Thrinax, Sargent, 1899
|
publication ID |
https://doi.org/ 10.11646/phytotaxa.614.1.1 |
|
DOI |
https://doi.org/10.5281/zenodo.8400285 |
|
persistent identifier |
https://treatment.plazi.org/id/03B387DA-FFF8-1F7E-FF50-FC75FE098CEB |
|
treatment provided by |
Plazi |
|
scientific name |
Thrinax |
| status |
|
COCCOTHRINAX View in CoL View at ENA
Sargent (1899) established Coccothrinax for specimens from Florida. The most important early works on the genus were those of Beccari (1907, 1913, 1931), Bailey (1939a, 1939b), and Bailey in Bailey & Moore (1949). The most important work on Cuban species was that of León (1939). Here several new species were described and species were classified into two series, each again divided into two subseries. Following León, two botanists, Muñiz and Borhidi, continued work on Cuban species, culminating in their catalogue of all Cuban palms ( Muñiz & Borhidi 1982).This work included a key to all Cuban species. Read worked on western Caribbean species (e.g. Read 1966a, 1966b). Nauman & Sanders (1991a) gave a key to the species in cultivation, and Nauman & Sanders (1991b) discussed the morphology of the genus in some detail and gave a preliminary classification. Most recently, Craft (2017) has given an illustrated account of the Cuban species, with descriptions and distribution maps but without a key, and Moya (2020, 2021) has brought nomenclature and typification of Cuban species up to date. Fernández & Gottschalk (2017) have given an illustrated account of Hispaniolan species, also without a key. Zona et al. (2007) have assessed the conservation status of all species, and Jestrow et. al. (2018) have discussed the conservation status of endangered species.
Currently 61 species of Coccothrinax are recognized ( POWO 2023, Henderson et al. 2023). Most recent authors agree that a modern revision is needed. However, the genus is considered difficult taxonomically (e.g. Bailey in Bailey & Moore 1949). León (1939, page.153) wrote: ‘las formas múltiples que occurren en muchas especies de este género, la variación considerable que se observa no sólo en la follaje, sino también en las diferentes partes de la inflorescencia y de la misma flor, por una parte dificultan grandemente el estudio de los Coccothrinaces…’ [The multiple forms that occur in many species of this genus, the considerable variation that is observed not only in the foliage, but also in the different parts of the inflorescence and of the same flower, on the one hand greatly hinder the study of Coccothrinaces]. Tomlinson et al. (2011) considered Coccothrinax to be the most variable genus anatomically of any genus of subfamily Coryphoideae , and one of the most variable in the whole family.
Morphology
In the following discussion, morphology is treated in detail and several attributes of Coccothrinax not used in delimiting species are discussed. A detailed discussion of morphology is given in Nauman & Sanders (1991a) and a detailed generic description can be found in Dransfield et al. (2008).
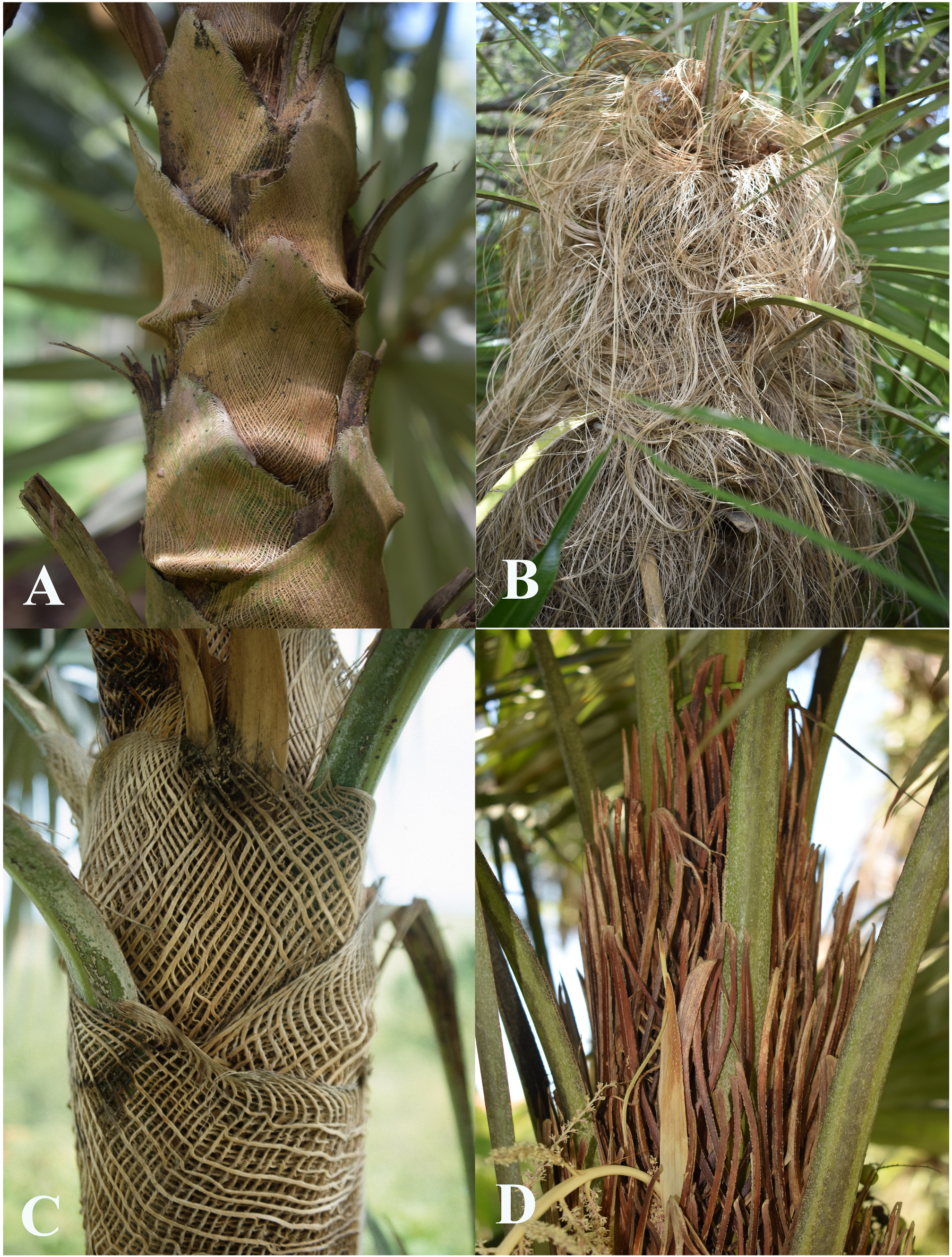
Stems are recorded as 0.03–20.0 m long and 2.9–50.0 cm diameter. The shortest stems are found in the mainland Florida population of C. argentata and the tallest in Hispaniola populations of C. argentea . Stem diameter is remarkably variable. Stems of C. argentea may be slender and flexible ( Fig. 1A View FIGURE 1 ) while those of C. spissa maybe swollen ( Fig. 1B View FIGURE 1 ). Within species, stems of C. spissa may be swollen or not swollen within the same population. Stems are usually solitary and only four species ( C. fagildei , C. jimenezii , C. pseudorigida , C. pumila ) are recorded as having clustered stems. Specimens of C. argentata from mainland Florida are scored as having solitary stems, but according to Presley (1934) they can have shoots at the base or on the stems. Rarely, specimens of C. argentea are recorded as having clustered stems. In three species ( C. borhidiana , C. garciana , C. pseudorigida ), stems are covered with persistent dead leaves, giving a ‘skirt’ of dead leaves ( Fig. 1C View FIGURE 1 ). Other species may have such skirts, for example C. fagildei illustrated in Moya (2021), but this cannot be scored from specimens.
The central, solid part of the leaf sheath of Coccothrinax is not split at the base ( Fig. 1D View FIGURE 1 ) (rarely specimens appear to have a short split, but this may be an artefact of pressing and drying). Apart from the central, solid part, leaf sheaths are fibrous, and here fibers are scored as seven different states: thin (usually <0.5 mm diameter), closely woven, forming persistent, triangular ligules at the apices ( Fig. 2A View FIGURE 2 ); thin (usually <0.5 mm diameter), closely woven, not forming persistent ligules and soon disintegrating at the apices; thin (usually <0.5 mm diameter), flimsy, loosely woven, free and greatly elongate at the apices ( Fig. 2B View FIGURE 2 ); stout (usually> 1 mm diameter), woody, loosely (rarely closely) woven, ± joined or briefly free at the apices ( Fig. 2C View FIGURE 2 ); stout (usually> 1 mm diameter), woody, loosely woven, the inner and outer layer combining at the apices to form erect , spine-like fibers ( Fig. 2D View FIGURE 2 ); stout (usually 0.3–0.5 mm diameter), loosely woven and forming a loose, hexagonal mesh, initially forming ligules at the apices; and thin (usually ca. 0.4 mm diameter), curled toward the apex, loosely woven and forming a loose, hexagonal mesh, truncate at the apex.
Leaf sheath fibers are usually arranged in two layers, one perpendicular to the other, but in a few species three layers are present. However, this does not appear consistent within species. In C. miraguama individuals can have either two or three layers. Nauman & Sanders (1991a) considered that C. miraguama specimens can have three layers nearer the base of the sheath and two layers nearer the apex. This variable is therefore not used here to delimit species.
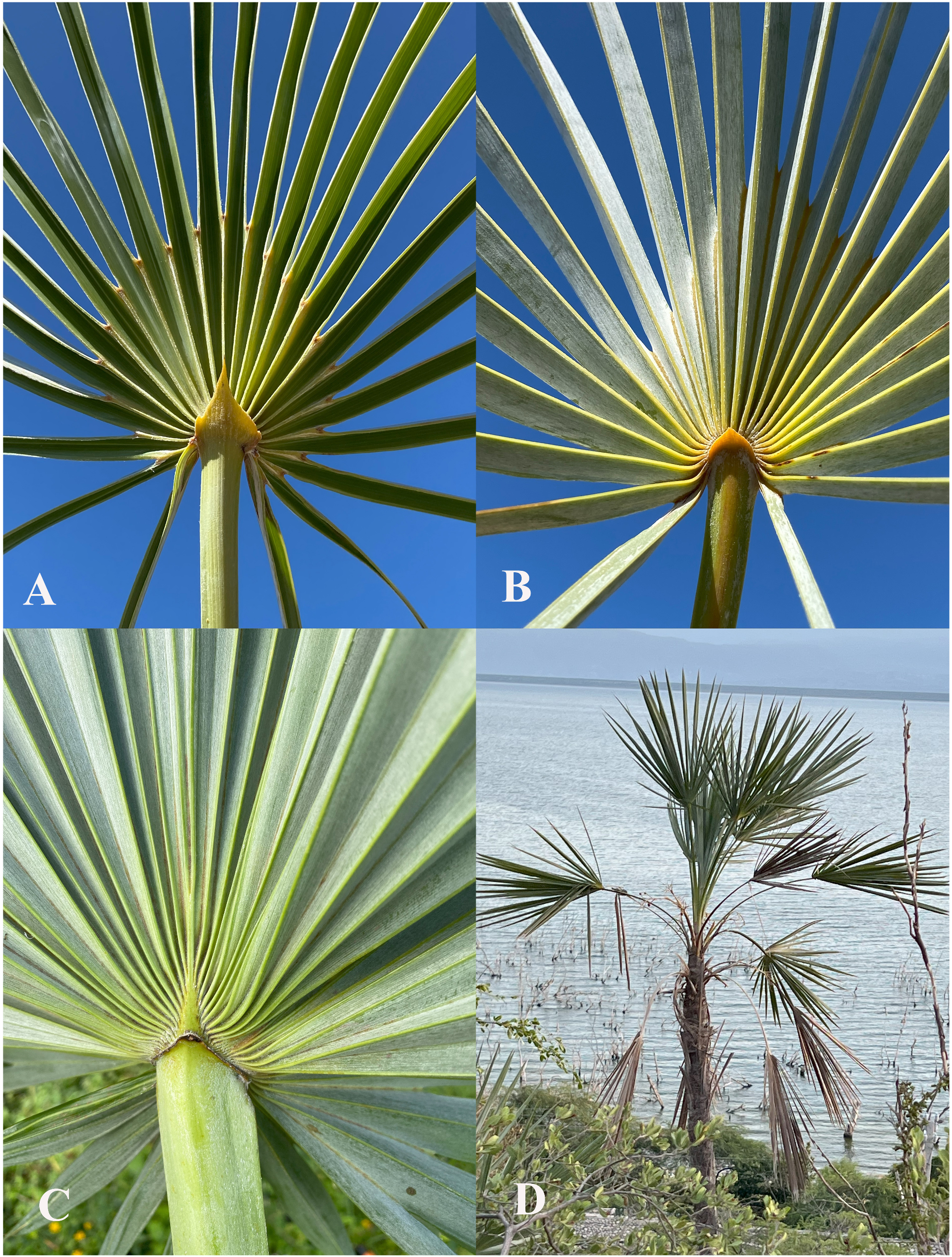
Petiole width ranges from 2 mm wide, on a specimen from the mainland Florida population of C. argentata , to 26.4 mm wide from a specimen of C. spissa . At the apex of the petiole is a hastula. As noted above in the general morphology section, hastulas are too variable to be useful taxonomically. The shape of the adaxial hastula varies from rounded to acuminate to cuspidate ( Fig. 3A View FIGURE 3 ), and sometimes may be briefly bifid at the apex. The abaxial hastula is much less developed, usually forming a low ridge ( Fig. 3B View FIGURE 3 ). Palman length ranges from 1.0–43.0 cm. As noted by Nauman & Sanders (1991a), occasionally the central adaxial vein is abnormally long, and then the palman length is measured along the adjacent adaxial vein. Palmans may be relatively short, with the adaxial veins prominent and terminating in a distinct pulvinus ( Fig. 3A View FIGURE 3 ). In this case, palmans appear somewhat sunken in relation to the rest of the leaf. Alternatively, palmans may be relatively long, without prominent adaxial veins, and not lower than the rest of the leaf. Rarely, a short costa is present abaxially ( Fig. 3C View FIGURE 3 ).
Nauman & Sanders (1991a), based on living plants, described the outline of the leaf blade as a series of fractions, i.e. 4/4 orbicular, 3/4 orbicular, etc. However this has been found to be somewhat impractical and difficult to score from specimens. Here, leaf blades are described as either wedge-shaped ( Fig. 3D View FIGURE 3 ) or not wedge-shaped. Nine species, from Cuba and Hispaniola, are scored as having wedge-shaped leaf blades. In these species, it is only the adults that have wedge-shaped blades, especially those in more exposed places. Species with wedge-shaped blades tend to have fewer segments per leaf, a mean of 23, as opposed to 36 in non-wedge-shaped blades.
Segment shape, taken from those segments from the middle of the leaf, is described by four states. In the first state, the segments are relatively long and narrow, tapering from base to apex, scarcely folded, flexible and not leathery, with a shoulder or constriction absent or poorly developed, and the apices are thin, deeply splitting and breaking off. The mean size of this type of segment is 56.3 cm long and 2.6 cm wide. In the second state, found in several Cuban species, the segments are relatively short and broad, abruptly narrowed (shoulder) toward the apex, otherwise parallel-sided, often strongly folded, stiff and leathery, the apices briefly splitting. The mean size of this type of segment is 42.9 cm long and 2.6 cm wide. However, two, somewhat different apices occur in this type of segment, short and elongate, and these can appear quite distinct. In fact, León (1939) used them to distinguish species, for example C. miraguama from C. yuraguana . However, the distinction between these two types of apex is more complex and may also be influenced by habitat. Moya (2020) illustrated this in C. acuminata , with plants with short segment apices (his Fig. 1 View FIGURE 1 ) growing in open habitats and those with long apices (his Fig. 2 View FIGURE 2 ) growing in shaded habitats. In the third leaf segment state, segments are tapering from base to apex, often folded, stiff and leathery, with or without scarcely developed shoulders, and with the apices sharply pointed and briefly splitting. The mean size of this type of segment is 41.8 cm long and 3.5 cm wide. In the fourth leaf segment state, found only in C. crinita , middle leaf segments are relatively long and broad, abruptly narrowed (shoulder) about the middle, stiff and leathery, and the apices are elongate beyond the shoulder and briefly split. Mean size is 77.4 cm long and 3.9 cm wide.
In a few species ( C. orientalis , C. yunquensis ), the middle leaf segments are blunt and rounded at the apices and only briefly splitting. However, this is difficult to score. In some species it appears that leaves from younger plants may have blunt segment apices, while adults have normal attenuate apices. Leaf segments may not be waxy adaxially or may have a deciduous, thin layer of wax abaxially. Rarely segments may have a persistent, dense, whitish layer of wax adaxially. This is a difficult variable to score and the amount of wax varies within species, and presumably also with the age of the leaves, with wax wearing off from older leaves. Some specimens of C. miraguama appear to have little or no wax abaxially, others appear to have more wax. Craft (2017) wrote that some forms of C. salvatoris have a layer of wax abaxially. Only four species are scored as having a dense, persistent layer of wax.
Leaf segments are usually indumentose abaxially. This is scored as four states. Segments may be densely indumentose abaxially, with irregularly shaped, persistent, interlocking, fimbriate hairs, each one with a conspicuous, reddish-brown, pale brown, or greenish elliptic center (e.g. C. argentata , Fig. 4A View FIGURE 4 ); densely indumentose abaxially, with irregularly shaped, persistent, interlocking, fimbriate hairs, each one with a rounded, raised, light green to greenish-brown or reddish-brown center (e.g. C. miraguama , Fig. 4B View FIGURE 4 ); densely indumentose abaxially, with irregularly shaped, semi-persistent, interlocking, fimbriate hairs without an obvious center (e.g. C. argentea , Fig. 4C View FIGURE 4 ); or segments may be without indumentum abaxially (e.g. C. bonettiana ). However, indumentum is often difficult to score. There may be intermediate states or indumentum may wear off from older leaves. Leaf segments in most species are ribbed abaxially as a result of their anatomy, although one species, C. ekmanii , appears not to be ribbed ( Tomlinson et al. 2011).
In several species leaf segments have well-developed transverse veinlets ( Fig. 4D View FIGURE 4 ). However, these are often difficult to see unless the leaf segments are illuminated from behind. It seems possible that transverse veinlets are more obvious in juvenile and younger leaves and obscure in older leaves, more obvious in fresh rather than dried leaves, and more obvious at the apices of the segments.
Inflorescences are scored either as erect, at least initially, amongst or above the leaves, with few to numerous partial inflorescences ( Fig. 5A View FIGURE 5 ), or as erect amongst the leaves, with few partial inflorescences at apex of inflorescence ( Fig. 5B View FIGURE 5 ), or as curving, arching, or pendulous amongst the leaves, with few to numerous partial inflorescences ( Fig. 5C View FIGURE 5 ). Inflorescences are usually branched to two orders, rarely some individuals have inflorescences branched to three orders (e.g. C torrida, Zona 842; C. barbadensis, Broadway 3067). In both these specimens, relatively well-developed bracts subtend proximal rachillae. Rachis bracts are either narrow, closely sheathing, sparsely tomentose, usually without hairs at the apex (e.g. C. boschiana , C. ekmanii ), or as somewhat flattened, loosely sheathing, usually tomentose with a dense tuft of erect hairs at the apex (e.g. C. argentata , C. argentea ). Rarely they are scored as swollen, woody, and not or sparsely tomentose. The number of partial inflorescences on an inflorescence ranges from 2–7. Coccothrinax scoparia consistently has two partial inflorescences. In species with elongate inflorescences, such as C. garciana and C. pseudorigida , there are two to four partials. On the other hand, species such as C. argentata may have up to seven partial inflorescences. The longest rachillae are found in C. spissa , with a mean length of 13.6 cm, and the shortest in C. scoparia , with a mean length of 4.0 cm. Rachillae are usually straight, rarely recurved. They may be more or less evenly spaced, seldom in pairs, or unevenly spaced, some in groups. In the latter case, usually in species with larger inflorescences, rachises are often somewhat flattened in cross-section. Rachillae at or near anthesis may be glabrous, or uneven, with lines of warty outgrowths, these often becoming more pronounced as fruits develop.
Flowers are solitary, spirally arranged along the rachillae, and are borne on pedicels ( Fig. 5D View FIGURE 5 ). Pedicel length, in fruiting specimens, ranges from 0.1–6.2 mm long. Pedicels are subtended by bracteoles, and these bracteoles are borne either at the base of the pedicel or sometimes on the pedicel itself (e.g. C. barbadensis ). The flowers themselves appear quite variable. Perianth lobes vary from small and narrow (e.g. C. garciana ) to large and broad (e.g. C. spissa ). In one species, C. scoparia , the lobes may be laciniate (illustrated in Beccari 1931, plate 31 IV). Perhaps most distinctive are the perianths of C. torrida which have a narrow base and deeply split lobes. Stamens are elongate and spread irregularly at anthesis, with latrorse anthers. Stamen number ranges from 5–13. Filaments are usually wider at the base and there fused into a shallow to deep cupule. The amount of basal fusion in filaments has sometimes been used to distinguish species (e.g. León 1939) but it does not appear to have any taxonomic significance.Anthers are often coiled and twisted ( Fig. 5D View FIGURE 5 ).
Fruits of Coccothrinax range in size from 3.0– 16.4 mm long and 3.8–15.4 mm diameter and are typically globose or nearly so. The largest fruits are found in C. montana and the smallest in C. samanensis . Fruit color is not discernible from herbarium specimens and is taken from specimen labels. Quite a range is found but usually fruits are described as black or various shades of purple. A few species (e.g. C. montana ) are reported to have white fruits. Fruit surfaces are usually smooth or sometimes with projecting fibers ( Fig. 6A View FIGURE 6 ), or rarely are densely muricate ( Fig. 6B View FIGURE 6 ). The latter kind of fruits are usually smaller than others and dry light brown. Some species (e.g. C. montgomeryana ) have small and nearly muricate fruits, apparently an intermediate state.
Seed surfaces of Coccothrinax are either deeply lobed, the lobes running from base of seeds almost to apices, or lobed, the lobes running from base of seeds approximately to equator. Rarely seed surfaces appear almost smooth or scarcely lobed ( C. samanensis ). Embryos are apical or subapical (but note that both Bailey in Bailey & Moore (1949) and Muñiz & Borhidi (1981) erroneously considered embryos to be basal).
Seeds from herbarium specimens can be divided into two states. In the first, seeds are light brown, with irregular, shallow lobes radiating from the base but not reaching the apex of the seed and not separating ( Fig. 6C View FIGURE 6 ). In the second state, seeds are dark brown, with the deep lobes separating almost to the apex ( Fig. 6D View FIGURE 6 ). Mayté Pernús Alvarez (pers. comm.) believes these states are based on differences in moisture content, and the separating lobes are caused by dehydration of the seeds. This variable is thus not used here to delimit species.
Results
Of the 61 preliminary species examined, 39 phylogenetic species are recognized. However, numerous difficulties in delimiting species and subspecies using the methods in the Materials and Methods section were found, as follows.
Two phylogenetic species are widespread— Coccothrinax argentata in the western Caribbean and C. barbadensis in the eastern Caribbean. Previous authors have recognized several different species within these two areas, each endemic to a particular island, or group of islands, or mainland area. Here, the two species are considered to comprise numerous, disjunct populations, with each island/mainland area having its own population. Coccothrinax argentata occurs on approximately 40 different islands as well as mainland Florida and Mexico, and C. barbadensis occurs on approximately 25 different islands as well as the larger islands of Hispaniola and Puerto Rico, and mainland Venezuela. Generally there are too few specimens from each island/mainland area to test for quantitative differences but it seems likely that each island has its own distinct population, and mainland areas may have more than one population. For example, Zona et al. (2018) showed that there were two distinct populations of C. argentata in mainland Florida. Furthermore, it appears that widespread species on larger islands ( Cuba, Hispaniola, Puerto Rico, Trinidad) are also made up of several, distinct, disjunct populations, each occurring in a different habitat or on a different substrate. Such areas may themselves be thought of as islands, in this case islands of some distinct substrate such as serpentine soils or limestone mogotes. Examples are C. miraguama , C. clarensis , and C. salvatoris in Cuba, as noted by León (1939), and C. argentea in Hispaniola, as discussed later under that species. Finally, even within species considered to be narrow endemics there may be distinct populations, such as in C. boschiana and C. jimenezii .
In summary, two Coccothrinax species ( C. argentata , C. barbadensis ) are widespread and made up of numerous, disjunct, different, usually insular populations. A few other species are widespread in Cuba and Hispaniola. This is a problem at the subspecies level. In the Materials and Methods section it was stated that if subgroups of phylogenetic species could be delimited by geographic/elevation disjunctions, and these subgroups differed in quantitative variables, then a phylogenetic subspecies concept could be applied. But as noted above, just within C. argentata and C. argentea there are dozens of disjunct, potentially different populations. The majority are represented by few specimens, too few to test for differences, and recognition of such populations as subspecies would lead to an unwieldly number of subspecies.
Further complications result from hybridization. There is evidence for extensive hybridization between species. Craft (2017) reported that Coccothrinax species commonly hybridize in cultivation and noted several naturally occurring hybrids in Cuba. These can occur between apparently unrelated species. For example, Suárez (2015) reported a naturally occurring hybrid between C. crinita and C. miraguama . Even intergeneric hybrids between Coccothrinax and Leucothrinax (as Thrinax ) have been reported ( Nauman 1989, 1990). Hybrids cause taxonomic problems because they are difficult to detect from specimens and may be part of the reason that several qualitative variables are difficult to score and consequently species difficult to delimit.
Another complication results from dispersal. The wide distribution of Coccothrinax species on almost every island in the Caribbean is evidence of successful dispersal, including long distance dispersal to such isolated islands as San Andrés, Providencia, and Islas del Cisne. The relatively small seeds are dispersed by birds, bats, deer, and turtles ( Zona & Henderson 1989, Davis et al. 2007). Dispersal causes taxonomic problems if plants from one population have dispersed to another. For example, Zona et al. (2018) found that an aberrant specimen from mainland Florida appeared similar to specimens from the Florida Keys and considered that it could be explained as a result of dispersal from the Florida Keys to the mainland.
Thus it can be seen that population structure, hybridization, and dispersal complicate the taxonomy of Coccothrinax . This means that qualitative variables are difficult to define and score from specimens and relatively few variables have been found in this study. Several potential qualitative variables were not used because of their infraspecific variability (e.g., hastula shape, pedicel shape). Some qualitative variables that were used are difficult to score because of intermediate states. There is also the problem of changes during development or aging. Indumentum, for example, can wear off as leaves age, and transverse veinlets may be most easily scored from younger leaves. Other variables, such as those based on stem branching are problematic because they can only be scored from labels, where such data are usually lacking. Other authors have experienced the same problem. Nauman & Sanders (1991b) scored 22 morphological variables for their study of Coccothrinax phylogeny, and noted that most were highly homoplasious and only four were reliable for classificatory purposes at the infrageneric level (elongate sheath fibers, spine-like sheath fibers, transverse veinlets, muricate fruits). In the present study, only about seven of 22 qualitative variables used are unambiguously scoreable.
These problems are compounded by the limitations of the available specimens.As in many palm genera, specimens are relatively few and often fragmentary because the plants are large in size and difficult to collect. Type specimens of some species are either non-existent or fragmentary. Some areas are poorly collected. In consequence there are many missing data.
In summary, population structure, dispersal, hybridization, and the limitations of herbarium specimens means that relatively few qualitative variables have been found, their states are difficult to define, they are often difficult to score because of intermediates between states, and there are many missing data. All these problems mean that a specimenbased revision using morphology and employing the Phylogenetic Species Concept is problematic. Molecular data are needed to understand Coccothrinax , not only to delimit species and identify hybrids but to resolve species relationships. Two studies, Cano et al. (2018) and Roncal et al. (2008), have begun to understand relationships based on molecular data, but included rather few species. Nevertheless, the present study will at least provide hypotheses of species that can be tested by molecular data.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.