leg. P.A.H., det. K.A. Wilson, IRCZM- 089: 03321; (12) 1 male, cl 3.3, Niaklubir Island, Holandes Cay, San Blas Islands, Panama, 0 9o 35’ 07” N 78o 44’ 27”W, Collection TEPE 70-35, depth 0–1.2m., snorkel, R/V “Alpha Helix”, leg. W. Newman & S. Luke, 7 October 1970, (as Odontozona sp.), SIO – BIC 3068; (13) 1 ov. female, cl 7.2, Long Key, Dry Tortugas, leg. H. Doochin, 25 April 1948, det. F.A. Chace, Jr., UMML 32-348; (14) 1 ov. female, cl 5.3, Pigeon Rocks, British West Indies, leg. Voss, Williams & McKenney, 14 December 1957, UMML 32-1698; (15) 1 male, cl 5.8, Lesser Lameshur Bay, St. John, Virgin Islands, leg. Work & Kumpf, 10 June 1959, det. Thomas, UMML 32-1597; (16) 1 female, cl 4.1, Long Reef, Florida, leg. R.B. Manning, 6 June 1960, det. R.B. Manning, UMML 32-1787; (17) 1 male, 1 ov. female, cls 5.0, 4.7, Lesser Lameshur Bay, St. John, Virgin Islands, leg. L.P. Thomas, 7 December 1958, det. R. B. Manning, UMML 32-1234; (18) 1 male, cl 6.3, Long Reef, Dade County, Florida, leg. L.P. Thomas, 16 May 1959, det. R.
Manning, UMML 32-1995; (19) 1 ov. female, cl 5.0, reef front off Pajaros, Mexico, under dead coral, depth 0.6–1.2m., Texas A&I Alacran Expedition, leg. H.H. Hildebrand, 16 July 1959, ULLZ 2972; (20) 1 ov. female, cl 5.7, Carib Point, Roatan Island, Bay Islands, Honduras, under rocks in heavy surf, leg. E. Garcia, 7–8 August 1981, det. J.W. Goy, ULLZ 10700; (21) 1 female, cl 4.6, First Bight, Roatan Island, Bay Islands, Honduras, under rock, depth 0.9m., leg. E. Garcia, August 1982, det. J.W. Goy, ULLZ 10699; (22) 1 male, cl 5.9, Looe Key, Florida Keys, Florida, broken from coral rubble, depth 6–10m, leg. Felder, Goy, Lovett & Howell, 23 June 1984, det. J.W. Goy, ULLZ 9778; (23) 1 female, cl 6.0, Bird Key Reef, Tortugas, #3-31, 19 June 1931, USNM; (24) 1 male, cl 6.1, Bush Key Reef, Tortugas, #29-30, 23 July 1930, USNM; (25) 1 ov. female, cl 6.8,Bush Key Reef, Tortugas, # 25-4, 5 June 1925, USNM; (26) 1 female, cl 6.5, Loggerhead Key, Tortugas, #7-32, under rock, leg. R.G. Stone, 20 June 1932, USNM; (27) 1 ov. female, cl 6.6, Tortugas, #24-31, 11 July 1931 USNM; (28) 1 male, 2 females (1 ov.), cls 6.0, 5.3, 5.7, west side of Loggerhead Key, Dry Tortugas, #8-32, leg. W.L. Schmitt, 21 June 1932, USNM 78407; (29) 1 male, 1 female, cls 6.9, 7.2, Bird Key Reef, Tortugas, Florida, leg. H.H. Darby, det. W.L. Schmitt, USNM 68473; (30) 1 female, cl 3.4, Hutchinson Island, Seminole Shores, Indian River, Martin County, Florida, seawall, hand, snorkel, rotenone, Indian River survey 137-74, leg. R.H. Gore, 20 June 1974, USNM 170124; (30) 1 male, 1 ov. female, cls 6.1, 6.3, Cat Island, Bahamas, Bennett's Creek, South Point, littoral, mangroves inside mouth of creek, leg. K. Gosner, 31 June 1968, det. F.A. Chace, Jr., USNM 128528; (31) 1 male, 2 females (1 ov.), cls 5.5, 5.5, 5.9, Abaco, Bahamas, U.S. Fisheries Commission Steamer "Albatross", USNM 23389; (32) 1 female, cl 5.2, Pelican Island, Barbados-Antigua Expedition, tide pools, State University of Iowa, 11 May 1918, det. W.L. Schmitt, USNM 68716; (33) 1 ov. female, cl 7.0, English Harbor, Barbados, University of Iowa, 1918, det. W.L. Schmitt, USNM 68789; (34) 1 female, cl 7.0, English Harbor, Barbados, #1 Nutting, University of Iowa Barbados- Antigua Expedition, 1918, det. W.L. Schmitt, USNM 57945; (35) 1 male, cl 4.4, St. Christopher, British West Indies, coral reef just off Windward Beach opposite Frigate Bay, associated with anemones ( Bartholomea annulata ), sta. 103-56 " Freelance", Smithsonian-Bredin Expedition, 12 April 1956, det. L.B. Holthuis, USNM 105282; (36) 1 ov. female, cl 7.6, Caribbean Sea, Lesser Antilles, Bonaire, depth 5–10 ft., leg. R.V. Harrison, 10 February 1975, det. F.A. Chace, Jr., USNM 155675; (37) 2 males, cls 4.1, 4.3, St. Lucia, British West Indies, Pigeon Island, near breakwater north of Pigeon Island Club, Smithsonian-Bredin Expedition Sta. #60-59, 15 April 1959, det. F.A. Chace, Jr., USNM 136536; (38) 1 ov. female, cl 6.1, Boekoeti Reef, Aruba (Oranjestad), between corals and coral debris on the "Scharrenfläche" #25, leg. P. Hennelinch, 25 June 1930, det. W.L. Schmitt, USNM 67414; (39) 1 ov. female, cl 6.6, Dominica, British West Indies, west of Portsmouth, off Shingle Beach, depth 3–6ft., Smithsonian-Bredin Expedition Sta. 76-59, leg. Nicholson, Jordan & Finlay, 19 April 1959, det. F.A. Chace, Jr., USNM 136537; (40) 1 ov. female, cl 4.5, Antigua, British West Indies, reef off Black's Point, Falmouth Bay, Smithsonian-Bredin Expedition Sta. 113-59, leg. D.V. Nicholson, 30 April 1959, det. F.A. Chace, Jr., USNM 136540; (41) 1 female, cl 4.1, Carriaeou, Grenadines, British West Indies, Tyrrell Bay, sand flats inside reef, Smithsonian-Bredin Expedition Sta. 17-56, leg. D.V. Nicholson, 16 March 1956, det. F.A. Chace, Jr., USNM 136531;(42) 1 male, cl 5.0, Barbuda, West Indies, east side of Cocoa Point, rotenone, Smithsonian-Bredin Expedition Sta. 102-59, 27 April 1959, det. F.A. Chace, Jr., USNM 136539; (43) 1 ov. female, cl 5.2, Anquilla, British West Indies, Sandy Island, depth 1.8–3.7m., Smithsonian-Bredin Expedition Sta. 54-58, 13 April 1958, det. F.A. Chace, Jr., USNM 136534; (44) 1 male, cl 5.2, Tobago, West Indies, west of Pigeon Point, sand flats off beach, Smithsonian-Bredin Expedition Sta. 31-59, 10 April 1959, det. F.A. Chace, Jr., USNM 136535; (45) 1 male, 1 female, cls 3.3, 3.9, Antigua, British West Indies, Charlotte Point, English Harbor, Smithsonian-Bredin Expedition Sta. 73-56, leg. Schmitt, Chace, Nicholson & Smith, 2 April 1956, det. F.A. Chace. Jr., USNM 136533; (46) 1 male, cl 6.0, Tobago Cays, Grenadines, British West Indies, west side of Borodol, coral rock, depth 0.9m, Smithsonian-Bredin Expedition Sta. 22-56, leg. D.V. Nicholson, 17 March 1956, det. F.A. Chace, Jr., USNM 136532; (47) 1 male, 1 female, cls 4.5, 4.5, Port Antonio, Jamaica, leg. J.E. Overden, USNM 4805; (48) 2 females, cls 4.8, 4.4, Jamaica, exchange from Marine Biological Laboratory, Woods Hole, Massachusetts, USNM 47358; (49) 2 males, cls 7.3, 2.7, Caribbean Sea, Ascension Bay, Quintana Roo, Mexico, along shore near Suliman Point, Smithsonian-Bredin Expedtion Sta. 85-60, leg. Schmitt, Daiber, Bousfield, & Rehder, 17 April 1960, det. F.A. Chace, Jr., USNM 136541; (50) 1 male, cl 2.3, Caribbean Sea, Ascension Bay, Quintana Roo, Mexico, 183– 274m. southwest of Suliman Point, sand shallows, depth 0.6–7.6m., Smithsonian-Bredin Expedition Sta. 87-60, leg. Bousfield, Daiber & Rehder, 17 April 1960, det. F.A. Chace. Jr., USNM 136542; (51) 3 males, 2 ov. females, cls 5.0, 6.6, 7.0, 5.5, 8.1, Caribbean Sea, Ascension Bay, Quintana Roo, Mexico, Suliman Point to 183 m. to southwest shore, Smithsonian-Bredin Expedition Sta. 95-60, leg. Schmitt, Bousfield & Daiber, 19 April 1960, det. F.A.
Chace, Jr., USNM 136543; (52) 1 male, 1 female, cls 4.3, 5.0, Old Providence Island, Colombia, shore, reef and tidepool collecting, Presidential Cruise 1938, Sta # 30, leg. W.L. Schmitt, 6 August 1938, USNM 77864.
Diagnosis. Moderately small spongicolid shrimp with subcylindrical, depressed body, with few spinous processes; carapace covered with small, sometimes blunt spines, with cervical groove reaching far posteriorly; propodus and dactylus of third pereiopod with distinct dorsal crista, numerous spinules along dorsal and ventral margins, surfaces of third pereiopod covered with spinules, except ischium, spines and spinules on dorsal and ventral margins of carpus and meri; second pereiopod with 1 or 2 spines anterodorsally on merus; first pereiopod with all segments without spinules; transverse ridges on first and second abdominal somites; longitudinal ridge on uropodal endopodite with median spine; antennular peduncle with distinct, curved stylocerite; scaphocerite lobate with 4–6 very strong teeth on outer margin; first maxilliped with unsegmented or 3-segmented endopodite; body coloration red with white patches dorsally on carapace and abdomen; third maxilliped red; pereiopods red, white at joints of segments and tips of dactyl.
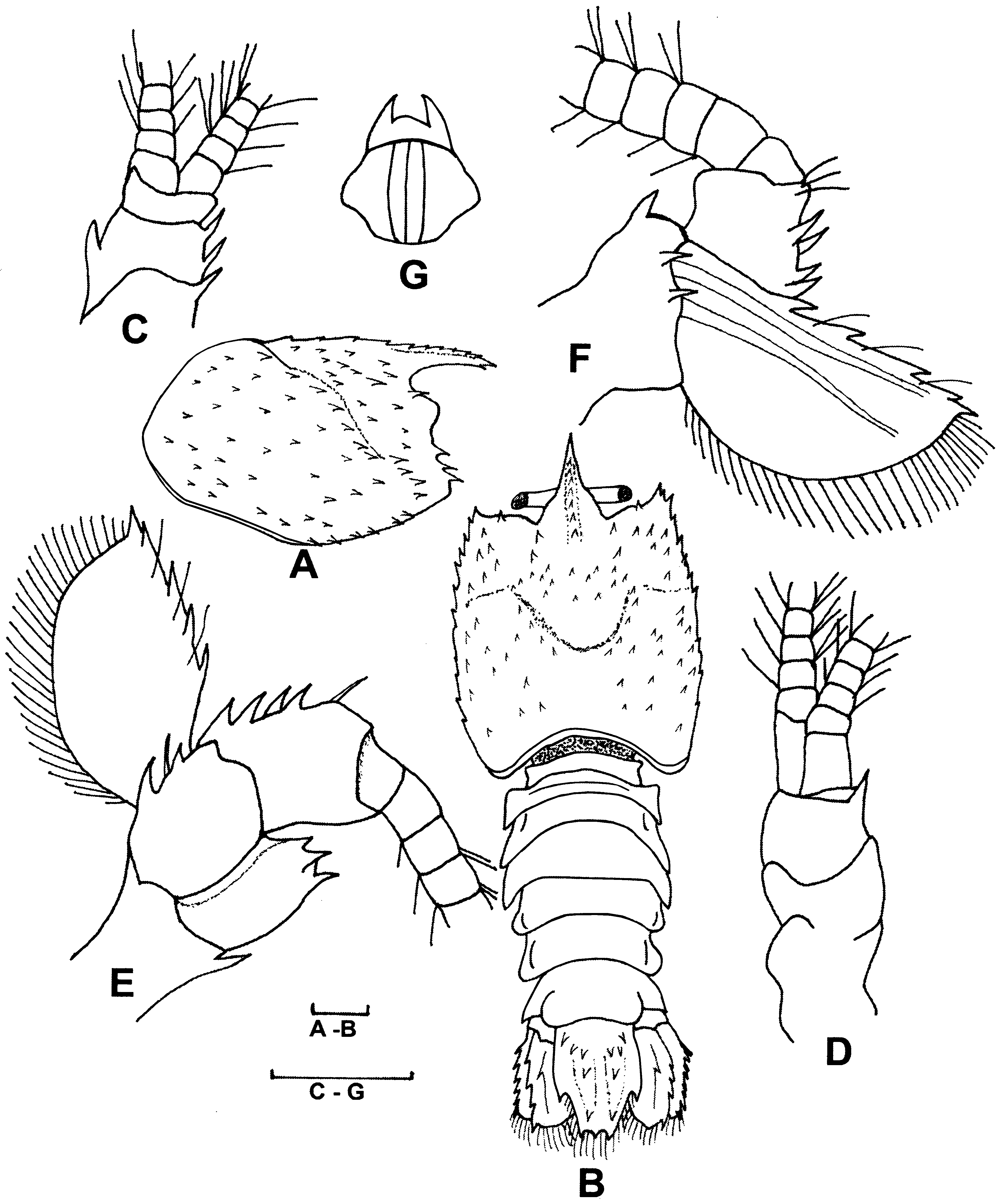
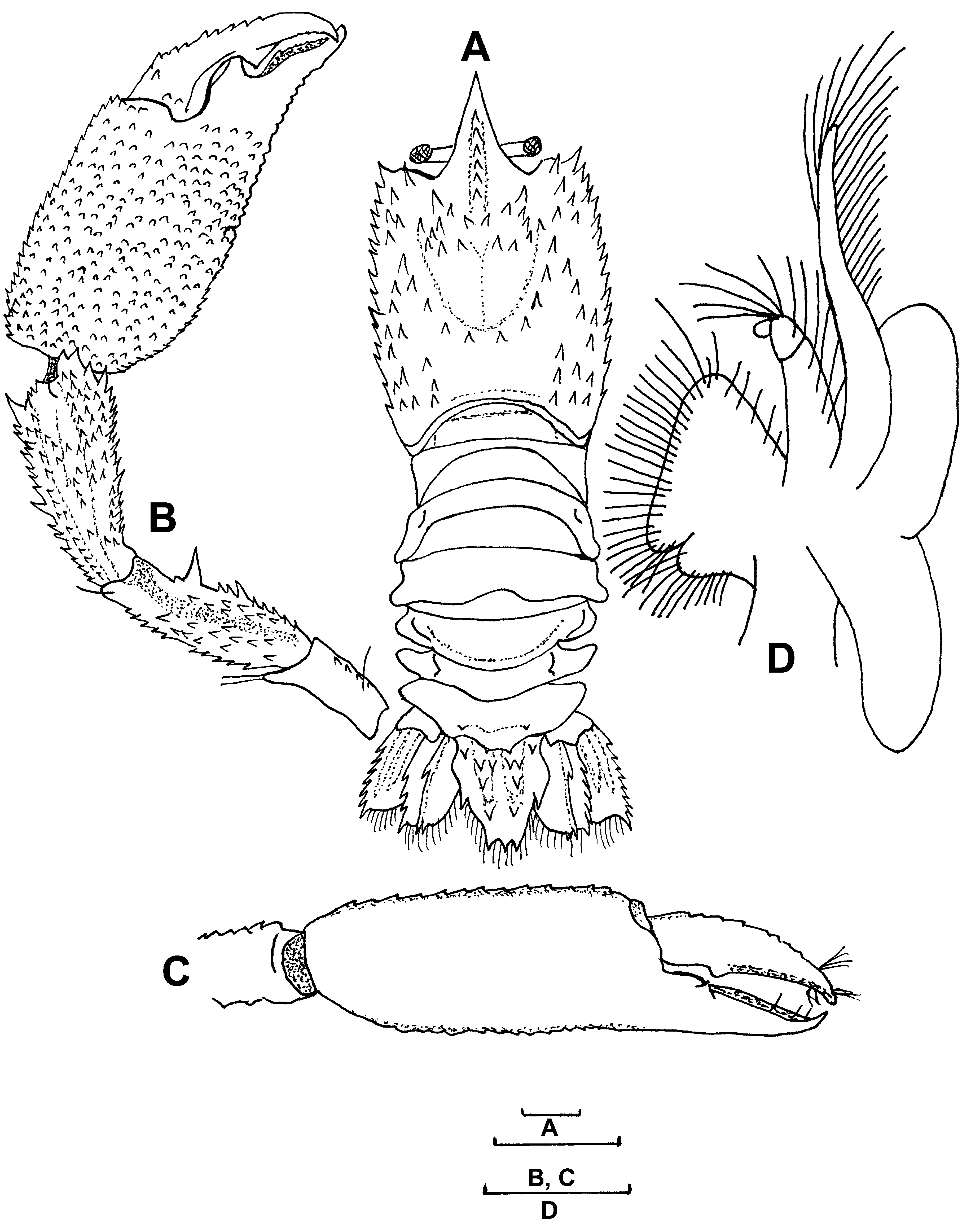
Redescription. Lectotype (female, ZMB 3006). Rostrum ( Fig. 1 View FIGURE 1 A, B) long, slightly deflexed, nearly reaching level of distal end of scaphocerite. Dorsal margin with 5 spines, ventrally with distal spine, laterally without spines.
Carapace ( Fig. 1 View FIGURE 1 A, B) covered with small forwardly directed spinules. Cervical groove reaching far posteriorly, with 5 spinules along each lateral margin. Antennal, branchiostegal and hepatic spines present; 2 small pterygostomial spines present. Ventrolateral carapacial angle and brachiostegite slightly rounded.
Abdomen ( Fig. 1 View FIGURE 1 B) broad, depressed, dorsally glabrous. First pleomere with posterior transverse ridge dorsally provided with row of setae; lateral margin of pleuron with anterior tooth. Second pleomere with median transverse ridge; lateral margin of pleuron broadly rounded. Third pleomere with transverse carina only evident at pleuron, which is laterally broadly rounded. Fourth and fifth pleomere with posterior margin near base of pleura with deep broad blunt incision; pleura broadly triangular with blunt top, each with short median carina. Sixth pleomere pleuron triangular, anterior margin rounded, posterior margin slightly concave. Thoracic sternites unarmed broadening from front to back.
Telson ( Figs. 1 View FIGURE 1 B, 5C) slightly longer than uropods, truncately triangular. Dorsal surface with 2 longitudinal ridges, ending considerable distance before posterior margin, bearing 4 strong teeth and 2 long setae; 2 anterior spines present at base of telson. Lateral margin at each side provided with large median spine; posterior margin with 3 small spines; posterior half of telson fringed with plumose setae.
Eyes ( Fig. 1 View FIGURE 1 B) well developed, cornea smaller, wider than peduncle. Facets, pigment distinct in cornea. Ophthalmic narrow, peduncle unarmed.
Basal segment of antennular peduncle ( Figs. 1 View FIGURE 1 C, D) with short straight stylocerite. Middle, distal segments with some spinules. Both flagella short, provided with numerous plumose setae; upper flagellum with 18 aesthetascs, 2 on articles 3–11.
Antenna ( Figs. 1 View FIGURE 1 E, F) with strong basal segment, outer margin ending in acute spine. Other segments of antennal peduncle with some spines. Scaphocerite reaching slightly beyond tip of rostrum, broad at base, tapering towards tip, outer margin straight, bearing 5 teeth, 3 plumose setae. Inner margin strongly convex, fringed with plumose setae. Dorsal surface with 2 distinct longitudinal carinae, ventral surface glabrous. Antennal flagellum well developed, extending slightly beyond abdominal somites, covered with numerous short plumose setae.
Epistome ( Fig.1 View FIGURE 1 G) triangular anteriorly with 2 stout submedian spines. Labrum normally developed. Paragnath bilobed with lobes separated by median fissure.
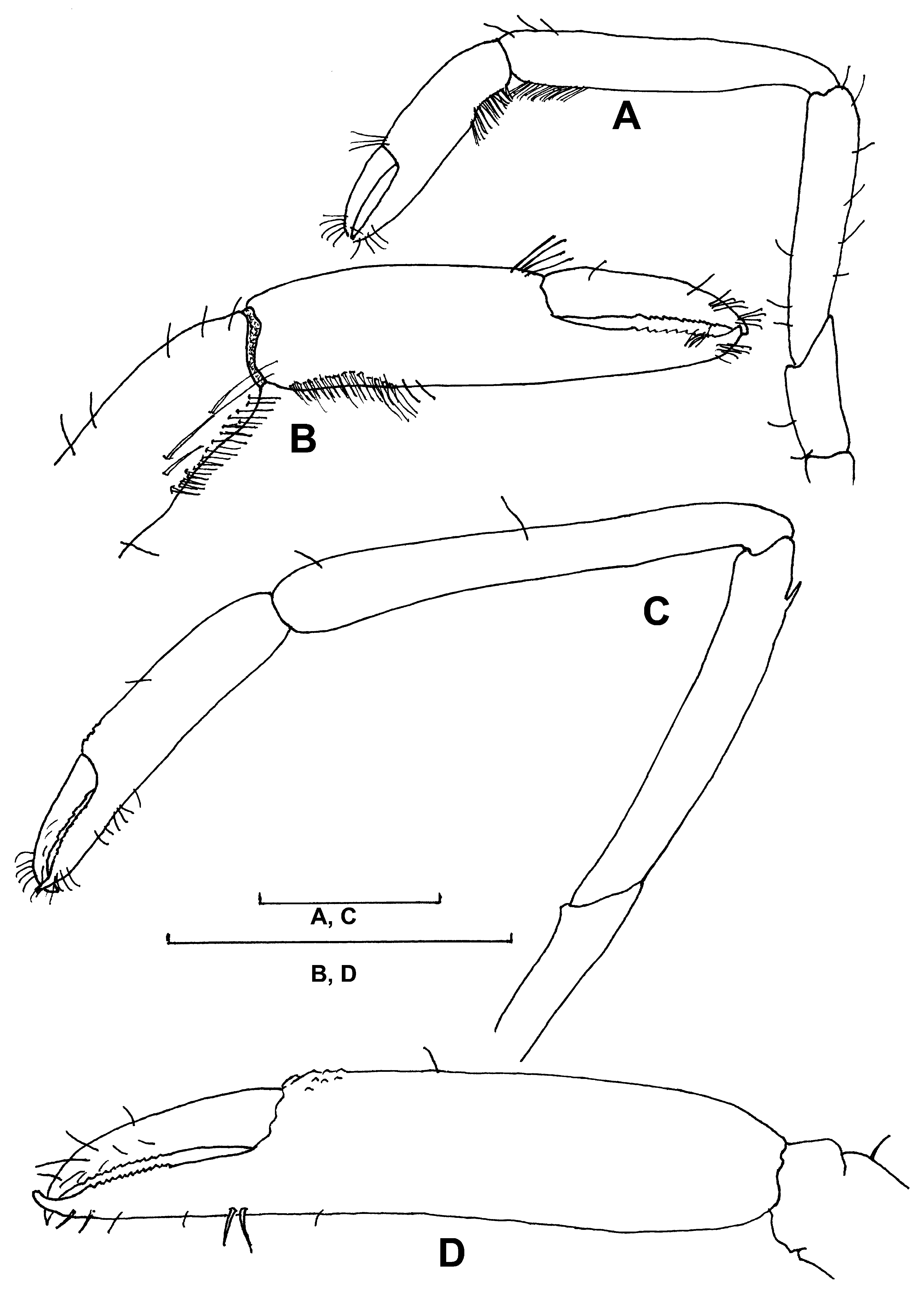
Mandible ( Fig. 2 View FIGURE 2 A) robust with short, fused molar and incisor processes. Molar surface without teeth; incisor thickened with 7 small median teeth. Palp well developed, 3-segmented. Proximal segment with 3 small lateral plumose setae; middle segment with 5 small lateral plumose setae; distal segment broad, fringed with plumose setae.
Maxillule ( Fig. 2 View FIGURE 2 B) with slender undivided endopodite bearing 5 lateral, 5 distal plumose setae. Proximal endite moderately broad, truncate distally with 3 plumose setae laterally, 5 compound spinose setae, 14 simple setae distally. Distal endite slightly broader, rounded distally bearing numerous simple setae.
Maxilla ( Fig. 2 View FIGURE 2 C) with setose coxal and basal endites. Endopodite long, slender, not exceeding anterior margin of scaphognathite, 21 long plumose setae laterally and distally. Scaphognathite long, narrow, fringed with numerous plumose setae.
First maxilliped ( Fig. 2 View FIGURE 2 D) with unsegmented endopodite bearing 16 plumose setae. Basipodite large, rounded anteriorly with slight concave outer border bearing dense fringe of plumose setae; coxopodite short, unilobed with numerous plumose setae. Exopodite well developed with numerous distal and lateral plumose setae.
Second maxilliped ( Fig. 2 View FIGURE 2 E) with 4-segmented endopodite. Dactylus suboval withdense fringe of setae along distodorsal margin. Propodus slightly longer than dactylus, densely setose on dorsal margin. Carpus short, about half length of propodus, with 2 long simple setae at distodorsal angle. Merus 2 times length of dactylus, with straight inner border bearing 6 long simple setae distally; outer border convex with numerous long simple setae. Ischium and basis short setose lobes; coxa larger, rounded with dense fringe of setae. Exopodite long, slender, undivided with distal half bearing 24 long plumose setae. Basipodite with 4 long, simple setae.
Third maxilliped ( Fig. 2 View FIGURE 2 F) endopodite strongly developed, 5-segmented. Dactylus slender with fringe of simple setae. Propodus slightly longer than dactylus, with numerous simple setae, setiferous organ distally on inner margin.
Carpus equal to dactylar length, with numerous simple setae. Merus about 2 times longer than carpus, robust, with 3 sharp spines on outer margin, 2 dorsomesial spines distally, numerous simple setae dorsomesially and on margins. Ischium robust, slightly longer than merus, with 5 spines on outer margin; distal spine, numerous simple setae on inner margin. Basis and coxa fused, unarmed. Exopodite long, slender, reaching middle of merus, with distal half fringed with numerous long, plumose setae. Epipod proximally rounded, tapering distally, with 7 long simple setae on outer margin.
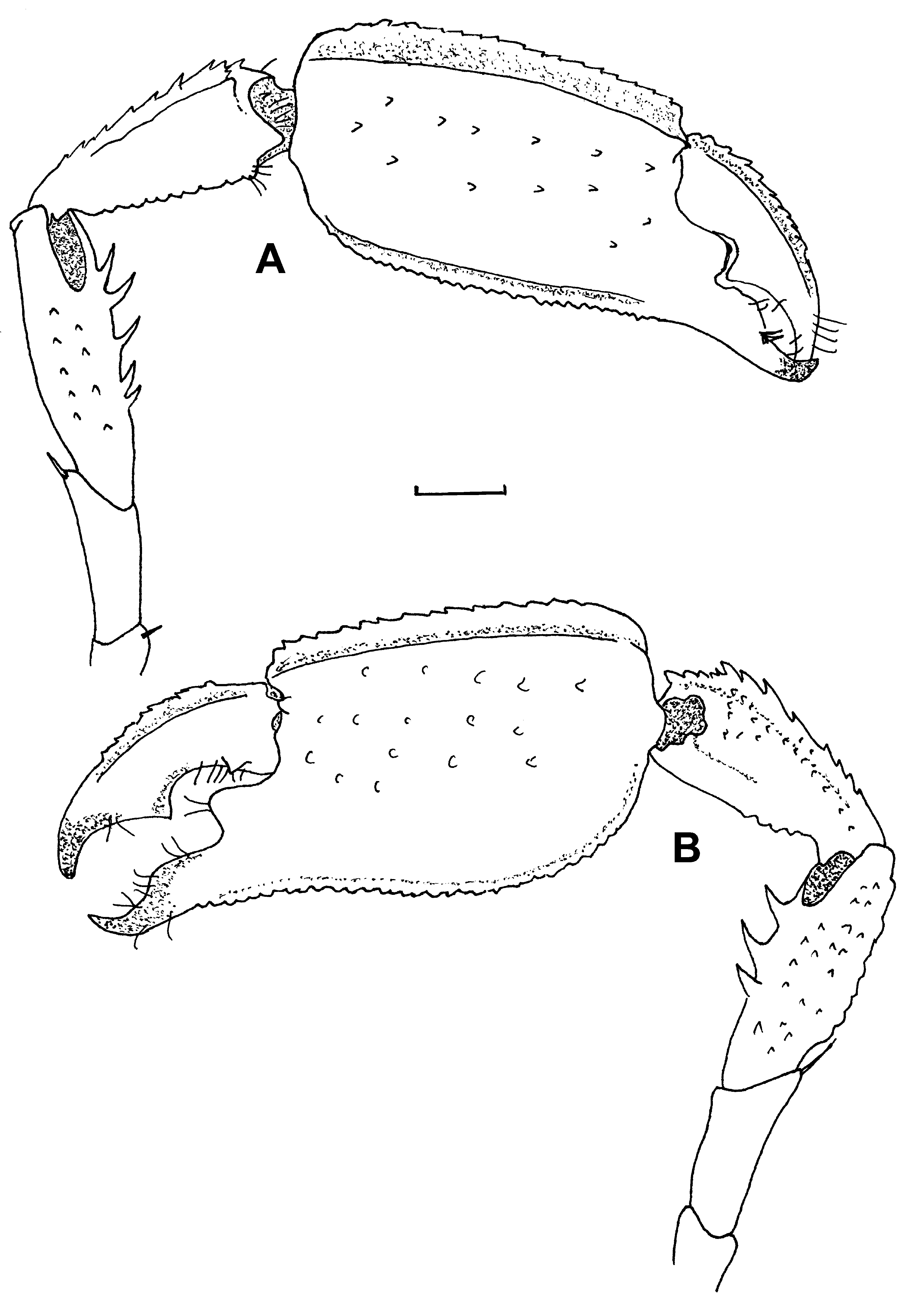
First pereiopod ( Figs. 3 View FIGURE 3 A, B) small,slender, reaching past scaphocerite, all segments without spines. Fingers slightly compressed, with hooked tips, cutting edge indistinct with tiny series of small teeth. Fingers with small tufts of short setae; distodorsal extremity of palm with 4 long simple setae. Distoventral part of carpus and proximoventral part of propodus provided with poorly developed setiferous organ. Carpus longest segment about 2 times propodal length, merus slightly shorter than carpus, ischium half of meral length; all these segments bear few short simple setae. Basis and coxa short, unarmed.
Second pereiopod ( Figs. 3 View FIGURE 3 C, D) similarly built as first, including setation, but longer. No setiferous organ present. Fingers slightly compressed, with hooked tips, small tufts of short setae, cutting edge indistinct with series of small teeth. Anterodorsal part of merus with 1 spine; distodorsal end of propodus with series of small, blunt tubercules; other segments glabrous.Carpus and merus of equal length, about twice propodal length.
Third pereiopod ( Fig. 4 View FIGURE 4 A, B) robust, largest, strongest of pereiopods, reaching with entire carpus beyond scaphocerite. Fingers with sharp hooked crossing tips. Dactylus with dorsal crista and finely serrated edge; cutting edge with large triangular tooth midway, large spine proximally. Cutting edge of propodus with large, rounded tooth; palm of chela longest segment with distinct dorsal crista, serrate dorsal and ventral margins, scattered blunt spinules on rest of surface. Carpus half propodal length with dorsal carina, 10–12 spines on dorsal margin, serrate ventral margin, few scattered blunt spinules on surface. Merus equal carpal length with 3–5 strong ventral spines and scattered blunt spinules on rest of surface. Ischium 0.8 meral length without spinules, single long simple seta at distodorsal extremity.
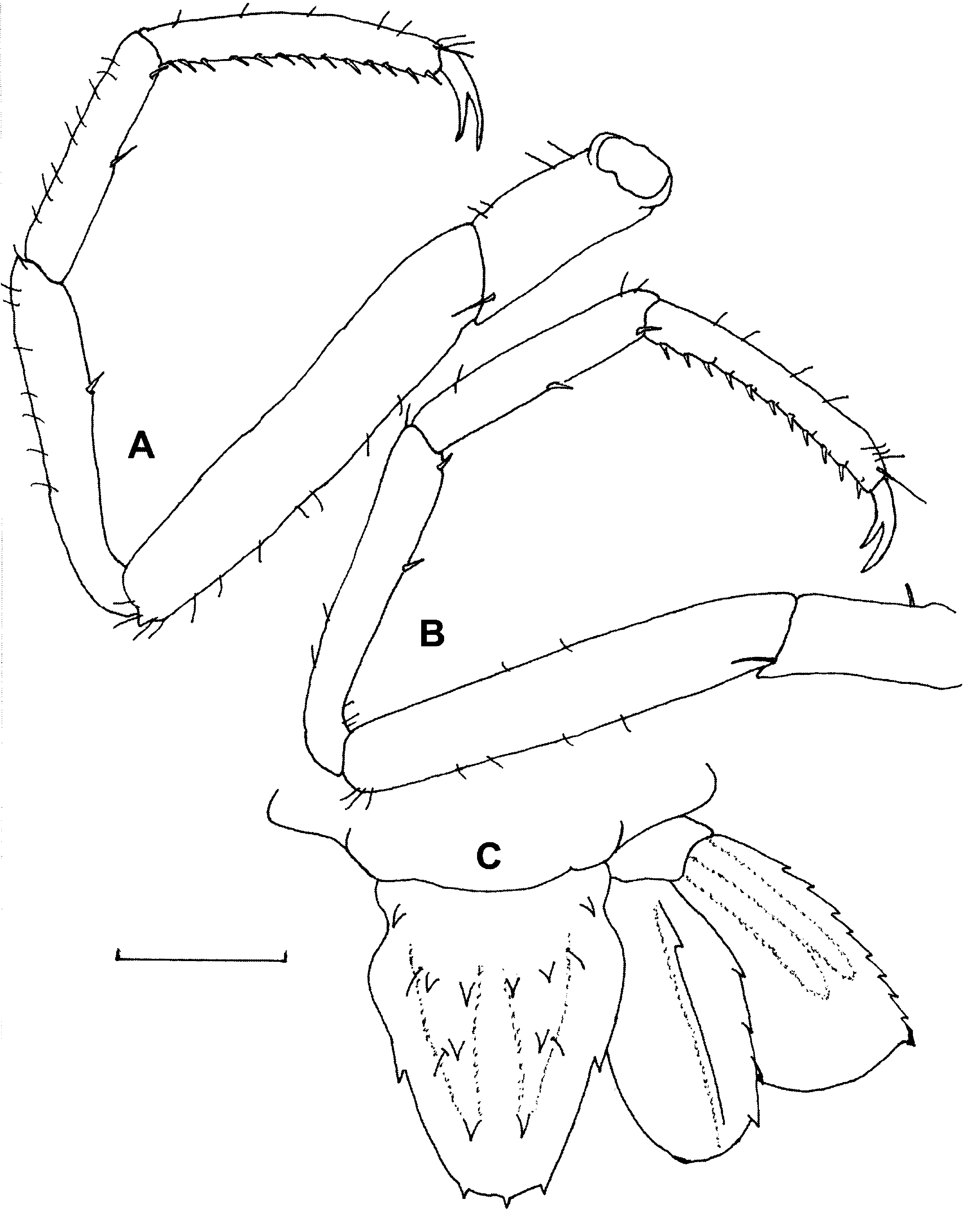
Fourth and fifth pereiopods ( Figs. 5 View FIGURE 5 A, B) long, slender, propodus undivided, carpus 2-segmented. Dactyli biunguiculate with unguis long, slightly curved, not separated from dactylar corpus. Propodi with ventral row of 11–13 movable spines, dorsally with few short setae. Carpi longest segment, 3 or 4 ventral movable spines, dorsally with few short setae. Meri slightly shorter than carpi with few short setae. Ischia short with proximal short ventral seta; bases, coxae short, unarmed.
First pleopod uniramous, others biramous, all lacking appendices. First pleopod smallest, with exopodite about twice length of basipodite, margins with dense fringe of plumose setae. Rami of other pleopods 1.5 times length of basipodite, margins of rami fringed with plumose setae.
Uropods ( Figs. 1 View FIGURE 1 B, 5C) well developed, almost reaching tip of telson. Exopodite with outer margin slightly rounded, bearing 6–9 teeth; inner margin semicircular fringed with plumose setae; dorsal surface with 2 distinct longitudinal ridges without spinules. Endopodite subovate, outer margin with 4 teeth; inner margin fringed with plumose setae; dorsal surface with distinct median longitudinal ridge bearing strong spine.
Branchial formula:
Maxillipeds Pereiopods I II III I II III IV V Pleurobranchs _ _ 1 1 1 1 1 1 Arthrobranchs 1 1 2 2 2 2 2 _ Podobranchs _ 1 _ _ _ _ _ _ Epipods 1 1 1 1 1 1 1 _ Exopods 1 1 1 _ _ _ _ _
Variations. The species shows considerable variation in the number of body and appendage spines. Male specimens have the abdominal pleura more acute than the rounded ones of females; there is a ventral median spine on each male abdominal somite; male thoracic sternites 4–6 bear 2 submedian blunt spines; and males have 1 or 2 small distodorsal spines on the sixth abdominal somite ( Fig. 6 View FIGURE 6 A). Spination of the 3rd pereiopod ( Figs. 6 View FIGURE 6 B, C) shows much variation; that of the allotype ( Fig. 6 View FIGURE 6 C) may be due to regeneration of a lost appendage. The endopodite of the 1st maxilliped ( Fig. 6 View FIGURE 6 D) is 3-segmented in most of the specimens examined. Further variation is as follows: 4–11 rostral dorsal spines; 2 or 3 dorsomedial spinules on ophthalmic peduncle; 4–6 spines on outer margin of scaphocerite; 4–6 ischial outer margin spines on 3rd maxilliped; 2 or 3 meral outer margin spines on 3rd maxilliped; 1 or 2 anterodorsal meral spines on 2nd pereiopod; 11–20 propodal movable ventral spines on 4th and 5th pereiopods; 3 or 4 carpal movable spines on 4th and 5th pereiopods; 7–16 teeth on outer margin of uropodal exopodite; and 3–6 teeth on outer margin of uropodal endopodite.
Measurements. (mm) Postorbital carapace length: females 1.9–5.9, males 1.0–6.0; carapace and rostrum length: females 3.4–8.1, males 2.3–7.0; total body length: females 7.2–20.9, males 5.1–17.7.
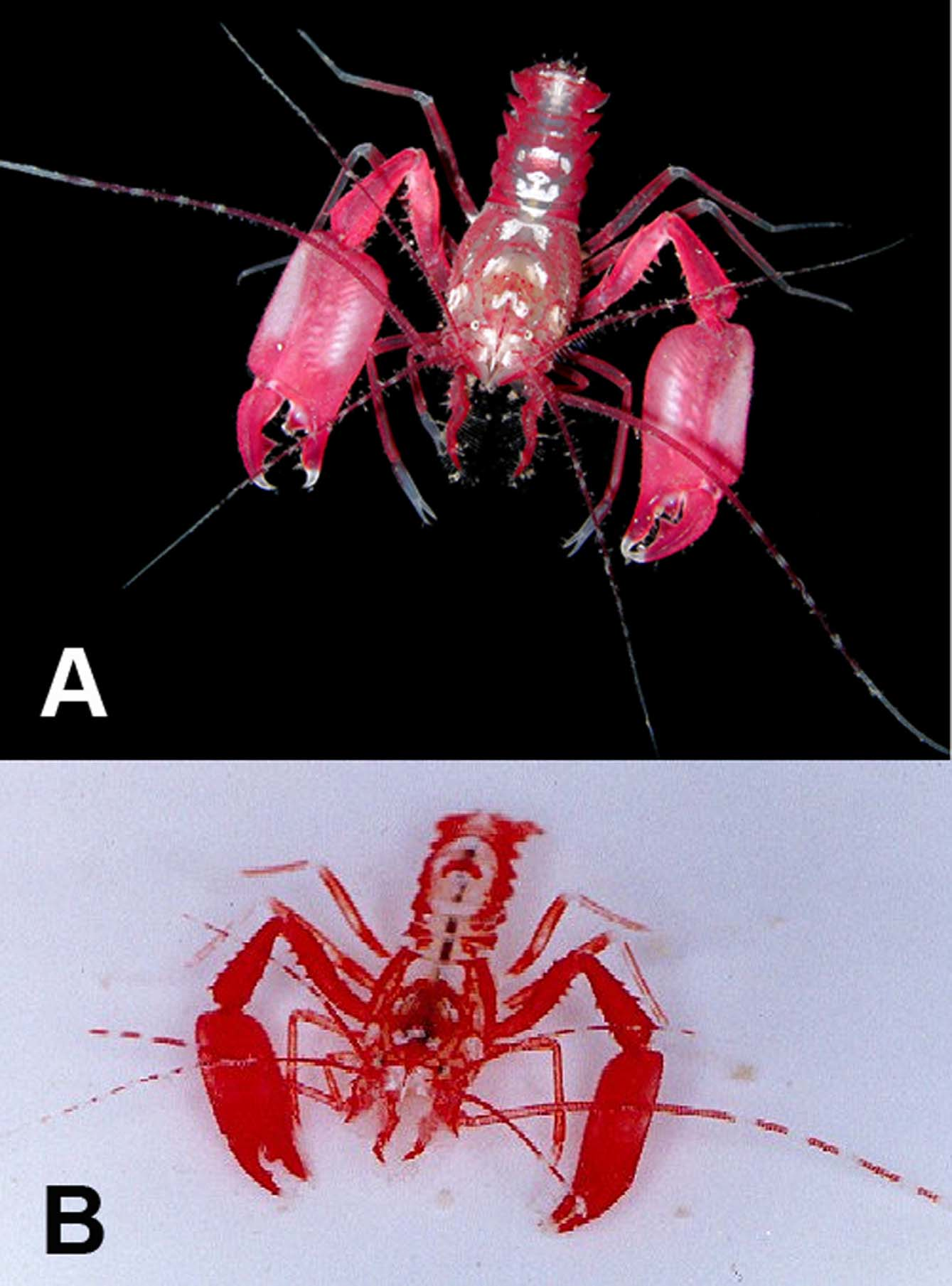
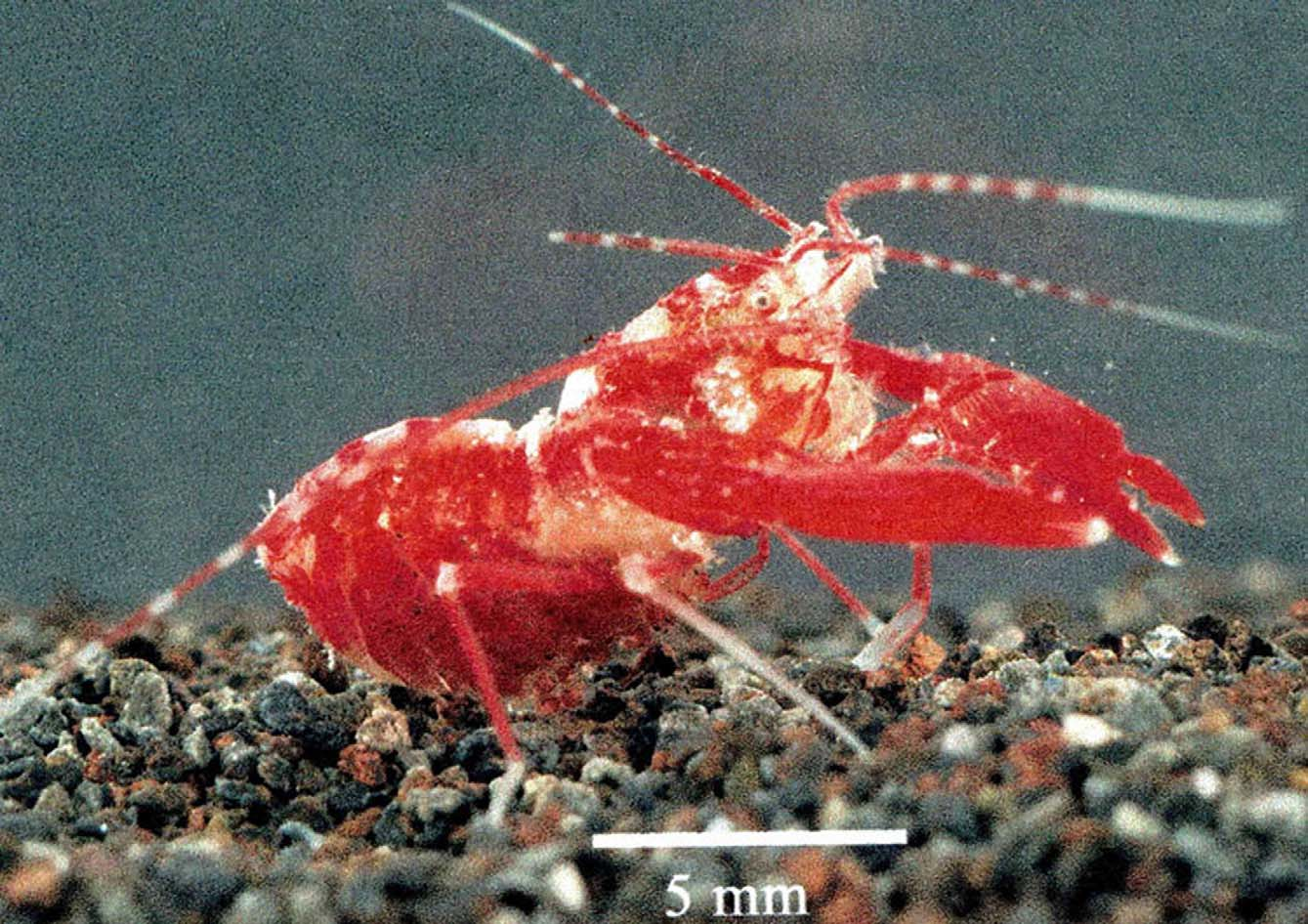
Color pattern. The color pattern of Microprosthema semilaeve is very consistent over its zoogeographic range ( Figs. 7 View FIGURE 7 , 8 View FIGURE 8 ). Manning (1961) gave a thorough description of this pattern from a specimen (UMML 32-1787) collected from Long Reef, Florida, which is not repeated here. In general, the body coloration is a deep red with white patches dorsally on the carapace and abdomen. The third maxillipeds are red and all the pereiopods are red with white at the joints and tips of the dactyli. Zeiller (1974) presented a color photo of this species taken in association of the rough fileclam, Lima scabra , off southern Florida. The shrimp’s coloration blended in very well with the scallop’s red tentacles. Manning (1961) stated that this striking color pattern camouflages the shrimp quite well where it blends in completely with coral rocks encrusted with red foraminifera and coralline algae.
Development. Ovigerous females ranged in size from 3.0– 4.1 mm postorbital carapace length, 9.3–20.9 mm total body length, and carried 67– 565 eggs. Eggs at blastula with undifferentiated yolk cells were 0.38 × 0.41 mm in size, while eggs with embryos having pigmented eyes and well developed appendages were 0.43 × 0.73 mm in size. The prezoeal and first zoeal stages were described by Martin & Goy (2004).
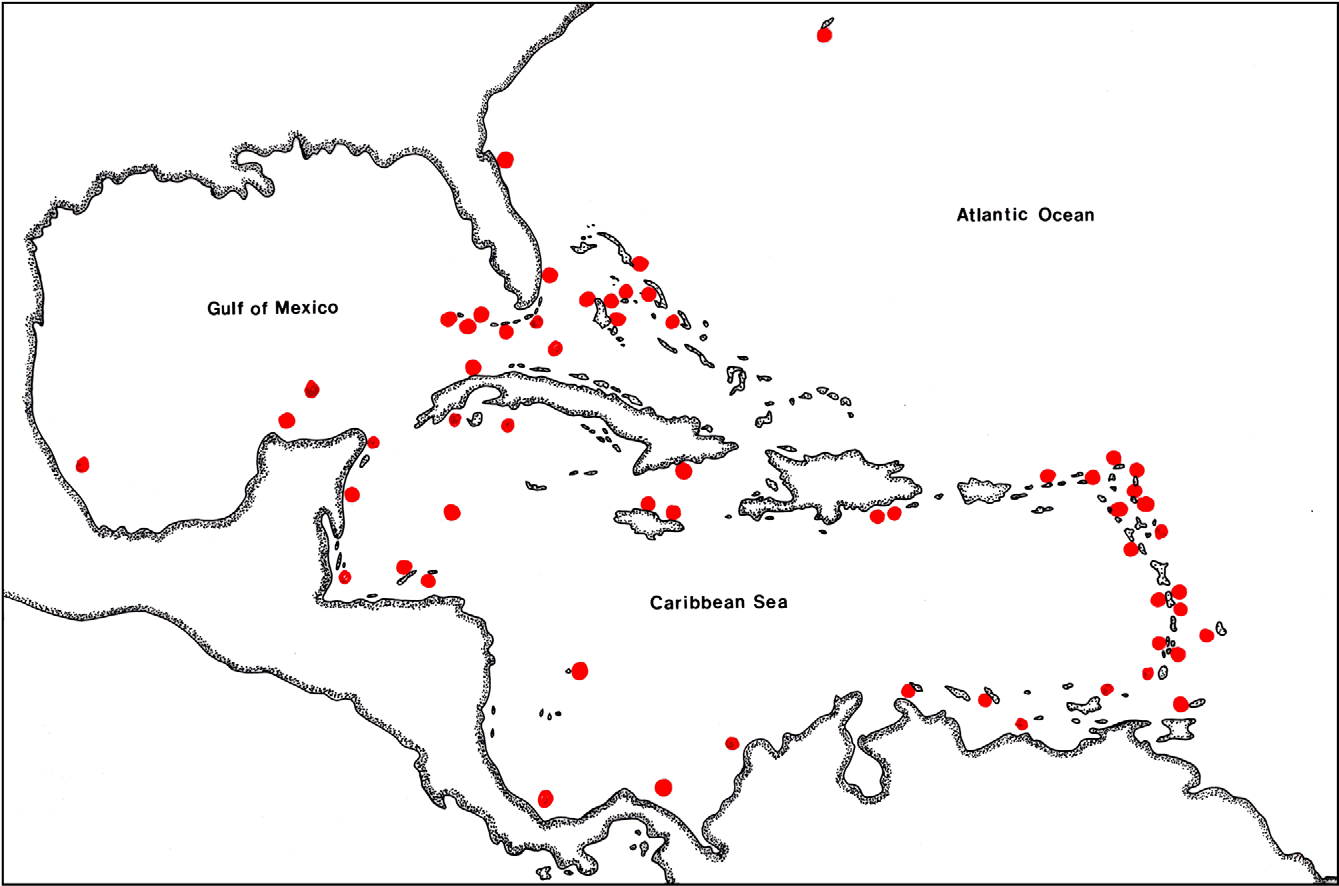
Distribution. The type material for Microprosthema semilaeve was described by E. von Martens (1872) based on material in the Berlin Museum obtained from Mr. Wessel in Hamburg and collected in the West Indies. Von Martens did not design a holotype, so the female is herein designated the lectotype and the male a paralectotype. The species is known from numerous locations in the Caribbean and Gulf of Mexico ( Fig. 9 View FIGURE 9 ). Its range extends from Bermuda, along the east coast of Florida, through the Caribbean, Gulf of Mexico to the northeast coast of Brazil (Fernando de Noronha, Pernambuco, and Bahia).
Habitat. This species has been collected in coral rubble or under rocks down to depths less than 10 m, which is consistent for other members of the genus (Holthuis 1946). Specimens have also been taken in tide pools, on sand flats and in empty conch shells. One male specimen was collected in association with pink-tipped sea anemone, Bartholomea annulata and the species has been collected or observed numerous times amongst the red tentacles of the rough fileclam, Lima scabra , justifying its common name “crimson lima shrimp”(McLaughlin et al. 2005).
Remarks. Microprosthema semilaeve closely follows the definition of the genus Microprosthema Stimpson given by Holthuis (1946). It is most closely related to the eastern Pacific geminate species M. emmiltum Goy 1987 , but differs in color, abdomen, uropods, and spination of the pereiopods. Among the other species in the genus, M. semilaeve is similar to the Indo-West Pacific M. validum , with which it has been confused with in the past (Mahadevan et al. 1962).
Microprosthema validum differs markedly in coloration from M. semilaeve , has median longitudinal carinae on the 3rd, 4th and 5th abdominal tergites, the scaphocerite is relatively narrow with 2 or 3 strong obtuse teeth on the outer margin, the 2nd pereiopods bear 2–6 dorsal meral spines, and the uropodal endopodites do not have any dorsal spines. Comparison of the larval development of M. semilaeve (see Lebour 1941; Raje & Ranade 1978; Martin & Goy 2004) and M. validum (see Ghory, Siddiqui & Kazmi 2005) show they represent three different species. Indian and Pakistani specimens of Microprosthema identified as M. semilaeve (see Mahadevan et al. 1962; Ranade 1973; Raje & Ranade 1978) or M. validum (see Pillai 1962; Tirmizi & Kazmi 1979) represent an undescribed species of Microprosthema (Saint Laurent & Cleva 1981; Felder et al. 1985; Goy 1987; Goy & Felder 1988; Martin & Goy 2004).
The degree of morphological variation seen in Microprosthema semilaeve and compared to the degree of variation reported for M. manningi Goy & Felder, 1988 leads us to conclude that M. jareckii Martin, 2002 needs to be considered a junior synonym of M. manningi . The holotype of M. jareckii is ~ 10.5 mm total length while the smallest paratypes of M. manningi are 12.5 mm (USNM 77866a) and 13.7 mm (USNM 77865) total length. The body spination of these two paratypes of M. manningi is very similar to that of the holotype of M. jareckii and the morphology of the mouthparts of both species is essentially the same. The differences seen in M. jareckii are probably due to its smaller size.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.