Victoria boliviana Magdalena and L. T. Sm., 2022
|
publication ID |
https://doi.org/ 10.3389/fpls.2022.883151 |
|
DOI |
https://doi.org/10.5281/zenodo.7576648 |
|
persistent identifier |
https://treatment.plazi.org/id/03B287DB-FFFD-FFC6-AD77-FBEC491FFDC7 |
|
treatment provided by |
Juliana |
|
scientific name |
Victoria boliviana Magdalena and L. T. Sm. |
| status |
|
Victoria boliviana Magdalena and L. T. Sm. View in CoL , sp. nov.
Type: Bolivia, Beni Department, Provincia Ballivían, subiendo el Río Yacuma desde Puerto Espíritu, laguna en conexíon al Río Yacuma, unos 20 m al N, 29 Mar. 1988, S. G. Beck 15173 (holotype: LPB; isotype: K ( K000798309 ) .
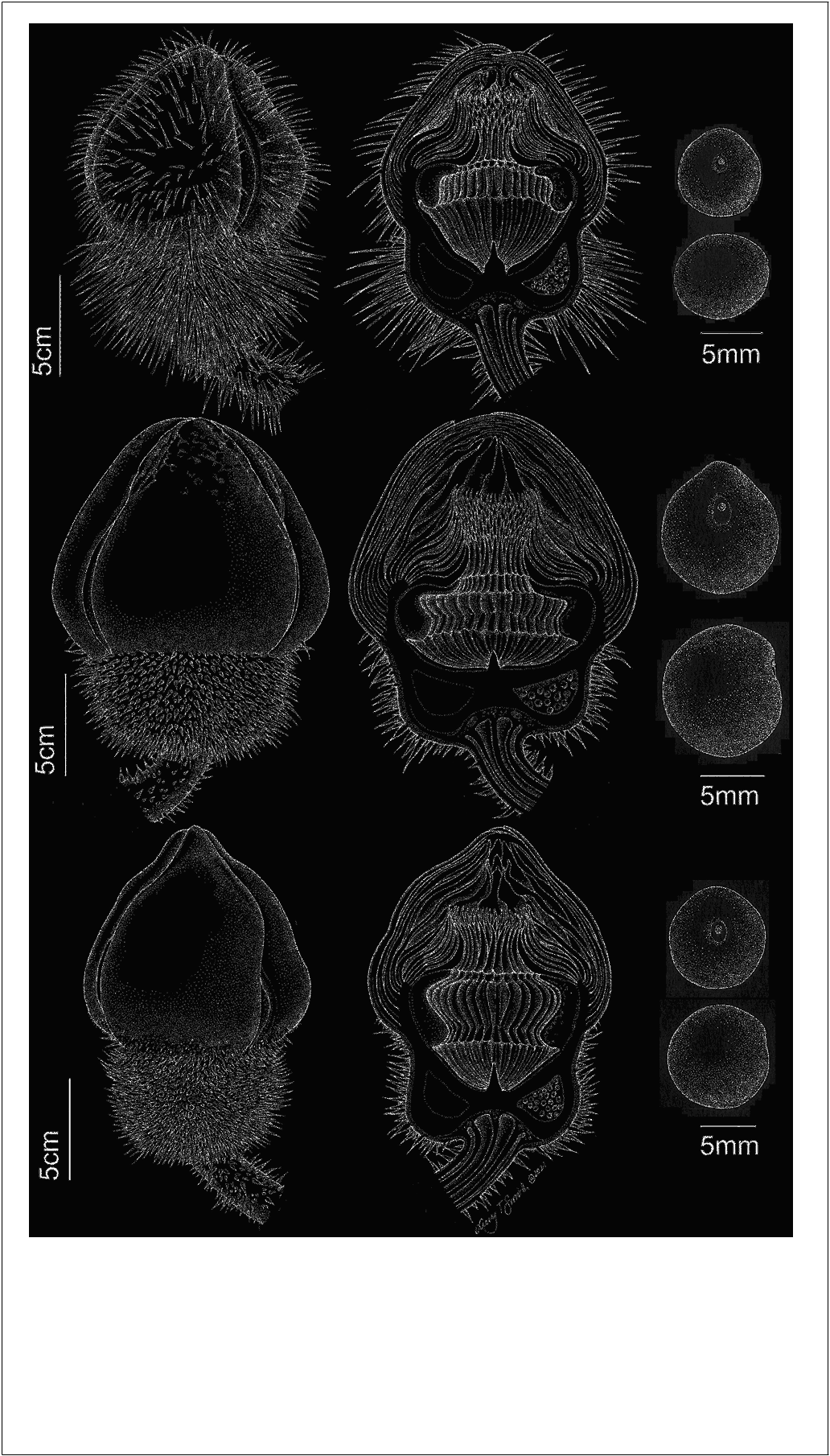
Vernacular names: Reina Victoria , Victoria regia . Figures 1B View FIGURE 1 , 3D–F View FIGURE 3 , 5 View FIGURE 5 , 10 View FIGURE 10 .
Most similar to V. cruziana Orb. , from which it can be distinguished by the lower rim of the floating leaf, convex apex of the flower bud, length of the upper part of the carpellary appendages exceeding that of the lower part and the larger seeds. The V. boliviana plastid genome differs from that of other Victoria species by a 14 bp insertion between plastid genes ndhC and trnV in the large single copy region (LSC), a 5 bp deletion between trnK and rps16, a 7 bp deletion adjacent to trnC in the LSC and a 42b p deletion in the CDS of gene ycf1, within the SSC. Finally, a 4 bp transversion unique to V. boliviana sp. nov. was found inthe LSC.
Leaves up to 3.2 m broad, adaxial surface of lamina green; abaxial surface of lamina dark green, maroon or dark-blue, radial and reticulate ribs yellow or green; leaf margins upturned to form a moderate rim c. 4–7% of leaf length, rim recurving strongly over blade surface at base and curving inwards or flared outwards at top, abaxial surface of rim deep maroon or very pale green/white in color, glabrous or with hairs, where present 1.2–3 mm, simple, multicellular, 6–15 segmented. Flower bud broadly ovoid, convex at apex, up to 36 cm in diameter at second-night anthesis. Ovary 8–10 cm diameter, outer surface covered in prickles, 1–10 mm (dried) glabrous; prickles abruptly tapering from c. half of length to sharp apex; inner surface of ovary with shallowly concave stigmatic surface, oblong in longitudinal profile, ridged with lines corresponding with 25–36 radially arranged locules, each containing 8–14 ovules, 2–2.5 mm diameter (fresh). Outer tepals 4, 10–15 × 8–10 cm when fresh, abaxial surface predominantly green, or tinged maroon, prickles absent or present, where present up to 10 per tepal, prickles tapering abruptly at their midpoint to a sharp point, 1–10 (dried), distributed irregularly over entire surface, glabrous. Inner tepals 6–15 × 1.5–9 mm (fresh), innermost remaining white or turning pale pink at their base at the second-night anthesis; outer staminodia> 50,3– 4 × 0.5– cm, thick, rigid, apiculate; stamens, 4–5 × 0.5–1 cm; inner staminodia 4– 5 × 0.5–0.7 base of lower parts of carpellary appendage angular in shape and arising at 45 degree angle from stigmatic surface, length of upper parts exceeding that of lower parts. Flower at first night of anthesis, inner tepals white, outer staminodia tipped blue-violet; second night anthesis, inner tepal adaxial surface pink, inner tepals pale pink at base, white or pink towards the apex, outer staminodia dark pink for basal twothirds of their length, white then violet towards the apex, inner staminodia pink at base. Seeds c. 300 per fruit, 12–13 × 16– 17 mm, globose with a prominent raphe (especially when dry), dark brown to black, surrounded by a mucilaginous aril.
Distribution and Conservation status Victoria boliviana Magdalena and L. T. Sm. is restricted to Bolivia and the flood plains of the Llanos de Moxos, Mamoré watershed, identified as a Centre of Plant Diversity and Endemism (Site SA24) (Beck and Moraes, 1997). These area is considered by Langstroth Plotkin (2012). Moxos is surrounded by the forests of the Upper Madeira basin and is an area of largely open vegetation – herbaceous wetlands, grasslands, savannas, and woodlands ( Beck, 1983, 1984; Langstroth Plotkin, 2012). Images (not included in the minimum calculation of the EOO or AOO) suggest that V. boliviana ’s range extends further west (natural or cultivated) to Rurrenabaque. We estimated a minimum and maximum EOO and AOO. The maximum range was based on the potential habitat across the Llanos de Moxos region (including geographical information from public unverified images) and verified herbarium collections and iNaturalist images. The minimum range was based on the coordinates of herbarium collections and iNaturalist images alone. We estimated that the EOO of V. boliviana ranges between 8,006 km 2 (minimum) and 33,151 km 2 (maximum), falling close to the thresholds of the Vulnerable category under criterion B. We estimated that the AOO of V. boliviana ranges from 32 km 2 (minimum) to 2,000 km 2 (maximum), falling between the Endangered and Vulnerable categories. There are less than five known locations for V. boliviana . We could find no information about population fragmentation but believe that V. boliviana may be vulnerable to fluctuations in flooding and drought throughout the year. For example, Beni Department has been recently affected by seasonal floods, fires and droughts due to El Niño and La Niña climate events ( Vásquez, 2015). Arecent increase in agriculture-lead deforestation has been documented along the Trinidad-Santa Cruz highway ( Langstroth Plotkin, 2012), to the south of known V. boliviana populations and satellite images from Google Earth Pro and i-Terra suggest extensive deforestation along the edges of roads ( i-Terra, 2021; NASA, 2021; GoogleEarth, 2022) which we use to infer an active decline in habitat quality.
Taking a precautionary approach and based on the small EOO and AOO, small number of locations (5), and continuing decline in habitat, we assess V. boliviana as Vulnerable (VU), according to criteria B1ab(iii)+B2ab(iii).
Notes. Victoria boliviana sp. nov. has the largest observed leaves of the three species, with laminae> 3 m in length having been observed. The abaxial surface of the upturned rim surface varies between individual plants in the same locality from dark maroon to very pale, almost “white” green, a characteristic not found in other species. Prickles on abaxial outer tepal surfaces are absent or very few, and if present are not confined to the lower portion of the outer tepals unlike V. cruziana where the smaller number of prickles are confined to the lower one-third of the abaxial tepal surface.
Victoria boliviana is the only species of Victoria whose carpellary appendages have upper portions that are longer than the lower portions. In addition, V. boliviana ’s stigmatic chambers are the shallowest of the three Victoria species. Haenke saw Victoria plants in Yacuma in 1801, during the Malaspina expedition ( Gickelhorn, 1966, p. 105; Ibañez Montoya, 1984), but no identifiable description is available and no voucher has been found. d’Orbigny (1840, p. 57) reported seeing this species on the banks of the Mamoré river in 1832 and mistakenly assigned this species to V. amazonica when publishing his description of V. cruziana .
Further investigations and surveys are required to better understand the species’ current range, population fluctuations and habitat and thus better predict the impacts of the threats identified.
Field observations of the flowers from a single population in the Llanos de Moxos suggest that whilst pollinated by beetles, V. boliviana sp. nov. flowers may host fewer individuals of pollinators than V. amazonica , only 4–10 individuals being observedintheflowersof theformer, comparedto> 20 inthe flowers of the latter. This could be due to a lower density of pollinators in their area of occupation.
Additional Material – BOLIVIA. Beni: Santa Ana del Yacuma, –65.4236, –13.74, –/6–7/1845, Bridges, T. s. n. (K); Cercado: Laguna Suarez 5 km sur de la ciudad de Trinidad, –64.864167, – 14.872222, 08/05/2019, Magdalena, C. Melgar, D. G., Salazar, C. D., Alvarez, C., Gutierrez, G., Arias, J. 154 (German Coimbra Sanz Herbarium, Jardin Botanico Municipal); Moxos, pasando el Río Mamoré, cerca al puente del Río Tijamuchi a lado del camino, –65.145278, –14.851111, 09/05/2019, Magdalena, C. Melgar, D. G., Salazar, C. D., Alvarez, C., Gutierrez, G., Arias, J.155 (Herbario German Coimbra Sanz Jardín Botanico Municipal).
| LPB |
LPB |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.