Dushia nigra ( Stimpson, 1855 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4691.4.2 |
|
publication LSID |
lsid:zoobank.org:pub:875FB4E7-148A-4AC2-904C-174416B11256 |
|
persistent identifier |
https://treatment.plazi.org/id/03B387A4-FFFA-CC37-FF73-C289FCC8FCDE |
|
treatment provided by |
Plazi |
|
scientific name |
Dushia nigra ( Stimpson, 1855 ) |
| status |
|
Dushia nigra ( Stimpson, 1855) species complex comb. nov.
( Figs 1 View FIGURE 1 B–D, 4A–F, 5A–F, 6A–F, 7A–C)
Meckelia nigra Stimpson, 1855, p. 382 View in CoL .
Meckelia rubella Stimpson, 1855, p. 382 View in CoL .
Cerebratulus niger: Stimpson 1857, p. 161 .
Serpentaria rubella: Stimpson 1857, p. 162 View in CoL .
Cerebratulus rubellus: Bürger 1904, p. 112 ; Kajihara 2017, p. 427.
Micrura formosana Yamaoka, 1939, p. 286 .
Micrura japonica Iwata, 1952, p. 139 ; Kajihara 2007, p. 301; Kajihara 2017, p. 431.
Dushia sp.: Chernyshev 2016, p. 292.
Composition. Dushia nigra species complex comb. nov. is comprised of at least two cryptic species, three color morphs (orange, brown, and black), and four nominal species ( Meckelia nigra , Meckelia rubella , Micrura formosana , and Micrura japonica ). These cryptic species cannot be separated by external or internal morphological characters. At least a part—or possibly even all—of the four nominal species are likely to be synonymous. Future DNA taxonomic studies based on materials from wider geographic areas would be necessary to reveal actual genetic diversity of this complex (see below).
Material examined. Unless otherwise mentioned, the following description is based on ICHUM 3456, collected on 14 October 2004, among sand on a rocky beach in Seragaki, Onna, Kunigami, Okinawa, Japan, 45 slides in serial transverse plane of section.
Additional material. Several specimens were collected from the beach in the vicinity of Sesoko Marine Laboratory, and in Nakijin, Okinawa, Japan, under rocks in sand in the mid- to high-tide line in July 1999. From these samples, USNM 1098170 View Materials consists of serial transverse sections of whole specimen, 29 slides ; USNM 1098171 View Materials consists of 9 slides, serial transverse sections of midbody and 9 slides in the frontal plane of midbody ; USNM 1097172 View Materials consists of 49 slides, some are frontal sections and others are transverse of a single specimen ; USNM 1107399 View Materials , serial frontal sections of anterior (4 slides) and serial transverse sections of mid-body and hind-end (36 slides) ; USNM 1011553 View Materials , one anterior end ; USNM 1011554 View Materials , single whole specimen ; USNM 1107396–1107398 View Materials , three complete specimens . ICHUM 3457 View Materials , serial transverse sections, 82 slides, collection data same as ICHUM 3456, and serial sections (some are transverse and others are longitudinal ones) deposited as ICHUM 5504–5511 View Materials , collected in Kakeroma in February 2018 and in Okinawa in May 2018, were also examined. Four unsectioned specimens, orange in color when alive, collected by Svetlana Maslakova from Pelorus Island in the Palm Island group, Queensland , and preserved in 70% ethanol, are deposited under MTQ G20038 .
Diagnosis. Heteronemertean with mostly black (but also sometimes orange or brown) body, and head with white tip on the both dorsal and ventral surface; head shape is retuse at the anterior and ending in truncate, horizontal and lateral cephalic furrows; foregut region moderately rounded, intestinal region moderately dorsoventrally flattened without sharp lateral margins; caudal cirrus present; ocelli absent.
Description. External features. The living worm is contractile, and 9 cm long, 1.5 mm wide when extended ( Fig. 1B View FIGURE 1 ). The body is uniformly black without any mottles, bands, or stripes; other specimens assume orange ( Fig. 1C View FIGURE 1 ) or brown ( Fig. 1D View FIGURE 1 ) body color. The head is slightly wider than the body, overall oblong with an anteriorly tapered point that is retuse. The posterior portion of the lateral, horizontal cephalic furrows flare slightly but are truncated. The cerebral ganglia are visible as a reddish hue at the widest portion of the head. Horizontal cephalic furrows are deep and extend on both sides of the head, reaching to the anterior end of the mouth opening. The lateral margins of the furrows are closed except at where it flares open at the tip of the snout and at the posterior margin. The tip of the head is whitish and the lateral sides of the head and the edges of the mouth are paler. The tail is rounded, possessing a white caudal cirrus.
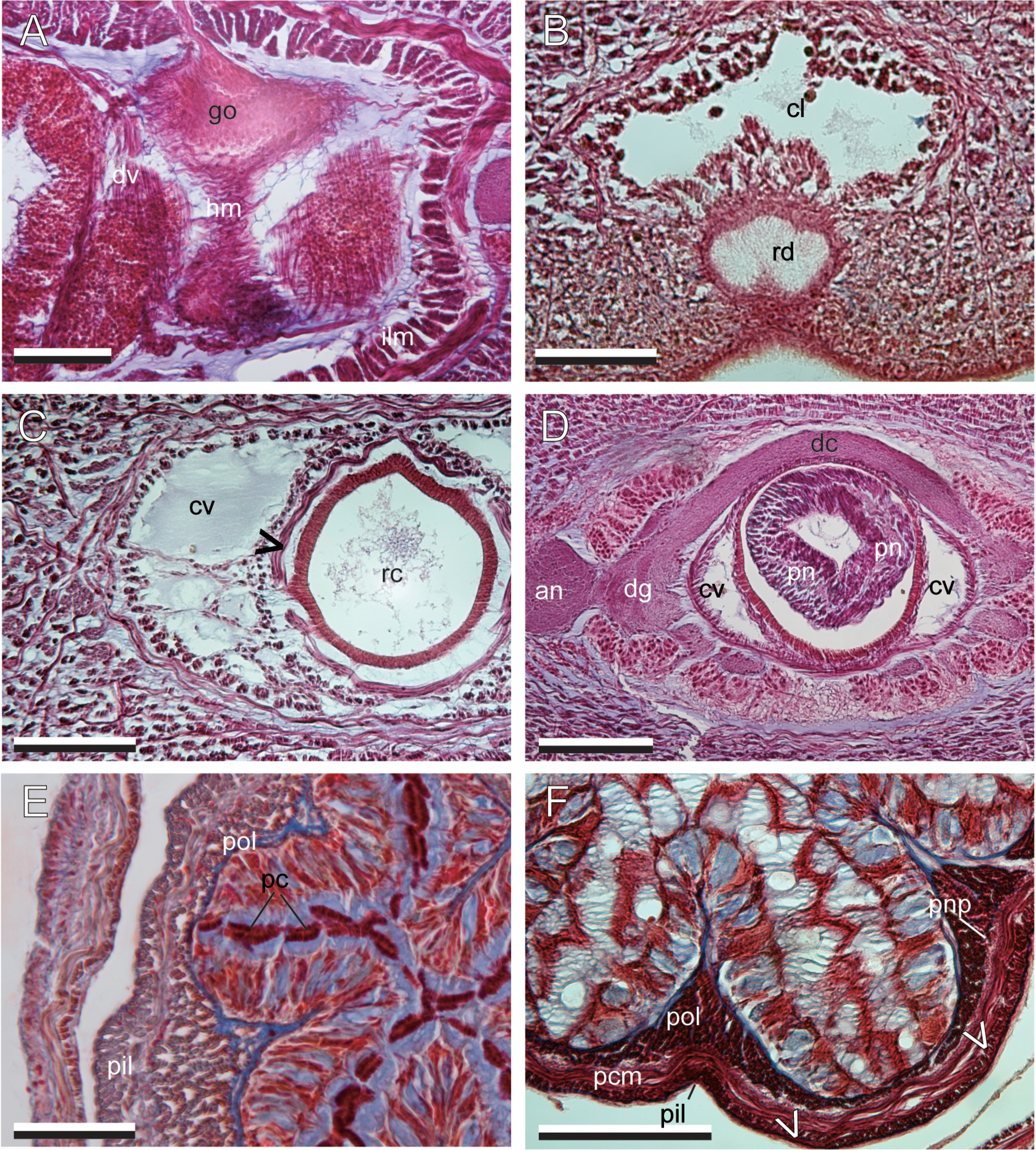
Body wall and musculature. The ciliated epidermis ( Fig. 4A View FIGURE 4 ) contains numerous red and gold to yellow goblet-shaped cells. Below the epidermis is a thin basal lamina. The basal lamina rests on cutis (= dermis), which consists of three components: from exterior inward, a thin, mostly 1–3 muscle fibers thick, diagonal muscle layer with fibers oriented as a lattice, a thin, 1–5 fibers thick, dermal longitudinal muscle layer, and a glandular layer containing acidophilic and basophilic components ( Fig. 4A, B View FIGURE 4 ). Dark pigments are non-cellular and distributed between the basement membrane of the epidermis to the mcm. The body wall below the cutis is typically three tiers of muscles with a thick layer of longitudinal muscles (olm), a thick layer of middle circular muscles (mcm) upon which the nerve cords run laterally, and a variable-sized inner longitudinal layer (ilm) ( Fig. 4C View FIGURE 4 ).
Precerebral body-wall musculature consists of three muscle layers; these are, from exterior inward, an outer longitudinal muscle layer, a middle circular muscle layer, and an inner longitudinal muscle layer ( Fig. 4D View FIGURE 4 ). The inner longitudinal muscles are peripheral to the cephalic blood vessels and rhynchodaeum.
The outer longitudinal muscles on the periphery of the brain are enclosed by connective tissues, forming discrete bundles around the brain; these bundles lead to the proboscis insertion just in front of the brain ( Fig. 4E View FIGURE 4 ). The middle circular muscle layer in the precerebral region disappears at the proboscis insertion, posteriorly becoming evident again from the posterior brain region backward. In the anterior mouth region, horizontal muscles run above the dorsal wall of buccal cavity ( Fig. 4F View FIGURE 4 ); these fibers become less distinct posteriorly and disappear in the posterior mouth region.
The foregut region of approximately eight serial sections in ICHUM 3456 had a band of mcm fibers, which separate the main trunk and looped around the lnc and insert on the opposite side into the mcm. This arrangement was pronounced on one side of the animal and consisted only of a few fibers on the opposite side. Most likely this is a mutation as it was not found in any other specimens examined and was only present in a short stretch of the worm.
The intestinal diverticula are marked by a thin layer of dv musculature. In sexually mature, gravid specimens, the dv musculature forms a wall of fibers inserting into the mcm dorsally and ventrally and is more extensively developed. It also contains several longitudinal fibers which are associated with the gonad epithelium; however, these fibers are not present in immature specimens ( Fig. 5A View FIGURE 5 ).
Proboscis apparatus. The rhynchodaeum opens subterminally and ventrally as a thin slit ( Fig. 5B View FIGURE 5 ) and a rhynchodeal sphincter is present just in front of the proboscis insertion. The rhynchodaeum is unciliated for most of the length ( Fig. 4D View FIGURE 4 ), except at its intersection with the epidermis at the anterior ( Fig. 5B View FIGURE 5 ). The musculature of the rhynchocoel consists of inner longitudinal and outer circular muscle layers ( Fig. 5C View FIGURE 5 ) that are not interwoven with the body wall muscle layers ( Fig. 6A View FIGURE 6 ).
The proboscis insertion is just anterior to the cerebral ganglia and laterally fenestrated to allow two cephalic blood vessels to pass through ( Fig. 4E View FIGURE 4 ). The proboscis is thin anteriorly and a pair of proboscis nerves is evident ( Fig. 5D View FIGURE 5 ) Posteriorly, the main part of the proboscis is stouter, with its epithelium differentiated, and the nerves forming a complete sheath ( Fig. 5E, F View FIGURE 5 ).
The main proboscis is unbranched and consists of nine layers; these are, from exterior inward when protruded, an epithelium containing acido- and basophilic glandular cells and pseudocnidae ( Fig. 5E, F View FIGURE 5 ), a thin connective tissue layer, an outer longitudinal muscle layer, a neural sheath, a circular muscle layer, a diagonal muscle layer, an inner longitudinal muscle layer, an inner circular muscle layer, and an endothelium. Two muscle crosses are present between the inner circular and the diagonal muscle layers ( Figs 5F View FIGURE 5 , 7 View FIGURE 7 A–C). The inner strands of the proboscis inner longitudinal muscle layer are different in size between the two muscle crosses ( Fig. 7B, C View FIGURE 7 ); the muscle crosses are thus unequal. There is no pre-overlapping part of the muscle cross (cf. Chernyshev 2015), and thus no outer strand of inner longitudinal muscle layer is present ( Fig. 7B, C View FIGURE 7 ).
Alimentary canal. The mouth is a short ventral slit posterior to the horizontal cephalic furrows without obvious salivary gland cells. Foregut and intestinal musculature consist of a few sparsely distributed strands of longitudinal muscle fibers; no circular muscle fibers were observed, and there is no distinct layer of muscles present. The junction of the foregut and intestine is not marked by a distinctive sphincter and no caeca are present. The anus opens dorsally at the root of the caudal cirrus.
Circulatory system. A single pre-rhynchostomal blood lacuna ( Fig. 5B View FIGURE 5 ) is divided into one mid-dorsal and two lateral vessels flanking the rhynchodaeum ( Fig. 4D View FIGURE 4 ); the mid-dorsal vessel terminates blindly anterior to the proboscis insertion. Posterior to the proboscis insertion, at the level of the brain commissures, the two lateral vessels ventrally anastomose to form a U-shaped vessel, from which a mid-dorsal vessel originates and posteriorly enters the rhynchocoel wall to form a vascular villus.
In the anterior foregut region, the lateral vessels are situated laterally to the rhynchocoel wall, branching to give rise to ramified blood vascular plexus surrounding the foregut. Posteriorly, the lateral vessels gradually move to the position ventral and lateral to the foregut, with the vascular plexus converging into the lateral vessels.
At the posterior of the foregut the rhynchocoelic villus leaves the floor of the rhynchocoel to become a middorsal vessel between the foregut and rhynchocoel. In the intestinal region, vascular system consists of a mid-dorsal vessel, situated below rhynchocoel and above intestine, and a pair of lateral vessels, running in connective tissue inside the body-wall inner longitudinal muscle layer. The lateral vessel is linked to the mid-dorsal vessel by transverse connectives, running along the lateral and dorsolateral sides of the intestine in connective tissue.
Excretory system. Nephridial tubules run inside blood vessels, distributed from just posterior to the cerebral organs to the level of the posterior foregut region. Although the tubules are found in almost every section throughout the distribution area, not all of them are continuous, i.e., the nephridial system is compartmentalized into several sub-systems. The tubules in each sub-system are often ramified and convoluted, mostly attached to the distal wall of the blood vessel. The tubules lead to efferent canals, each dorsolaterally passing through the body wall and opening to a nephridiopore in the epidermis ( Fig. 4C View FIGURE 4 ). Approximately 30 nephridiopores are found on each side; the number of the efferent canals from each single sub-system is uncertain, but apparently plural canals lead from one sub-system.
Nervous system and sense organs. The central nervous system consists of paired dorsal and ventral cerebral ganglia connected by transverse commissures ( Fig. 5D View FIGURE 5 ). The dorsal ganglia are larger than the ventral and are forked posteriorly into an upper and lower neuropil. The ventral commissure is thicker (50 µm) than the dorsal (30 µm). Bürger type I, II, III and neurocord cells can be found in both pairs of ganglia ( Fig. 6B, C View FIGURE 6 ). A nerve stem arises from lateral side of the dorsal ganglion on each side and runs forward; it is ramified anteriorly, giving rise to many nerve branches in the body-wall outer longitudinal muscle layer. A neural sheath encircles the body-wall mcm layer, with a mid-dorsal bulge. A mid-dorsal rhynchocoel nerve is present above the rhynchocoel circular muscle layer. A pair of foregut nerves originates from medial surface of the ventral ganglia and extends into the foregut wall.
Three apical organs are present at the anterior tip of the snout. The horizontal cephalic furrows begin at the anterior end of the head and terminate at the level of the anterior most portion of the mouth. The cerebral organ canal opens at a pit anterior to the end of the furrows and is surrounded by an upraised “U” shaped ridge of ciliated cells that appear neuroglandular. The cilia on these cells are much longer than in other portions of the furrow ( Fig. 6D View FIGURE 6 ). The neuroglandular cerebral organs adjoin the dorsal ganglia and are typical in construction for heteronemerteans. There are no ocelli.
Reproductive system. The gonads are arranged serially between the intestinal lateral diverticula as a single row on each side of the intestine ( Fig. 6E View FIGURE 6 ). Each gonad is bordered by the dv fibers of the diverticula, as well as a longitudinal layer of muscle fibers which encircle the basal lamina of the gonad wall. A single gonoduct extends from each gonad to the exterior through a dorsolateral gonopore. Primary spermatocytes develop from the wall of the gonad closest to the lateral nerve cord and maturate inward, towards the lumen of the gonad into conical spermatozoa. The head of spermatozoa, in histological sections, were 4 µm ( Fig. 6F View FIGURE 6 ).
Body fragments (including ones without head) capable of posterior regeneration ( Fig. 1E View FIGURE 1 ) but no anterior regeneration confirmed.
Distribution and habitat. Members of the Dushia nigra species complex comb. nov. are found intertidally under stones embedded in coarse sand, co-occurring with the ophiuroid cf. Ophiocoma scolopendrina (Lamarck, 1816) . Specimens have been collected or observed from the following locations in the West Pacific: Sado, Niigata, Japan ( Iwata 1960; Honma and Kitami 1978); Hayama, Kanagawa, Japan ( Iwata 1957); Niisaki, Kanagawa, Japan ( Saito and Suzuki 1974); Shimoda, Shizuoka, Japan ( Iwata 1952); Fukue, Goto Islands, Nagasaki, Japan ( Iwata 1952); Tomioka, Kumamoto, Japan ( Iwata 1952); Sakurajima, Kagoshima, Japan ( Iwata 1960); Kakeroma, Kagoshima, Japan (present study); Okinawa (Nakijin, Bisezaki, Sesoko, and Seragaki), Japan ( Kajihara 2017; present study); Su’ao, Taiwan (as Suô, Yamaoka 1939); China ( Stimpson 1855); Vietnam (Van Phong Bay, Nha Trang Bay, and Hon Cau Island) ( Chernyshev 2016). Occurrence of the orange form in Pelorus Island, Queensland, Australia (present study) was only confirmed by external appearance of the body but not by molecular data.
Species delimitation. The specimens included in the TCS analysis formed three networks ( Fig. 8 View FIGURE 8 ). Network A consisted of 12 specimens having black, brown, and orange body color, collected in Kakeroma and Okinawa. Network B was comprised of four black individuals from Kakeroma, Okinawa, and Van Phong Bay. Network C was formed by two black individuals from Okinawa. Among them, the maximum genetic distance was 0.168 (K2P) and 0.148 (p-distance) with respect to the 513-bp COI sequence, observed between specimens in Networks A and C ( Table 3 View TABLE 3 ). Average genetic distances in terms of uncorrected p-distance among the networks varied from 0.022 (Networks A–B), 0.139 (Networks B–C), and 0.147 (Networks A–C); mean within-network distances were 0.003 (Network A), 0.002 (Network B), and 0.010 (Network C), both in terms of uncorrected p-distance and K 2P. Networks A–C differed from D. wijnhoffae sp. nov. by 0.198 –0.200 p-distances ( Table 3 View TABLE 3 ). The ABGD and PTP analyses resulted in two groups ( Fig. 9 View FIGURE 9 ) in the D. nigra species complex. These results suggest presence of at least two cryptic species among the complex, one represented by Network A + B and the other by Network C; Hiebert & Maslakova (2015) discovered 0.129 –0.180 uncorrected p-distance for COI among five species of Maculaura from the Northeast Pacific.
Nomenclature. Our earlier interpretation in 1999 when we had only the black and orange forms was that the former would represent ‘ Dushia nigra ’ and the latter ‘ Dushia rubella ’, and ‘ D. nigra ’ might be a senior synonym of Micrura formosana Yamaoka, 1939 and Micrura japonica Iwata, 1952 . Later, a preliminary COI analysis upon finding the third, brown form from Kakeroma, suggested that all the three color forms formed a single species, which could have been referred to as ‘ Dushia nigra ’, with all the four nominal species (Me. nigra, Me. rubella , Mi. formosana , M. japonica ) possibly being synonymous. However, the final analysis with expanded dataset from Okinawa showed i) a single locality can harbor individuals from three Networks, and ii) a single Network may contain three different color morphs (i.e., orange, brown, black). In future studies, individuals from multiple Networks may occur in a single type locality of any of the four nominal species. This would complicate an objective allocation of the four available names (i.e., formosana , japonica , nigra , and rubella ) to each of the Networks discovered in the analysis. Furthermore, the exact type localities for Me. nigra and Me. rubella are not pinpointed, as Stimpson (1855) only noted “ China ”, although these must be somewhere along the southeast Chinese coast from Hong Kong to Shanghai ( Habersham 1858). If the same taxonomic species turns out to contain the two nominal species Me. nigra and Me. rubella , nomenclatural precedence should be given to one of them by the act of the First Reviser under Article 24.2.2 of the Code (ICZN 1999), because these two names were published in the same work ( Stimpson 1855).
Phylogeny. In the resulting phylogenetic tree ( Fig. 10 View FIGURE 10 ), Dushia wijnhoffae sp. nov. and Dushia nigra species complex comb. nov. showed a sister-taxon relation with full support values (100% bootstrap in ML; 1.0 posterior probability in BI), corroborating our taxonomic placement of the latter in the genus Dushia . They are further nested within a more extensive, well supported clade along with Gorgonorhynchus albocinctus Kajihara, 2015 and Cerebratulus leucopsis ( Coe, 1901) sensu Kvist et al. (2014) . The latter is represented by a specimen deposited in the Museum of Comparative Zoology (MCZ), Harvard University, with the catalogue number MCZ IZ 135331. However, judging from the images taken in life and available at the MCZ (https://mczbase.mcz.harvard.edu), the specimen is not likely to be C. leucopsis , as it had a uniformly pale orange body color. On the other hand, Coe (1901) originally described that “As preserved in formalin the color is homogeneous slaty blue or purplish, with a tinge of gray, except on the head. The color of the head is similar to that of the body, but is clearer and not so grayish. The tip of the snout, both above and below, is pure white (at least after preservation in formalin)”. Our phylogeny indicates that branched proboscis has evolved independently multiple times in the Heteronemertea , as Gorgonorhynchus albocinctus and Polydendrorhynchus zhanjiangensis ( Yin & Zeng, 1984) did not form a clade.
The western Pacific and Caribbean distribution of the genus may indicate that the lineage predates the rise of the Panamanian isthmus and spans the Pacific. Unfortunately, nemerteans are undersampled, especially from the Pacific coasts of the South and Central America, as well as the Pacific Islands including Polynesia, Micronesia, and Melanesia. From the Indo-Pacific, at least 14 nominal species have been described that possess a uniformly colored body without any distinctive color pattern, and are thus potentially members of Dushia ( Bürger 1890; Punnett 1900a, b, c, 1903; Staub 1900; Punnett & Cooper 1909; Coe 1947; Table 4 View TABLE 4 ). Thorough investigations at the type localities for those nominal species may reveal their actual systematic position.
TABLE 3. Range of within-Network genetic distance (on diagonal, the values were the same in terms of uncorrected p-distance and K2P) and average between-Network genetic distances (K2P below diagonal; uncorrected p-distance above diagonal).
| D. nigra species complex A B | C | D. wijnhoffae | |||
|---|---|---|---|---|---|
| A | 0.000–0.010 | 0.022 | 0.147 | 0.198 | |
| D. nigra complex | B | 0.023 | 0.002–0.004 | 0.139 | 0.199 |
| C | 0.166 | 0.155 | 0.001 | 0.200 | |
| D. wijnhoffae | 0.233 | 0.234 | 0.235 | — | |
TABLE 4. Lineid heteronemertean species that occur intertidially in the Indo-Pacific, with a body color being uniformly red, brown, or blackish (not whitish or greenish), having non-branched proboscis.
| D. nigra species complex A B | C | D. wijnhoffae | |||
|---|---|---|---|---|---|
| A | 0.000–0.010 | 0.022 | 0.147 | 0.198 | |
| D. nigra complex | B | 0.023 | 0.002–0.004 | 0.139 | 0.199 |
| C | 0.166 | 0.155 | 0.001 | 0.200 | |
| D. wijnhoffae | 0.233 | 0.234 | 0.235 | — | |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Dushia nigra ( Stimpson, 1855 )
| Hookabe, Natsumi, Schwartz, Megan L., Kajihara, Hiroshi & Norenburg, Jon L. 2019 |
Dushia
| , Diva Correa 1963 |
Micrura japonica
| Iwata 1952: 139 |
Micrura formosana
| Yamaoka 1939: 286 |
Cerebratulus rubellus: Bürger 1904 , p. 112
| Burger 1904: 112 |
Cerebratulus niger:
| Stimpson 1857: 161 |
Serpentaria rubella:
| Stimpson 1857: 162 |
Meckelia nigra
| Stimpson 1855: 382 |
Meckelia rubella
| Stimpson 1855: 382 |