Nematocarpus ramuliferus (Allman, 1874)
|
publication ID |
https://doi.org/10.11646/zootaxa.3737.5.1 |
|
publication LSID |
lsid:zoobank.org:pub:B5FE322D-4D0A-45E6-84BF-F00FA6308DE1 |
|
DOI |
https://doi.org/10.5281/zenodo.6149350 |
|
persistent identifier |
https://treatment.plazi.org/id/03AEB111-0A58-BD08-99AC-FA67FA527F69 |
|
treatment provided by |
Plazi |
|
scientific name |
Nematocarpus ramuliferus (Allman, 1874) |
| status |
|
Nematocarpus ramuliferus (Allman, 1874) View in CoL
( Figs. 1 View FIGURE 1 E, 6A–J, tables 1, 7, 9, 11–12)
Halicornaria ramulifera Allman, 1874: 477 , pl. 67, fig. 3, 3a–d.—Kirchenpauer 1876: 26.—Bale 1886: 90.—Schneider 1898: 538.—Nutting 1900: 126.—Broch 1903: 9.—Jäderholm 1909: 109.
Halicornaria pluma Broch, 1903: 8 , pl. 4, fig. 15–21.
Nematocarpus ramuliferus: Broch 1918: 74 , fig. 37a–d, fig. 38.—Kramp 1938: 38.—Kramp 1943: 44.—Ramil & Vervoort 1992: 175.
Cladocarpus ramuliferus: Schuchert 2000: 413 (table 1).—Schuchert 2001: 144, fig. 124A–F.—Bouillon et al. 2006: 283.
Material examined. NEREIDA0609 RD27, 3 fertile colonies, largest 2.6 cm high; NEREIDA0610 RD62, one sterile colony 1.5 cm high; NEREIDA0610 RD64, one fertile colony 3.0 cm high; NEREIDA0610 RD74, one sterile colony 3.8 cm high; NEREIDA0710, RD88, one fertile colony 4.2 cm high.
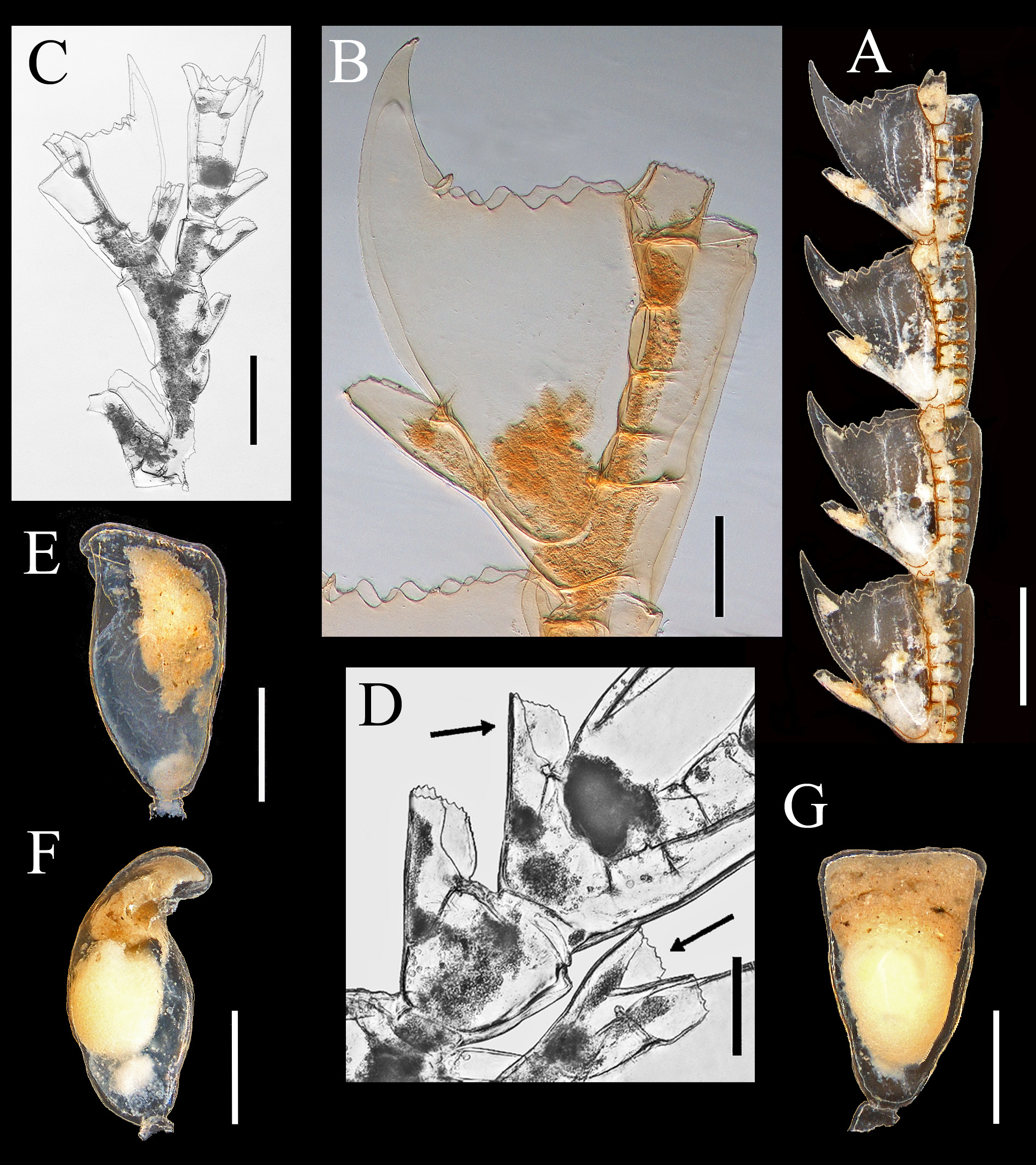
Description. Colonies light brown in alcohol, delicate, plumose, ramified, up to 4.2 cm high and 2.0 cm wide, arising from a poorly developed hydrorhiza consisting of a network of tubules that supports a polysiphonic stem basally, thinning out to monosiphonic distally. Stem flaccid, thin, erect, 0.4–0.5 mm wide proximally, tapering distally. Ramification in one plane, scarce. Branches up to 1.5 cm long, with thin axis (100 µm), polysiphonic only basally, not opposite, arising at acute angles.
Main tube in front of stem and branches, divided into short (340–386 µm) internodes by straight nodes. Accessory tubes parallel, not jointed, without hydrothecae and nematothecae. Internodes without internal septa, with one apophysis located in the lower half of internode, directed alternately left and right. Apophyses with two nematothecae: one frontal basally, the other distal, slightly above apophysis, somewhat lateral.
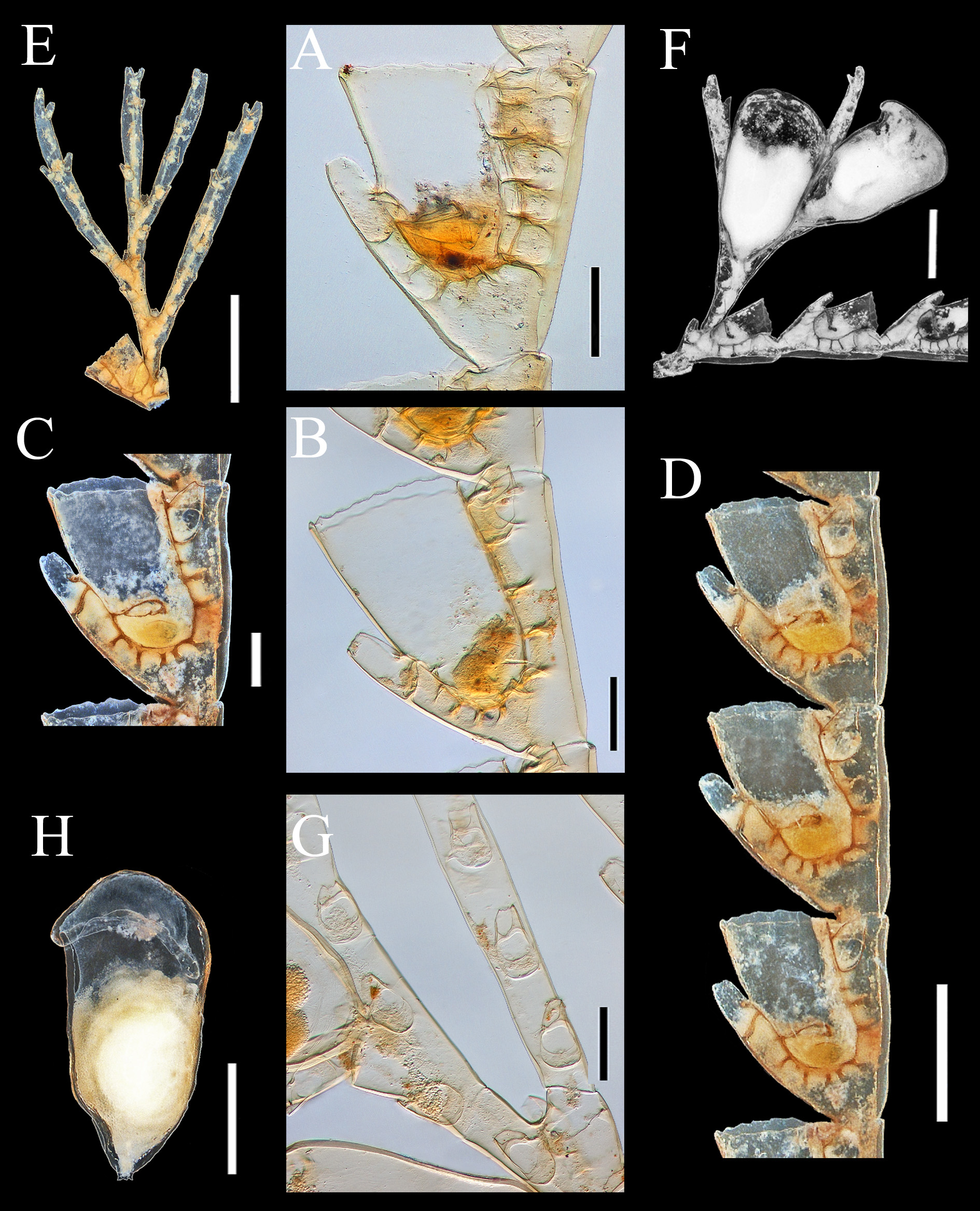
Hydrocladia abundant over entire colony, up to 6.0 mm long, given off at acute angles from apophyses of main tube, formed by up to 12 short internodes ( Figure 6 View FIGURE 6 A). All internodes thecate, separated by more or less transverse nodes provided with 4–7 internal septa. Internodes with one hydrotheca and three one-chambered nematothecae. Hydrothecae widening distally, 45–65 % adnate to internode and 45–35 % free, strongly bent outwardly; perisarc thin, no intrathecal ridge. Margin with 7–11 pointed cusps, most commonly 10, not uniform in shape and size; typically with one mesial abaxillar, one mesial adcaulinar (sometimes doubled), and 4 symmetric pairs on both sides of hydrotheca; unpaired mesial abaxial cusp slightly tilted inwardly; first pair of adcaulinar ones longer than the others. Nematothecae include one mesial proximal, distant from hydrotheca, and two distal laterals ( Figure 6 View FIGURE 6 C, F). Mesial with wide aperture and crenulated margin; laterals 58–51 % adnate to internode and hydrotheca and 42– 49 % free, with aperture adcaulinar, slightly tapering distally; margin crenulated.
Most thecate internodes provided with long, segmented branches carrying nematothecae (ramuli), arising on abcauline side of hydrothecae between the insertion of mesial nematotheca and the hydrothecal base ( Figure 6 View FIGURE 6 A, B, G). Ramuli 1587–1132 µm long, 48–65 µm wide proximally, and 28–30 µm distally, arching over the hydrothecae, gradually tapering distally; sometimes branched. Each ramulus jointed, with a short basal segment followed by 2–4 longer ones. Basal segment with no internal septa and no nematothecae, the others with 1–2 nematothecae aligned vertically on abcauline side and numerous septa, giving ramuli a chambered aspect. Distal end of ramulus with a nematotheca, with growing point slightly below ( Figure 6 View FIGURE 6 I). Nematothecae of ramulus more or less tubular, slightly tapering distally, with two apertures: one rounded, terminal, with crenulated margin, and another one basal, ovoid, with smooth margin. Ramuli sometimes bearing a hydrotheca on one of their segments, hydrotheca similar to those from hydrocladia (only one hydrotheca per ramulus observed); if a hydrotheca is formed, a new ramulus arises from the space between the mesial nematotheca and the hydrothecal base.
Gonothecae ovoid, arising singly from the distal end of apophyses of main tube of stem and branches, one per internode. Pedicel short and thin, aperture almost round, wide, distal, lateral. Sex indeterminable.
Remarks. Broch (1903) described Halicornaria pluma as a new species, his hydroid closely resembling the present species, but having more ramified colonies and more numerous phylactocarps. These were present on all hydrocladia, and had a single hydrotheca. In our material, we observed both types of colonies, some having a few ramified ramuli with one hydrotheca, as described in H. pluma . The colony from Stn. NEREIDA0710 RD88 is the largest specimen of the species reported to date ( 4.2 cm in height).
As a rare and interesting species, N. ramuliferus has been mentioned infrequently in the literature. It is clearly distinct from other known aglaopheniids from the study area. Of the five species discussed here, it has the smallest hydrothecae. They are also distinctive in shape. Each is strongly bent forwards (up to 90º), with the aperture tilted abaxially and with a significant part of the adcauline side free. Its most striking feature, however, is the presence of segmented appendages with nematothecae (and sometimes also with a few hydrothecae) ( Figure 6 View FIGURE 6 A, B, G). These appendages occur on all colonies collected, including those without gonothecae. Each of these ramuli is long and jointed, and given off somewhat laterally (as with phylactocarps of most Cladocarpus species) between hydrothecal base and mesial nematotheca from almost all the thecate internodes of the hydrocladia. The appendages are strongly septate and they arch over the hydrothecae. Their structure clearly resembles a phylactocarp, and they have been regarded as such by Schuchert (2001).
Confusion exists over the generic identity of this species because of the presence of hydrothecae on the ramuli. Moreover, uncertainty persists about the nature and function of the latter. It is unclear whether or not they are protective appendages for gonothecae. Originally assigned by Allman (1874) to the new genus Halicornaria (not Halicornaria Hincks, 1865 ), this hydroid has subsequently been referred to Nematocarpus (a monotypic genus containing Halicornaria ramulifera ) by Broch (1918), to Cladocarpus by Schuchert (2001) and Bouillon et al. (2006), and tentatively to Aglaophenopsis by Bogle (1975) and Calder (1997a). Halicornaria Allman, 1874 is an invalid junior homonym of Halicornaria Hincks, 1865 , while Halicornaria auct. is a junior synonym of Gymnangium Hincks, 1874 , a genus in which gonangia lack protective organs and appendages with nematothecae. In Nematocarpus , the gonothecae occur on apophyses of the main tube and are said to be unprotected, although appendages with nematothecae, arising between the mesial nematotheca and the hydrothecal base, occur on almost all thecate internodes. In Aglaophenopsis and Cladocarpus , the gonothecae are borne on the stem, branches, and/or hydrocladia, and are protected by phylactocarps.
Inclusion of this species in Halicornaria and Nematocarpus shows that neither Allman (1874) nor Broch (1918), respectively, regarded the ramuli as protective organs for gonangia, a point of view held also by Leloup (1932). According to the latter author, ramuli were secondary hydrocladia having a different function from the phylactocarps of Cladocarpus and Aglaophenopsis . Moreover, the diagnosis of Nematocarpus by Broch (1918) clearly states that “gonangia are not surrounded by any protective organs”. In describing Halicornaria pluma (= H. ramulifera ) earlier, Broch (1903) noted that a new genus should be erected for this peculiar species, and he did so later on (Broch 1918, as Nematocarpus ).
Other authors, however, have considered the ramuli as protective organs and have rejected Aglaophenopsis , Nematocarpus , or both, and merged them into Cladocarpus (Stechow 1913: 26; Bedot 1921; Bogle 1975; Millard 1975; Bouillon 1985; Schuchert 2001; Bouillon et al. 2006). Their function remains obscure. Vervoort (1966) stated that even in the presence of phylactocarps, the limitations of Cladocarpus from Aglaophenopsis and Nematocarpus are far from clear. Calder (1997a) and Ramil & Vervoort (2004) for instance, regarded Nematocarpus as congeneric with Aglaophenopsis and distinct from Cladocarpus . Their opinion was based on the supposition that ramuli were phylactocarps occurring on several internodes.
In our opinion, ramuli of this species are not solely protective organs for the gonothecae. They function instead, in part or in whole, as a protection for the hydranths. Moreover, the structure of phylactocarps of Cladocarpus differs from those of Aglaophenopsis (see Bogle 1975) and from ramuli of Nematocarpus . Thus, in our opinion Allman’s Halicornaria ramulifera belongs to a genus other than Cladocarpus , either Aglaophenopsis or Nematocarpus ( Table 8). We offer five reasons to support our conclusion: i) all the colonies examined had many ramuli, but not all bore gonothecae ( Figure 6 View FIGURE 6 A), the presence of the former not being necessarily related to the presence of the latter; ii) ramuli occur on almost all of the thecate internodes of the colony, rather than only the proximalmost ones, as in Aglaophenopsis and Cladocarpus (with one phylactocarp per hydrocladium); iii) the gonothecae are borne on the apophyses of the main tube that support hydrocladia, and not on the ramuli; iv) the ramuli are always curved over the hydrothecae (with the nematothecae occurring in a row on the outer, convex side) and not over the gonothecae ( Figure 6 View FIGURE 6 B); v) the ramuli are strongly septate, with a chambered structure. As for point ii), see discussion below on Cladocarpella multiseptata Bale, 1915 .
Gonothecae Phylactocarps/ramuli
Aglaophenopsis On apophyses of main tube Borne only on proximalmost cormidia; they are either a modified mesial
and/or on phylactocarp nematophore (or a modification of one of the funnels when the nematotheca is bifid), or arise laterally from the hydrothecal base; hydrothecae always present. Internodes of phylactocarp strongly septate. Protection of gonothecae.
Cladocarpus On apophyses of main tube Borne only on proximalmost cormidia, between the mesial nematotheca
and/or on phylactocarp and the hydrothecal base. Protection of gonothecae.
Nematocarpus On apophyses of main Borne on almost all thecate internodes of hydrocladia, between mesial tube, never directly on the nematotheca and hydrothecal base; they can develop into secondary ramuli hydrocladia; hydrothecae sometimes present. Internodes of ramuli strongly septate, giving them a chambered structure. Doubtfully protecting gonothecae; certainly protecting the hydranths.
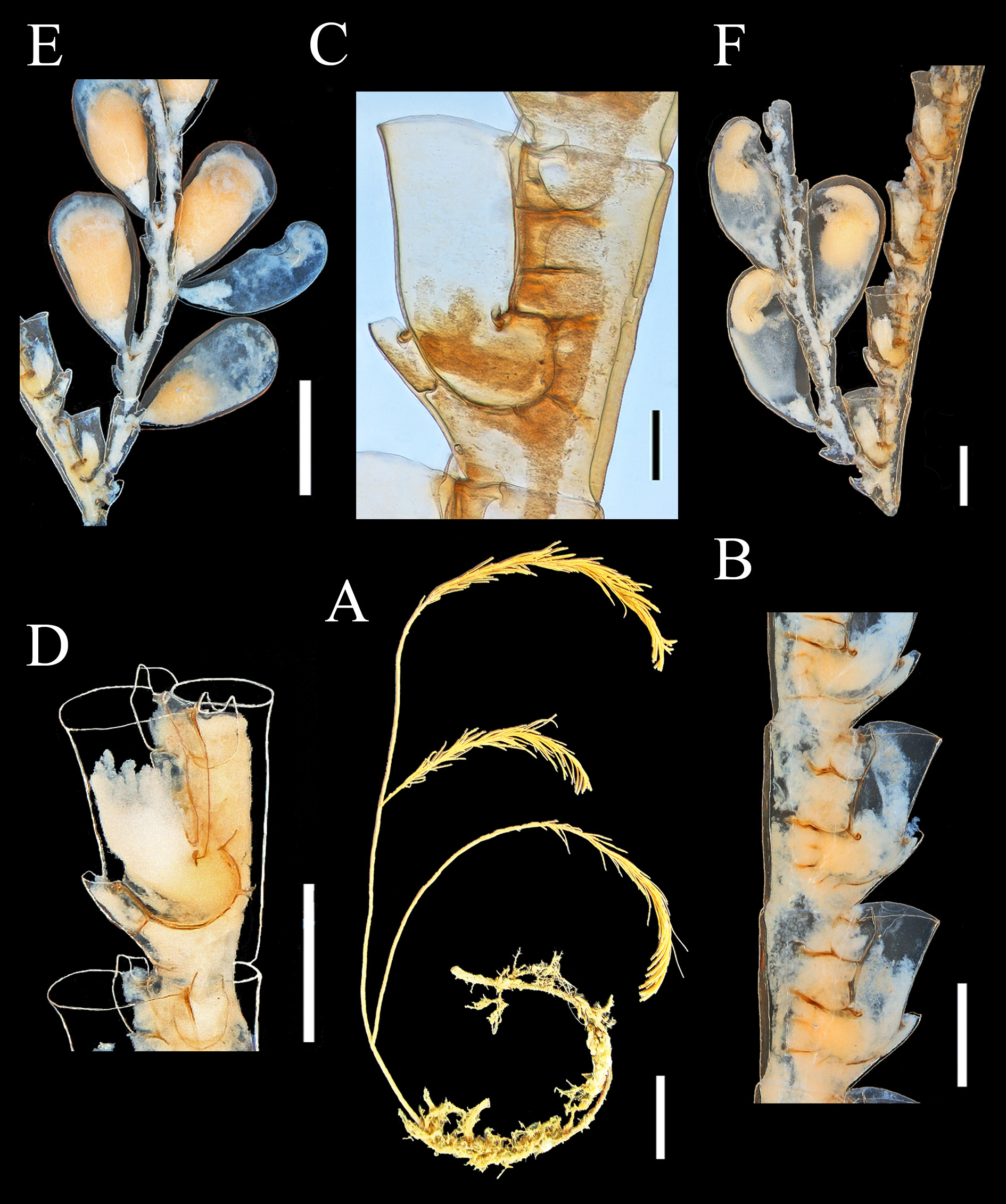
In Aglaophenopsis , protective structures (phylactocarps) occur only on the proximalmost internodes of hydrocladia, are strongly septate, and bear hydrothecae ( Figure 2 View FIGURE 2 C). In the type species, Aglaophenopsis hirsuta Fewkes, 1881 (but not in all species currently assigned to the genus), they are a modification of the mesial nematophore, replacing it (Fewkes 1881; Bale 1886) ( Table 8). In Cladocarpus , the phylactocarp is not so strongly septate, lacks hydrothecae, and does not replace the mesial nematophore; instead, it is an appendage originating generally in the space between the mesial nematotheca and the hydrothecal base. In both genera, gonothecae arise both from apophyses of the main tube and from the phylactocarps.
In Nematocarpus , protective structures are strongly septate and may have hydrothecae on the ramuli, as in Aglaophenopsis . However, these structures commonly occur on all of the thecate internodes (only the basalmost of which afford significant protection to gonothecae), are borne between the mesial nematotheca and the hydrotheca (i.e., they do not replace the mesial nematotheca), and the gonothecae never arise from the ramuli. For these reasons, we recognize Nematocarpus as a valid monotypic genus, distinct from Aglaophenopsis and Cladocarpus , with N. ramuliferus as its only known species.
Appendages similar to these ramuli occur in the genus Calvinia Nutting, 1900 . A genus included in Halopterididae Millard, 1962 by Bouillon et al. (2006), it is poorly known and not possible, at present, to determine its systematic position (see Peña Cantero et al. 2010). These appendages were considered as protective structures for the hydrothecae (hydranths) by Nutting (1900) and Fraser (1944), and are present on all of the thecate internodes of a cladium. The gonothecae are borne only on the first internode of the hydrocladia, “springing from the side of the proximal nematophorous branch” (Nutting 1900). According to Nutting, his newly described species― Calvinia mirabilis Nutting, 1900 ―was the only “Eleutheroplean” that “has produced a nematophorous branch for the protection of each hydrotheca”. In both C. mirabilis and Nematocarpus ramuliferus , these protective structures occur on most, if not all, thecate internodes of the hydrocladia. The genus Calvinia was accepted as valid by Bouillon et al. (2006) based on the development of these structures, and they were used as a discriminatory character in their identification key to genera of Halopterididae . Recognition of Nematocarpus as a distinct genus appears warranted on the same grounds.
Cladocarpella Bale, 1915 , synonymized with Cladocarpus by Billard (1918), likewise has appendages with nematothecae on more than one internode per hydrocladium. According to Billard, the genus fully agrees with the diagnosis of Cladocarpus by Allman (1874) in that protective branches are appendages from the hydrocladia. However, Cladocarpella multiseptata was moved into Streptocaulus Allman, 1883 by Ramil & Vervoort (2008) due to the structure of the phylactocarps. Fertile colonies of Streptocaulus multiseptatus from the Bay of Biscay (Avilés Canyon System, north of Spain, INDEMARES 2010 project, stn. RD01, 06º06.8150’W – 43º52.000’W, 266 m depth, unpublished data), have phylactocarps on 2–3 thecate internodes of the same hydrocladium, that arise between the mesial nematotheca and the hydrothecal base, and not only on the proximal thecate internode. Unlike Nematocarpus ramuliferus and Calvinia mirabilis , all phylactocarps have gonothecae, and nematothecae are paired and opposite.
Observed depth range: 605–1091 m, according to data from the literature.
Fertile material. Collected in June 2009 ( 846–859 m depth), and June–July 2010 ( 676–1091 m depth).
Distribution. Thought to be a visitor to northern European waters from warmer and deeper parts of the Atlantic Ocean (Broch 1918; Kramp 1938). It is a rare species, known previously only from a few stations in the northeastern North Atlantic. Records exist from east and west of the Faroes and Shetland Islands (Broch 1903, 1026 m), Iceland (Broch 1918, ca. 260 m; Kramp 1938, 260 m; Schuchert 2001, 196– 1508 m), and now the Flemish Cap and slope of the Grand Banks (present study). Present records constitute the first report of the species in the western Atlantic, and they extend its known latitudinal range further to the south.
Discussion
The discovery of five aglaopheniid species off the east coast of Newfoundland, two of them not recorded previously in the western North Atlantic, is noteworthy. Aglaopheniids are predominantly a warm-water group of hydroids (Calder 1997a), and their diversity is low in cold Atlantic waters (Fraser 1944). Only eight species are known previously in the region from 40ºN (New York Bight) northwards to the Arctic Ocean in northeastern North America (Table 1), and most of those have been reported infrequently. However, biodiversity of the deep-water fauna in this region is comparatively less well known than at equivalent latitudes in the northeast Atlantic. For example, 12 species of aglaopheniids (11 of Cladocarpus Allman, 1874 and one of Lytocarpia Kirchenpauer, 1872 ), were reported by Schuchert (2001) from waters around Iceland, and a greater number are known from 40ºN northwards in European waters (Cornelius 1995; Altuna 2007). Based on current knowledge, species richness in the family therefore appears to be higher at a given latitude in the northeastern than in the northwestern Atlantic. The disparity in species richness, particularly at shallow depths, may be due in part to differences in sea temperatures at a given latitude in North America and Europe. However, there has also been unequal sampling effort and taxonomic work in the two regions. For instance, Kramp (1943, table 2), listed six species from the west coast of Greenland, while only three were known previously from eastern Canada.
Aglaophenopsis cornuta , Cladocarpus formosus , and Cladocarpus integer are quite widely distributed (although infrequently reported) from the Davis Strait and Baffin Bay regions southwards at least to Newfoundland. In contrast, Cladocarpus diana and Nematocarpus ramuliferus are new records for the northwestern North Atlantic. Both occur on the Flemish Cap, a location where all species dealt with in this paper were recorded ( Table 11 View TABLE 11 ). The Flemish Cap is a plateau of approximately 200 km width, with depths of less than 150 m at its center. Situated eastward of the Grand Banks of Newfoundland, it is separated from those banks by the approximately 1200 m deep Flemish Pass ( Figure 1 View FIGURE 1 ). The Flemish Cap is located within an area of transition between cold subpolar waters and is influenced by fluctuations in the Labrador Current and in the North Atlantic Current (Gil et al. 2004). Compared with the Grand Banks, the Flemish Cap supports higher primary and secondary productivity due to its hydrodynamic conditions (Maillet et al. 2005). The mix of different currents in the area (Gil et al. 2004), a highly oxygenated environment rich in nutrients (Stein 2007), and greater substratum heterogeneity on the Flemish Cap compared with adjacent areas may explain differences in its faunal composition. It is a poorly studied area, and one in which rare and seldom-recorded species of other animal groups, such as deepsea corals (Murillo et al. 2011), are not uncommon. According to those authors, species exist on the Flemish Cap that are not usually recorded in deep-waters off the Canadian coast, and species richness is higher compared with more extensive areas immediately to the west. Such faunal differences probably also exist in the hydroid fauna, but no investigations have been undertaken to support this assumption.
As noted in the Introduction, genera of Aglaopheniidae are differentiated mainly on the basis of gonosomal characters. Of eight genera recognized in the family worldwide by Bouillon et al. (2006), Cladocarpus is second in terms of diversity, with 66 species of mostly deep-water forms. Two approaches have been adopted in the taxonomy of this group of aglaopheniids. One of these, adopted by Bouillon et al. (2006) and others, is to recognize a broadly defined genus Cladocarpus , treating it essentially as a collective group until questions of relationships become clearer. The second approach is to refer Cladocarpus -like hydroids to several genera, distinguishing them largely by differences in morphology of their phylactocarps that are thought to reflect relationships. Following this approach, Ramil & Vervoort (1992) distinguished between species having a phylactocarp homologous to the axis ( Cladocarpus s.l.) from those in which the rachis is similar structurally to the hydrocladia ( Streptocaulus ). Their proposals have not been widely accepted because of the existence of intermediate material with respect to this character (see Schuchert 2001). Given the present limited knowledge, actual relationships thus remain obscure. Phylogeny of the group should become clearer as cladistic studies and molecular analyses are undertaken. Particular attention in such investigations must be given to the type species of the various putative genera. Whichever taxonomic approach is followed now, revisions in classification and nomenclature can be expected, although we maintain that fewer changes will be likely to take place when the second alternative is adopted. We therefore support recognizing various genera within this group based on available evidence, rather than simply assigning a large and diverse assemblage of species to an excessively broad Cladocarpus . The genus is already species-rich, and adding many dissimilar taxa to it overextends its scope and renders it more difficult to conceptualize and refine. In our opinion, several nominal genera included in the synonymy of Cladocarpus sensu lato will almost certainly be recovered as valid based on differences in structure of the phylactocarp. In any case, reproductive structures in species of Cladocarpus , as conceived by Bouillon et al. (2006), are heterogeneous. All five species recorded herein were assigned to the same genus ( Cladocarpus ) in that work, notwithstanding significant variation in morphology of their gonosomes (and in one case colony structure). We conclude that the five species are referable to three genera, viz. Cladocarpus , Aglaophenopsis , and Nematocarpus .
The taxonomy of Cladocarpus formosus is unmistakable, as it is the type species of the genus. Cladocarpus diana is also referable to Cladocarpus based on characters of its reproductive structures. The generic identity of Cladocarpus integer is less clear. Its phylactocarp is an unbranched structure and as such is dissimilar to that of C. formosus . Still, species with both branched and unbranched phylactocarps are currently assigned to Cladocarpus , and we include C. integer in that genus for the time being.
The status of Nematocarpus , and the generic affinities of its sole species N. ramuliferus , are unclear. Nematocarpus is closely related to Aglaophenopsis , and the two were regarded as congeneric by Calder (1997a). Hydrothecae and ramuli/phylactocarps of Aglaophenopsis hirsuta Fewkes, 1881 ( type species of Aglaophenopsis ) and Nematocarpus ramuliferus (Allman, 1874) ( type species of Nematocarpus ) are quite similar, as apparent in illustrations of A. hirsuta by Vervoort (1972: 206) and N. ramuliferus by Broch (1918: 76) and Schuchert (2001: 144). Gonothecae and overall colony forms of the two are also similar. However, ramuli of N. ramuliferus are not restricted to the proximalmost cormidia, as occurs with the phylactocarps of Aglaophenopsis and Cladocarpus , and the gonothecae are borne only on the apophyses of the main tube of stem and branches, and not on the ramuli. Hence, ramuli do not appear to function primarily as reproductive structures protecting the gonothecae, as is the case with phylactocarps of A. hirsuta . Hydroids belonging to the genus Calvinia (Halopterididae) are recognized as distinct based on possession of such accessory tubules, and the same character provides justification for recognition of Nematocarpus . Thus, Nematocarpus is upheld here as a valid genus, containing its type species, N. ramuliferus .
Some seven species have been assigned to Aglaophenopsis , with six of them occurring in the North Atlantic (Table 10). The phylactocarp in the type species, A. hirsuta Fewkes, 1881 , arises in place of the mesial nematotheca (Fewkes 1881). Vervoort (1972) noted that it was borne on one of the funnels when the nematotheca is bifid. This difference was clearly stated by Bale (1886), who noted that “the phylactocarps of Aglaophenopsis are modified from the mesial nematophore of the proximal hydrothecae, while in Cladocarpus they are independent structures, and the genus must rest on this distinction”. The emended diagnosis of the genus by Fraser (1944) asserts that the gonosome is “protected by phylactogonia that appear to be modified mesial nematophores”. However, Bogle (1975) did not mention phylactocarps as modified mesial nematothecae in her diagnosis of the genus, stating: “phylactogonia arising singly from the proximal internode of unmodified hydrocladia from an area to the side and slightly above the mesial nematotheca”. In fact, not all species referred to Aglaophenopsis have modified mesial nematothecae (see Table 10). Bogle emphasized the presence of hydrothecae on the phylactocarps as a distinction from Cladocarpus in her key to genera, and proposed other characters for differentiation. Accordingly, we are uncertain about the correct generic assignment of Cladocarpus cornutus Verrill, 1879 (and Aglaophenopsis bonnevieae , which we regard as congeneric with A. cornuta ). While both species are usually referred to Aglaophenopsis , we question whether they are congeneric with A. hirsuta , type species of that genus, because their phylactocarps are different. In both A. cornuta and A. bonnevieae they are forked (‘Y-shaped’, or even alternately branched in large colonies of A. bonnevieae ) and consistently hydrothecate, usually throughout (e.g., see Kramp 1932a: 59; Schuchert 2001: 138) ( Figure 2 View FIGURE 2 C). Instead, those of A. hirsuta (see Nutting 1900: pl. 29, fig. 12; Vervoort 1972: 206) are unbranched, and hydrothecae are of sporadic occurrence. Gonothecae also seem to differ, especially those of A. cornuta , apertures of which are lateral and oval in shape, rather than terminal and circular as in A. hirsuta . These two species ( A. cornuta and A. bonnevieae ) do not correspond readily to any of the described genera in the tribe Cladocarpini Calder, 1997a, and a case could be made for a new genus to accommodate these two. For now, however, we have taken a conservative approach and have retained them in Aglaophenopsis .
In Cladocarpus formosus , type species of the genus, the mesial nematotheca on thecate internodes is almost entirely adnate to the abcaulinar wall of the hydrotheca except on the first internode, where there is a distinct gap between distal end of nematotheca and hydrothecal base ( Figure 4 View FIGURE 4 F). Phylactocarps arise from this area. The same morphology is seen in Cladocarpus integer ( Figure 5 View FIGURE 5 E, F) and Cladocarpus diana , with the mesial nematotheca being in a different position on the first thecate internode than on the others. In C. diana , however, the gap is not so apparent, with the phylactocarp arising clearly from the lateral side ( Figure 3 View FIGURE 3 E). In Aglaophenopsis cornuta , the mesial nematotheca of the first cormidium is adnate to the hydrotheca, but it is displaced to the side as seen in frontal view. In all four of these species, the position of the mesial nematotheca with respect to the hydrotheca is different on the proximalmost internode of the hydrocladium than on the rest. In Nematocarpus ramuliferus , however, there is no such difference. Phylactocarps occur only on the first thecate internode in Cladocarpus s.l., and a gap exists between the mesial nematotheca and hydrotheca on this internode, even in species having nematothecae that are adnate to hydrothecae on other hydrocladial internodes. This difference also occurs in sterile colonies.
Gonothecae in A. cornuta and C. formosus occur on both apophyses (1–2 gonothecae) and on the phylactocarp. In C. diana , they arise from the phylactocarp. Gonothecae in hydroids of C. integer examined here were borne on the phylactocarp, although colonies with gonothecae on the stem, but lacking phylactocarps, are known in the species (Broch 1918). In N. ramuliferus , gonothecae occur only on apophyses of the main tube. Notably, colonies of A. cornuta and C. formosus with gonothecae, but with no phylactocarps, were observed in our material. Such colonies have also been reported in Cladocarpus campanulatus Ritchie, 1912 (Schuchert 2001) . This suggests that the absence of phylactocarps in fertile material is probably much more common within the Cladocarpini than previously thought.
The most abundant species in our collections was C. formosus . Cladocarpus integer was found at the shallowest depth ( 119 m), while the deepest recorded species were A. cornuta and C. formosus ( 1885 m) . The latter two species also had the widest bathymetric range ( 1645 m).
Hydroids of exceptionally large size were collected in some of the species (Table 9). The largest colony, a specimen of C. integer , was 78.0 cm high. The largest ones of C. formosus and A. cornuta were 53.5 cm and 28.7 cm high, respectively. Cladocarpus diana and N. ramuliferus are small hydroids not exceeding 7.0 cm in height.
Of particular research interest, the habitat provided by several deep-water anthozoans has been emphasized only recently. The question arises whether large hydroid colonies, such as those found here, might also provide important substrate and shelter. However, sessile epibionts and vagile invertebrates were scarce amongst the colonies examined. Observed associates included other hydroids, alcyoniids, brachiopods, barnacles, bryozoans, and caprellid amphipods. Such a limited number of associates suggests that these hydroids may have effective antifouling defenses.
Identification key to aglaopheniid hydroids reported from the western North Atlantic between 40ºN and Baffin Bay, including western Greenland
1 Gonothecae protected by a closed corbula formed by modified hydrocladia bearing nematothecae and hydrothecae.......................................................................................... Lytocarpia myriophyllum View in CoL
- Gonothecae solitary, protected by unbranched or branched phylactocarps or unprotected............................. 2
2 Colonies with a segmented appendage having nematothecae (ramulus) that arches over the hydrothecae on abaxial side of most hydrocladial internodes; aperture of hydrothecae strongly tilted towards abaxial side........... Nematocarpus ramuliferus View in CoL
- Colonies with or without a segmented appendage having nematothecae (phylactocarps) only on first proximal thecate internode (fertile specimens); aperture of hydrothecae not strongly tilted towards abcauline side.......................... 3
3 Phylactocarp strongly septate, forked (Y-shaped) or alternately branched (without secondary branching), with 1–2 terminal hydrothecae or having a series of hydrothecae............................................................... 4
- Phylactocarp not strongly septate, unbranched or dichotomously branched (antler-shaped), sometimes also with secondary branching, without hydrothecae......................................................................... 6
4 Hydrothecae with a strong and pointed median keel of perisarc arising above mesial nematotheca, largely surpassing level of hydrothecal rim; mesial nematotheca large, extending 1/3–1/2 up hydrotheca...................................... 5
- No median keel present; mesial nematotheca short, separate from hydrotheca or only slightly adnate to it................................................................................................. Aglaophenopsis verrilli View in CoL
5 Hydrotheca cone-shaped, notably wider distally, with no intrathecal septum; hydrothecal rim cusped; phylactocarp forked (Yshaped) with one terminal hydrotheca on each branch...................................... Aglaophenopsis cornuta View in CoL
- Hydrotheca of uniform width, with an intrathecal septum; hydrothecal rim slightly crenulated; phylactocarp alternately branched, each branch with a series of hydrothecae........................ Aglaophenopsis bonnevieae View in CoL (SW Greenland)
6 Hydrothecae bulging deeply into lumen of hydrocladium; hydrothecal rim even; phylactocarp unbranched, sometimes absent in fertile colonies...................................................................... Cladocarpus integer View in CoL
- Hydrothecae not bulging deeply into lumen of hydrocladium; hydrothecal rim undulated to decidedly cusped; phylactocarp branched; if unbranched, hydrothecae campanulate........................................................... 7
7 Hydrothecal rim with deep cusps; mesial nematothecae on thecate internodes very broad, sometimes notched in middle, not tapering distally in front view, with a wide aperture and distal end extending only 1/10–1/11 along hydrotheca.................................................................................................. Cladocarpus diana View in CoL
- Hydrothecal rim undulated or with shallow cusps; mesial nematothecae not as above.............................. 8
8 Mesial nematotheca extending 1/2–4/5 up hydrotheca; hydrotheca normally with two slightly prominent mesial outer cusps on abcauline side; intrathecal septum short to well developed, arising from abcauline wall of hydrotheca, frequently curved backwards; mature gonotheca forming a distinct hood overarching the opening; aperture lateral, narrowly oval................................................................................................... Cladocarpus formosus View in CoL
- Mesial nematotheca free from hydrotheca or only reaching hydrothecal base; hydrotheca with or without one abcauline cusp; intrathecal septum not well formed or absent, although an annular ridge along hydrothecal wall may occur; gonotheca without a hood; aperture terminal or subterminal, broadly oval....................................................... 9
9 Hydrothecae campanulate, with annular ridge at lower third; mesial nematotheca reaching hydrothecal base, opening into lumen of segment at adcauline side; phylactocarp poorly developed, unbranched..................................................................................................... Cladocarpus campanulatus View in CoL (SW Greenland)
- Hydrothecae elongate, rather distant, with no intrathecal septum; characteristic strong median abcauline cusp; mesial inferior nematotheca free from hydrotheca or nearly so, not opening into lumen; phylactocarp well developed, dichotomously branched, antler-shaped.................................................................. Cladocarpus flexilis View in CoL
TABLE 11. List of species, depth interval (m), depth range (m), and number of stations at which each species was collected in the three sampling areas. FC, Flemish Cap; FP, Flemish Pass; GB, Grand Banks. Depth range is the difference in meters between the shallowest and deepest records. The coldest bottom temperature (– 0.42 ºC), recorded with a collection of Cladocarpus integer, was from the shallowest collection site (on the Grand Banks).
| Species | FC | FP | GB | Depth interval | Depth range | Bottom temperature ºC | Gonosome |
|---|---|---|---|---|---|---|---|
| Aglaophenopsis cornuta | 14 | 19 | 18 | 240–1885 | 1645 | 2.68–4.42 | May to August |
| Cladocarpus diana | 13 | -- | -- | 600–1575 | 975 | 3.40–3.95 | June, July |
| Cladocarpus formosus | 26 | 15 | 24 | 240–1885 | 1645 | 2.68–4.12 | May to August |
| Cladocarpus integer | 6 | 3 | 15 | 119–1339 | 1220 | –0.42–4.11 | June to August |
| Nematocarpus ramuliferus | 2 | 2 | 1 | 605–1091 | 486 | 3.50–4.01 | June, July |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |