Molossops temminckii ( Burmeister, 1854 )
|
publication ID |
https://doi.org/ 10.1093/mspecies/sez006 |
|
publication LSID |
lsid:zoobank.org:pub:4829AAC6-98EC-4889-AAC4-9225DD2D034B |
|
persistent identifier |
https://treatment.plazi.org/id/03B187C0-1E43-F606-5E83-01743378FD12 |
|
treatment provided by |
Felipe |
|
scientific name |
Molossops temminckii ( Burmeister, 1854 ) |
| status |
|
Molossops temminckii ( Burmeister, 1854) View in CoL
Dwarf Dog-faced Bat
D [ysopes]. Temminckii Lund, 1842:128 . Nomen nudum.
Dysopes temminckii Burmeister, 1854:72 View in CoL . Type locality “Lagoa Santa,” Minas Gerais, Brazil.
[ Molossus (Molossops) View in CoL ]. Temminckii: Peters, 1866:575 View in CoL . Subgeneric description and name combination.
Molossus View in CoL [Myopterus] temminckii: Dobson, 1876:707 View in CoL . Name combination.
Molossus hirtipes Winge, 1892:17 . Type locality “Lagoa Santa,” Minas Gerais, Brazil.
[ Molossus (Myopterus) ] Temminckii: Trouessart, 1897:142 View in CoL . Name combination.
Molossops temminckii: Miller, 1907:248 View in CoL . Name combination.
Molossops temminckii sylvia Thomas, 1924:234 View in CoL . Type locality “Goya,” Corrientes, Argentina; may be a valid subspecies.
Molossops temminckii griseiventer Sanborn, 1941:385 View in CoL . Type locality “Espinal, west of Magdalena River on the plains of Tolima, Colombia;” may be a valid subspecies.
Molossops temminckii temminckii: Sanborn, 1941:386 View in CoL . Name combination.
Molossops View in CoL [( Molossops View in CoL )] temminckii View in CoL griseiventer: Cabrera, 1958:117. Name combination.
Molossops View in CoL [( Molossops View in CoL )] temminckii View in CoL sylvia: Cabrera, 1958:117. Name combination.
Molossops View in CoL [( Molossops View in CoL )] temminckii View in CoL temminckii: Cabrera, 1958:118 View in CoL . Name combination.
Molossops teminckii: Mares, Willig, Streilein, and Lacher, 1981b:113 . Incorrect subsequent spelling of Dysopes temminckii Burmeister, 1854 View in CoL .
Molossops temninckii: Coimbra, Borges, Guerra, and Mello, 1982:35 . Incorrect subsequent spelling of Dysopes temminckii Burmeister, 1854 View in CoL .
CONTEXT AND CONTENT. Context as for genus. Three subspecies have been assigned to Molossops temminckii View in CoL : M. t. griseiventer, M. t. temminckii View in CoL , and M. t. sylvia ( Thomas 1924; Cabrera 1930; Sanborn 1941; Tamsitt and Valdivieso 1963; Ximénez 1969; Vizotto and Taddei 1976; Gardner 1977; Anderson et al. 1982; Myers and Wetzel 1983; Ibáñez and Ochoa 1985; Simmons 2005). Although Cabrera (1958) recognized a fourth subspecies, M. t. mattogrossensis, most authors treated this taxon as a species of Neoplatynomps and more recent evidence shows that it effectively belongs to the genus Neoplatymops View in CoL ( Freeman 1981a; Eger 2008). A morphological study of M. temminckii View in CoL from Argentina, Bolivia, and Paraguay revealed variation consistent with the range expected for the species ( Barquez et al. 1999). Based on this evidence, Barquez et al. (1999) synonymized M. t. sylvia with M. t. temminckii View in CoL ; M. t. griseiventer needs further review.According to Eger (2008), the boundaries among these subspecies are not adequately established and they were not recognized because of conflicting evidence on geographic variation. Synonymies follow Eger (2008).
DIAGNOSIS
Molossops temminckii ( Fig. 1 View Fig ) differs from species of Cynomops by: the presence of triangular ears separated by a wide space, first and second phalanxes of digit IV similar in length, nostrils surrounded by small pointed warts, and one lower incisor in each ramus. In contrast, Cynomops has rounded ears, the second phalange of digit IV is about one-third the length of the first one, nostrils smooth, and two lower incisors in each ramus ( Barquez et al. 1999; Gregorin and Taddei 2002; Eger 2008; Díaz et al. 2016). Additional characters of M. temminckii include a relatively large tragus (at least one-half the size of the antitragus) and a short and wide antitragus (wider than high and notched posteriorly), a last upper molar with three commissures clearly marked, a complex last lower molar with two notable cusps, and lacrimal furrows that are less developed ( Barquez et al. 1999; Eger 2008). In contrast, Cynomops has a relatively small tragus (less than a one-third the size of the antitragus) and an antitragus that is about as high as wide, a last upper molar with two commissures, a simple last lower molar with one cusp, and lacrimal furrows that are strongly developed ( Barquez et al. 1999; Eger 2008). The characters that separate M. temminckii from the rufous dog-faced bat M. neglectus are: forearm less than 34 mm (versus> 36 mm M. neglectus ), postorbital constriction less than 4 mm (> 4.5 mm M. neglectus ), condylobasal length less than 15 mm (> 15.1 mm M. neglectus ), and general pale or dark gray coloration (versus reddish coloration— Barquez et al. 1999; Eger 2008; Díaz et al. 2016).
GENERAL CHARACTERS
Molossops temminckii is a small molossid bat with widely separated triangular ears, a small and triangular tragus, and a wide and slightly posteriorly inclined antitragus. The snout is elongated, flat, wide, and blunt, with a slightly prominent tip and an obtuse projection between the nasal orifices. Lips are smooth and bordered by a fine fringe of hook-shaped hairs, and a tuft of bristles below the nostrils ( Burmeister 1854; Dobson 1878; Barquez et al. 1999). The posterior margin of the dorsal side of the forearm has sparse hairs reaching halfway to the fifth metacarpal and to the union of the fourth and fifth digits. The thumb is small, but has a well-developed pad at its base. The wings attach at the midpoint of the tibia, and the calcar is well developed, extending more than one-half the distance between the tail and the foot ( Barquez et al. 1999).
The color of dorsal pelage in the holotype is chestnut brown at the tips and yellow at the base. Ventrally, pelage coloration is similar but paler. The integument and membranes are dark throughout ( Burmeister 1854; Dobson 1878). In Argentina and Paraguay, the pelage is variable in color, with specimens from forests being darker than those from arid zones (Myers and Wetzel 1983; Barquez et al. 1999). The dorsal coloration of the pelage varies from dark brown, with shades of gray, to light brown, and chocolate with lighter base hairs that are cream or white, and a pale venter with shades of gray ( Barquez et al. 1999; Díaz and Barquez 2002; Fabián and Gregorin 2007; Siles 2007). In Colombia and Venezuela, Ibáñez and Ochoa (1985) noted the presence of two size and pelage combinations: 1) larger specimens with longer fur, dorsally dark brown with black shades; tricolored ventral hairs with a white base, a middle grayish-brown band, and the distal half portion white, giving these bats a hoary appearance; and 2) smaller specimens, dorsally dark brown and ventrally grayish brown without a hoary appearance, and tricolored ventral hairs with a white base and a middle brown band and cream tip. Myers and Wetzel (1983) also found two distinct groups based on external appearance in Paraguay; the first consisting of smaller specimens with a light brown dorsum, and a tan venter; and the second consisting of larger specimens with a blackish dorsum and venter.
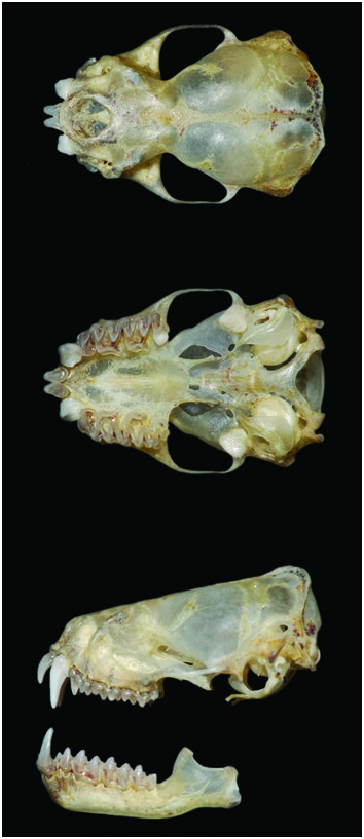
The skull of M. temminckii is flattened ( Fig. 2 View Fig ), with a slight elevation from the tip of the nasals to the back of the braincase; it is small but robust, widened at the mastoidal processes and anteriorly at the lacrimal furrows. The postorbital constriction is well marked; the lacrimal canals are deep; the sagittal crest is present but low, although it is more elevated posteriorly where it unites with the lambdoidal crests. The tympanic bullae are small; and the basisphenoidal pits are not differentiated ( Freeman 1981a; Anderson 1997; Barquez et al. 1999; Díaz and Barquez 2002; Fabián and Gregorin 2007). This species exhibits sexual dimorphism with females smaller than males (Myers and Wetzel 1983; Ibáñez and Ochoa 1985; Fabián and Gregorin 2007). Sexual dimorphism is more prominent in the skull than in the wing, and is more evident for measures of cranial breadth than cranial length (Myers and Wetzel 1983).
Ranges of external measurements (mm) of specimens from Argentina ( Barquez et al. 1999) were: total length, 60–84 (n = 33); tail length, 21–34 (n = 32); hindfoot length, 4.5–7 (n = 28); ear length, 10–15.3 (n = 33); forearm length, 28.9–32.5 (n = 45). Range of body masses (g) was 5–8 (n = 25— Barquez et al. 1999). In Bolivia, Anderson (1997) cited the following ranges of the external measurements (mm; n = 9): total length, 68–81; tail length, 19–25; length of hind foot, 5–8; ear length, 9.5–12; forearm length, 29–31; and body mass, 5–7 g (n = 3). Gregorin and Taddei (2002) reported a range of forearm length of 27.0– 33.5 mm, and range of body mass of 5–7 g for individuals from Brazil. Nunes et al. (2013) cited the following mean (and ranges) of body mass (g) and external measures (mm) for three females and three males, respectively, from Brazil: body mass, 6.83 (6.0–8.0), 7.67 (7.0–8.0); total length, 77.33 (74.0–84.0), 74.00 (72.0–76.0); body length, 53.67 (50.0–59.0), 51.67 (50.0–53.0); tail length, 23.67 (22.0–25.0), 22.33 (20.0–26.0); length of hind foot, 6.40 (5.7–7.0), 4.93 (4.2–6.0); ear length, 13.50 (12.5–14.0), 11.80 (11.4–12.0); forearm length, 32.00 (31.0–33.3), 31.37 (31.1–31.6). Means (and ranges) of external measurements (mm), given by Myers and Wetzel (1983) for 50 females and 48 males, respectively, from Argentina, Bolivia, and Paraguay, were: forearm length, 29.7 (28.5–31.0), 30.0 (27.8–32.8); length of third metacarpal, 31.4 (30.1–33.1), 32.1 (28.7–33.7); total length, 72.3 (61–85), 73.8 (66–79); tail length, 22.9 (14–29), 26.0 (20–33); length of hind foot, 7.5 (6–9), 7.6 (5–9); ear length, 13.1 (10–15), 13.0 (10–15). Ibáñez and Ochoa (1985) reported ranges of forearm length (mm) for females and males, respectively, from four countries: Brazil: 29.5–31.2 (n = 3), 30.0–30.8 (n = 3); Colombia: 30.3–31.9 (n = 3), 31.0– 32.5 (n = 2); Paraguay: 28.0–30.5 (n = 5), 29.1–31.9 (n = 9); and Venezuela: 28.3–31.3 (n = 18), 29.0–31.6 (n = 21).
Ranges of skull measurements (mm, n in parenthesis) of specimens from Argentina ( Barquez et al. 1999) were: greatest length of skull, 12.7–14.1 (40); condylobasal length, 12.1– 13.8 (42); least interorbital breadth, 5.0–6.3 (47); postorbital constriction, 3.4–4.1 (46); breadth of braincase, 6.8–7.6 (40); mastoidal breadth, 7.7–9.3 (38); palatal length, 5.5–6.4 (37); length of maxillary toothrow, 4.8–5.7 (47); length of mandibular toothrow, 5.4–6.3 (41); length of mandible, 9.5–10.8 (37); width across canines, 3.4–4.1 (44); width across molars, 6.0–6.8 (45). Gregorin and Taddei (2002) mentioned a range of greatest length of skull of 12.5–18.0 mm for specimens from Brazil. Means (and ranges) of skull measurements (mm) for three females and three males, respectively, from Brazil ( Nunes et al. 2013) were: maximum length of skull, 13.47 (13.2–13.7), 13.73 (13.4–14.2); condylobasal length, 13.07 (12.8–13.4), 13.23 (12.7–13.8); mastoidal breadth, 8.67 (8.3–9.1), 8.37 (7.8–9.0); zygomatic breadth, 9.30 (n = 1), 9.37 (9.1–9.6); breadth of braincase, 7.03 (6.8–7.3), 7.37 (7.2–7.5); postorbital constriction, 3.70 (3.6–3.9), 3.90 (3.8–4.0); palate length, 6.13 (6.0–6.2), 6.50 (6.4–6.7); palate breadth, 3.30 (3.1–3.6), 3.67 (3.6–3.7); length of maxillary toothrow, 5.23 (5.1–5.5), 5.43 (5.2–5.9); length of mandible, 10.13 (9.9–10.4), 10.60 (10.1–11.1); breadth across upper canine, 3.43 (3.4–3.5), 3.53 (3.2–4.0); breadth across upper molars, 6.50 (6.4–6.7), 6.73 (6.4–7.4); coronoid process height, 2.17 (1.8–2.4), 2.47 (2.3–2.7). Means (and ranges) of skull measurements (mm) for 50 females and 48 males, respectively, from Argentina, Bolivia, and Paraguay (Myers and Wetzel 1983) were: greatest length of skull, 12.9 (12.1–13.6), 13.3 (12.0–14.0); condylobasal length, 12.5 (12.1–13.0), 12.9 (12.3–13.2); greatest breadth across zygoma, 8.6 (8.1–9.4), 9.0 (8.2–9.9); least interorbital constriction, 3.6 (3.2–4.1), 3.7 (3.3–4.1); greatest breadth across mastoid processes, 8.1 (7.7– 9.0), 8.5 (7.8–9.4); breadth of palate and molars, 6.2 (5.8–6.8), 6.5 (5.8–7.1); length of maxillary toothrow, 5.1 (4.6–5.9), 5.3 (4.7–5.8); breadth across the labial cingula of the canines, 3.6 (3.3–3.9), 3.9 (3.3–4.2).
Ranges of skull measurements (mm, n in parenthesis) of females and males, respectively, from four countries (Ibáñez and Ochoa 1985) were: 1) Brazil: greatest length of skull, 13.5–13.8 (n = 3), 13.9–14.1 (n = 3); condylobasal length, 13.0–13.4 (n = 3), 13.5–13.6 (n = 3); lacrimal width, 5.5–6.0 (n = 3), 5.6–5.9 (n = 3); postorbital width, 3.5–3.7 (n = 3), 3.7–3.9 (n = 3); breadth at mastoids, 8.0–8.7 (n = 3), 8.0–8.6 (n = 3); lower toothrow, 5.0–5.0 (n = 3), 5.0–5.2 (n = 3); width at upper molars, 6.1–6.5 (n = 3), 6.4–6.7 (n = 3). 2) Colombia: greatest length of skull, 13.5–14.0 (n = 2), 14.1–14.8 (n = 3); condylobasal length, 13.0–13.4 (n = 2), 13.7–14.4 (n = 3); lacrimal width, 5.3–6.1 (n = 3), 5.9–6.6 (n = 3); postorbital width, 4.1–4.4 (n = 3), 4.1–4.2 (n = 3); breadth at mastoids, 8.4–8.5 (n = 2), 8.7–9.0 (n = 2); lower toothrow, 5.3–5.7 (n = 3), 5.5– 5.9 (n = 3); width at upper molars, 6.1–6.6 (n = 3), 6.4–6.6 (n = 3). 3) Paraguay: greatest length of skull, 13.0–13.8 (n = 3), 13.7–14.4 (n = 7); condylobasal length, 12.8–13.5 (n = 3), 13.1– 14.0 (n = 6); lacrimal width, 5.5–6.3 (n = 5), 5.7–6.6 (n = 9); postorbital width, 3.6–4.0 (n = 5), 3.6–4.1 (n = 9); zygomatic breadth, no measurement for females, 8.6–8.9 (n = 4); breadth at mastoids, 7.9–8.5 (n = 2), 8.2–9.1 (n = 7); lower toothrow, 4.8–5.2 (n = 5), 5.1–5.7 (n = 9); width at upper molars, 6.1–6.8 (n = 5), 6.5–7.0 (n = 9). 4) Venezuela: greatest length of skull, 12.6–13.6 (n = 13), 13.1–14.1 (n = 21); condylobasal length, 11.8–12.6 (n = 10), 12.6–13.5 (n = 21); lacrimal width, 5.3–6.3 (n = 14), 5.8–6.8 (n = 21); postorbital width, 3.6–3.9 (n = 14), 3.7–4.1 (n = 21); zygomatic breadth, 8.6–9.1 (n = 9), 8.6–9.7 (n = 16); breadth at mastoids, 7.4–8.1 (n = 10), 8.0–9.1 (n = 20); lower toothrow, 4.8–5.4 (n = 16), 5.0–5.7 (n = 21); width at upper molars, 5.9–6.5 (n = 14), 6.0–6.9 (n = 21).
Cranial measurements of two (unless otherwise noted) females from Bolivia ( Anderson 1997) were: condylobasal length, 13.1–13.3; maxillary length, 5.0–5.1; breadth at canines, 3.6–3.8; dental span, 6.4–6.6; molar width, 1.6–1.7; zygomatic breadth, 8.9–9.1; lambdoidal breadth, 8.2 (n = 1); breadth of braincase, 7.0–7.3; depth of skull, 4.8.
DISTRIBUTION
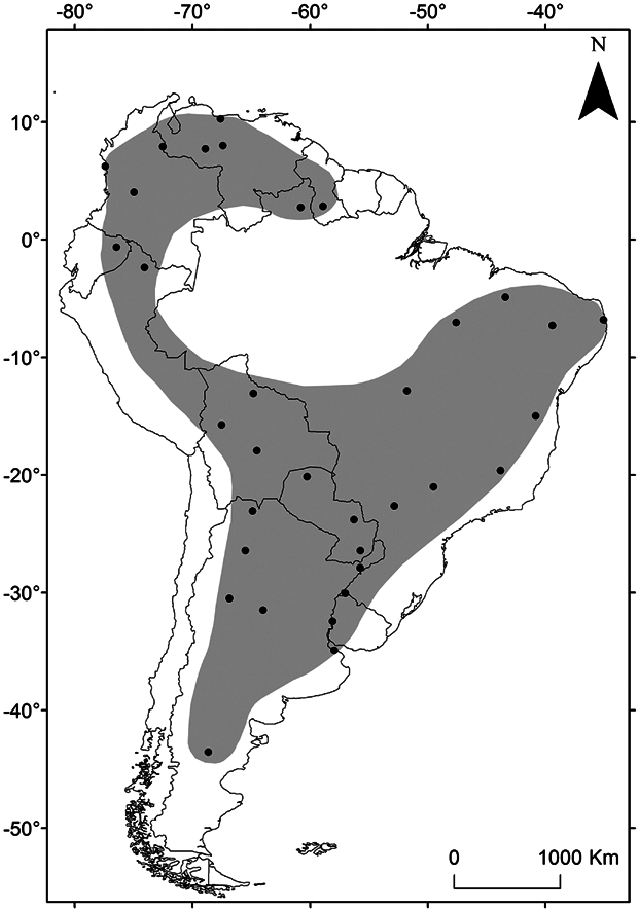
Molossops temminckii is restricted to South America; it is found from Colombia, Venezuela, and Guyana, southwestward through Ecuador, Peru, Bolivia, Paraguay, and Brazil, into western Uruguay, and Argentina ( Fig. 3 View Fig ; Barquez 1999; Simmons 2005; Eger 2008; Díaz et al. 2016). A single record of M. temminckii is known from Guyana ( Eger 2008). In Venezuela, this species is listed for the states of Apure, Aragua, Barinas, Carabobo, Cojedes, Guárico, Miranda, and Portuguesa ( Eisenberg et al. 1979; Ibáñez 1981; Ochoa and Bisbal 1982; Ibáñez and Ochoa 1985; Eger 2008). In Colombia, M. temminckii is found in the departments of Arauca (see Solari et al. 2013), Bolivar (Cuartas-Calle and Muñoz- Arango 1999), Chocó ( Eger 2008), Córdoba (see Solari et al. 2013), Cundinamarca ( Sanborn 1941; Tamsitt and Valdivieso 1963; Alberico et al. 2000), Huila (Pérez-Torres et al. 2007), Meta ( Gardner 1977; Ibáñez and Ochoa 1985), Norte de Santander ( Eger 2008), Santander (see Solari et al. 2013), Tolima ( Sanborn 1941; Tamsitt and Valdivieso 1963; Ibáñez and Ochoa 1985; Eger 2008; Ramírez Fráncel et al. 2015), and Vichada (see Solari et al. 2013). In Ecuador, it is known only from the province of Orellana ( Reid et al. 2000). Tuttle (1970), Koopman (1978), and Eger (2008) listed this species for the Peruvian departments of Loreto and Pasco. In Brazil, the species is widely distributed and it has been recorded in the states of Amazonas ( Bernard et al. 2011), Bahia ( Falcão et al. 2005), Ceará ( Mares et al. 1981b; Willig 1983), Distrito Federal (Bredt and Uieda 1996), Goiás ( Vieira 1942; Fracasso and Salles 2005; Zortéa and Alho 2008), Maranhão (Olímpio Massa et al. 2014; Gamboa Alurralde et al. 2016). In Uruguay, M. temminckii is known only from the departments of Artigas ( Ximénez 1969), Paysandú (Williams and Genoways 1980), and Río Negro (González and Botto 2009). The species is typically found in low altitude zones; the highest record is at 770 m in Colombia (see Solari et al. 2013).
FOSSIL RECORD
The fossil record of the Molossinae dates from the late Oligocene to the Pleistocene of South America ( Czaplewski 1996, 1997; Czaplewski and Cartelle 1998; Czaplewski et al. 2003). In a molecular analysis of the family Molossidae, Ammerman et al. (2012) suggested a Paleocene origin for the family with a geographic split between Old and New World groups around 29 million years ago. Fossils of Molossops temminckii have been recorded in Quaternary caves in the valley of the Rio das Velhas, near Lagoa Santa, in Minas Gerais, Brazil ( Lund 1840; Winge 1892; Paula-Couto 1946; Czaplewski and Cartelle 1998).
et al. 2016), Mato Grosso ( Allen 1916; Vieira 1955; Pine et al. 1970; Schaller 1983; Silva and Anacleto 2011), Mato Grosso de Sul ( Bordignon 2006; Cunha et al. 2009; Cunha et al. 2011), Minas Gerais ( Eger 2008; Tavares et al. 2010), Paraíba ( Nunes et al. 2013), Paraná (Miretzki and Margarido 1999), Pernambuco ( Willig 1983), Piauí ( Gregorin et al. 2008), Roraima ( Capaverde Junior et al. 2014), São Paulo (Vizotto and Taddei 1976; Reis et al. 1996), and Tocantins ( Nunes et al. 2005; Gregorin et al. 2011). In Bolivia, M. temminckii is known from the departments of Beni, La Paz, Pando, Santa Cruz, and Tarija ( Anderson 1997; Miserendino et al. 1998; Siles et al. 2003; Siles 2007). In Paraguay, this species is found in the departments of Alto Paraguay, Alto Paraná, Amambay, Boquerón, Canindeyú, Central, Concepción, Cordillera, Itapuá, Misiones, Ñeembucú, Paraguarí, Presidente Hayes, and San Pedro ( Cabrera 1958; Myers and Wetzel 1983; López- González 1998, 2005). In Argentina, it has a wide distribution that includes the provinces of Buenos Aires, Catamarca, Córdoba, Corrientes, Chaco, Chubut, Entre Ríos, Formosa, Jujuy, La Rioja, Misiones, Salta, Santa Fe, Santiago del Estero, and Tucumán ( Barquez et al. 1999; Barquez and Díaz 2009;
FORM AND FUNCTION
The dental formula is i 1/1, c 1/1, p 1/2, m 3/3, total 26. The downward curving upper incisors are procumbent and in contact at the base. The canines have a well-developed and extended cingulum that forms a platform on the lingual side. The P1 is well developed with a distinct protocone. M2 is slightly larger than M1; their cusps form the typical W-shape, and both have a distinct protocone and an obsolete hypocone. M3 has three commissures, the third being small, and with a well-developed protocone and absent hypocone. The pair of markedly bifid lower incisors is separated from the canines by a small space. The canines are well developed, and have the internal margin of the cingulum expanded posteriorly and forming a platform that meets the anterior margin of m1. The premolars are small and triangular in dorsal view; p2 is larger than p1 and both are flattened anteroposteriorly and have only a single cusp. The molars are well developed, and decrease in size from m1 to m3; their cusps are high and sharp. The third lower molar has a bicuspid talonid, that is the hypoconid and the entoconid. This distinguishes Molossops temminckii from members of Cynomops , which have a single cusp on the talonid ( Barquez et al. 1999; Fabián and Gregorin 2007; Eger 2008; Díaz et al. 2016).
The tongue is relatively short, as in other molossid bats. Its root-to-apex length ranges from 45% to 55% of the total length of the skull, and has a prominent median-dorsal lobe with a basal groove ( Gregorin 2003). This species has two types of gustative papillae, vallate and fungiform, and three mechanical ones: basal, scale-like, and posteromedian conical. The fungiform papillae are globose and fleshy, and proportionally larger than in other molossid taxa ( Gregorin 2003).
The hair in M. temminckii has coronal cuticular scales in which a single scale extends around the entire hair shaft. The distal scale margin has an irregular dentate pattern, with deep V-shaped incisions. The proximal one-third of hair shaft is narrow, widening from the middle to the distal one-third, with a gradual tapering at tip. The cuticle and cortex are lightly pigmented in its proximal one-half and moderately so in its distal one-half. The hair of M. temminckii has an average width of 7.87 μ m (scale index 1.09), and an average length of 4.84 mm (van Staaden and Jones 1997).
Males of M. temminckii have a well-developed gular gland restricted to the midline of the neck that is used to mark members of their colony with a distinctive odor (Gregorin and Cirranello 2016). This gland is less developed or absent in females (Gregorin and Cirranello 2016). The males of this species showed testicular epididymis complexes located within the abdominal cavity and dorsally arranged below the kidneys ( Beguelini et al. 2013). The testes are elongated and the epididymis is small and formed by a long and highly convoluted tubule divided into three main regions: caput, the initial portion that is juxtaposed with the confluence of the rete testes network; corpus, the medial part of the epididymis; and cauda, the end portion that communicates directly with the deferens ducts. M. temminckii has a low gonad mass and high Gonad-Somatic Index because of its low body mass ( Beguelini et al. 2013). Testicular regression was observed in this species; however, sperm retention (storage) was not observed ( Beguelini et al. 2013).
The glans penis in M. temminckii is small, stout, and oval in cross section at its base, distally rounded, and without a baculum. On the ventral surface, paired lateral ridges extend the length of the glans. A ventromedial ridge extends between these lateral ridges and continues beyond their distal margin. The urinary meatus is surrounded ventrally by this ventromedial ridge and dorsolaterally by a semicircular rim of tissue. Internally, the corpora cavernosa reach only to the midpoint of the glans. The corpus spongiosum and urethra course through the ventromedial ridge. Spines are present on the glans penis and absent from the rim of the urinary meatus ( Ryan 1991).
Mean values of white cell counts for M. temminckii show 47% neutrophils, 40% lymphocytes, 8% monocytes, 5% eosinophils, and 0% basophils (Valdivieso and Tamsitt 1971; Villalba-Alemán and Muñoz-Romo 2016). Menke (2007) described in detail the hyolaringeal region in M. temminckii , including the cartilages and muscles.
ONTOGENY AND REPRODUCTION
Despite limited data on reproduction of Molossops temminckii, Breviglieri and Uieda (2014) hypothesized that this species is polyestrous. They inferred that this reproductive strategy may reflect a regional pattern probably related to local environmental conditions such as seasonality, rainfall, and temperature.
In Venezuela, pregnant and lactating females as well as juveniles were reported in July (Ibáñez and Ochoa 1985). In Brazil, pregnant females were captured in Caatinga in September, while in the Cerrado, pregnant and lactating females were reported in December ( Willig 1985). The reproductive period in southeast Brazil ranges from June to September (Vizotto and Taddei 1976; Gargaglioni et al. 1998) and extends into October in the Tocantins State (Gonçalves and Gregorin 2004). Breviglieri and Uieda (2014) captured a male with abdominal testes in May and another one in February with descended testes in São Paulo State, Brazil; they recaptured the latter specimen in June with abdominal testicles. In Bolivia, two pregnant females, each with a single fetus, were reported in September ( Anderson 1997). For the Bolivian Chaco, juveniles were reported in February, April, and September, lactating females in September, and scrotal males in February, September, and October ( Siles 2007). In Argentina, pregnant females were reported in October and November from Buenos Aires and Salta Provinces; scrotal males were reported in May and October in Formosa and Tucumán Provinces, respectively ( Mares et al. 1981a; Barquez et al. 1999).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Molossops temminckii ( Burmeister, 1854 )
| Gamboa Alurralde, Santiago & Díaz, M Mónica 2019 |
Molossops teminckii:
| MARES, M. A. & WILLIG, K. E. & STREILEIN & AND T. E. & LACHER, Jr. 1981: 113 |
Molossops
| CABRERA, A. 1958: 117 |
Molossops
| CABRERA, A. 1958: 117 |
Molossops
| CABRERA, A. 1958: 118 |
Molossops temminckii griseiventer
| SANBORN, C. C. 1941: 385 |
Molossops temminckii temminckii:
| SANBORN, C. C. 1941: 386 |
Molossops temminckii sylvia
| THOMAS, O. 1924: 234 |
Molossops temminckii: Miller, 1907:248
| MILLER, G. S., Jr. 1907: 248 |
Molossus (Myopterus)
| TROUESSART, E. L. 1897: 142 |
Molossus hirtipes
| WINGE, H. 1892: 17 |
Molossus
| DOBSON, G. E. 1876: 707 |
Molossus (Molossops)
| PETERS, W. 1866: 575 |
Dysopes temminckii
| BURMEISTER, H. 1854: 72 |