Cephaloboides anisospiculus, Tahseen, 2017
|
publication ID |
https://doi.org/ 10.1080/00222933.2023.2235062 |
|
DOI |
https://doi.org/10.5281/zenodo.8270761 |
|
persistent identifier |
https://treatment.plazi.org/id/03B587FC-FF88-050E-3FF0-271AFD8EFB75 |
|
treatment provided by |
Plazi |
|
scientific name |
Cephaloboides anisospiculus |
| status |
|
Embryogenesis and gonad development in Cephaloboides anisospiculus View in CoL
Cephaloboides anisospiculus , a bacterivorous rhabditid, was collected from manure samples in Lado Saray, New Delhi, India. The natural population showed a male:female sex ratio of 1:1. A single gravid female was reared for the biological studies, which led to the production of progeny with a sex ratio similar to that found in nature.
Embryogenesis ( Figures 1 View Figure 1 , 3 View Figure 3 , 6 View Figure 6 )
The females of C. anisospiculus were largely oviparous, with rare instances of intra-uterine development. The eggs were laid in the single cell stage of embryonation ( Figure 6a View Figure 6 ). The uterine tract usually accommodated 1– 2 eggs, but in exceptional cases about 4–10 intra-uterine eggs were found.
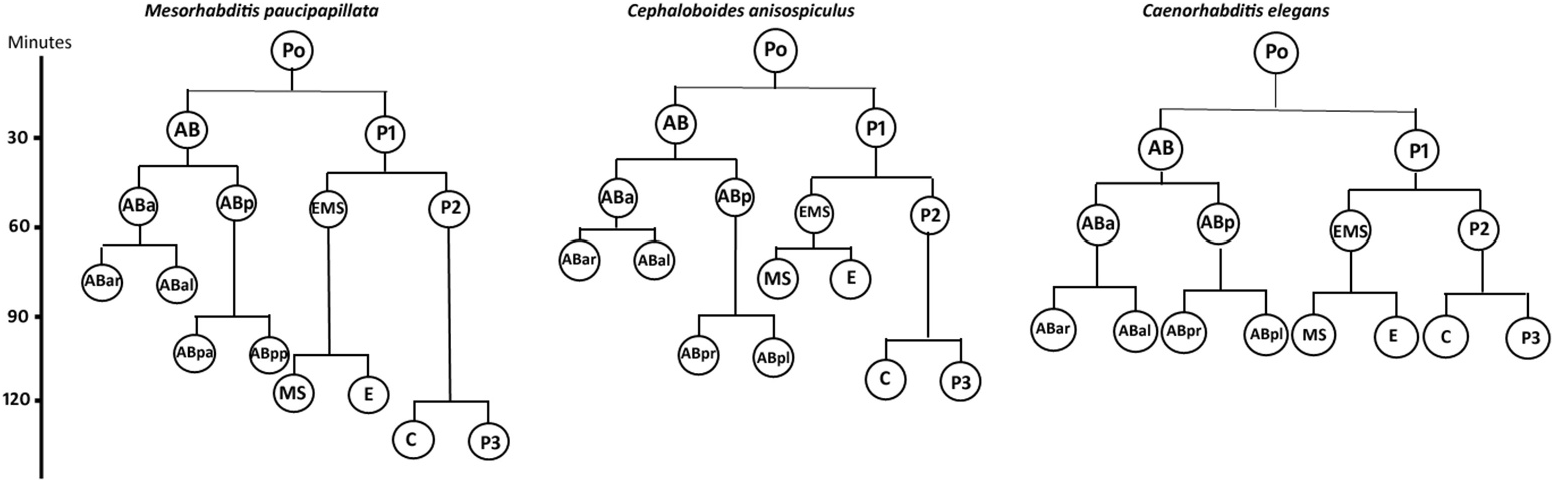
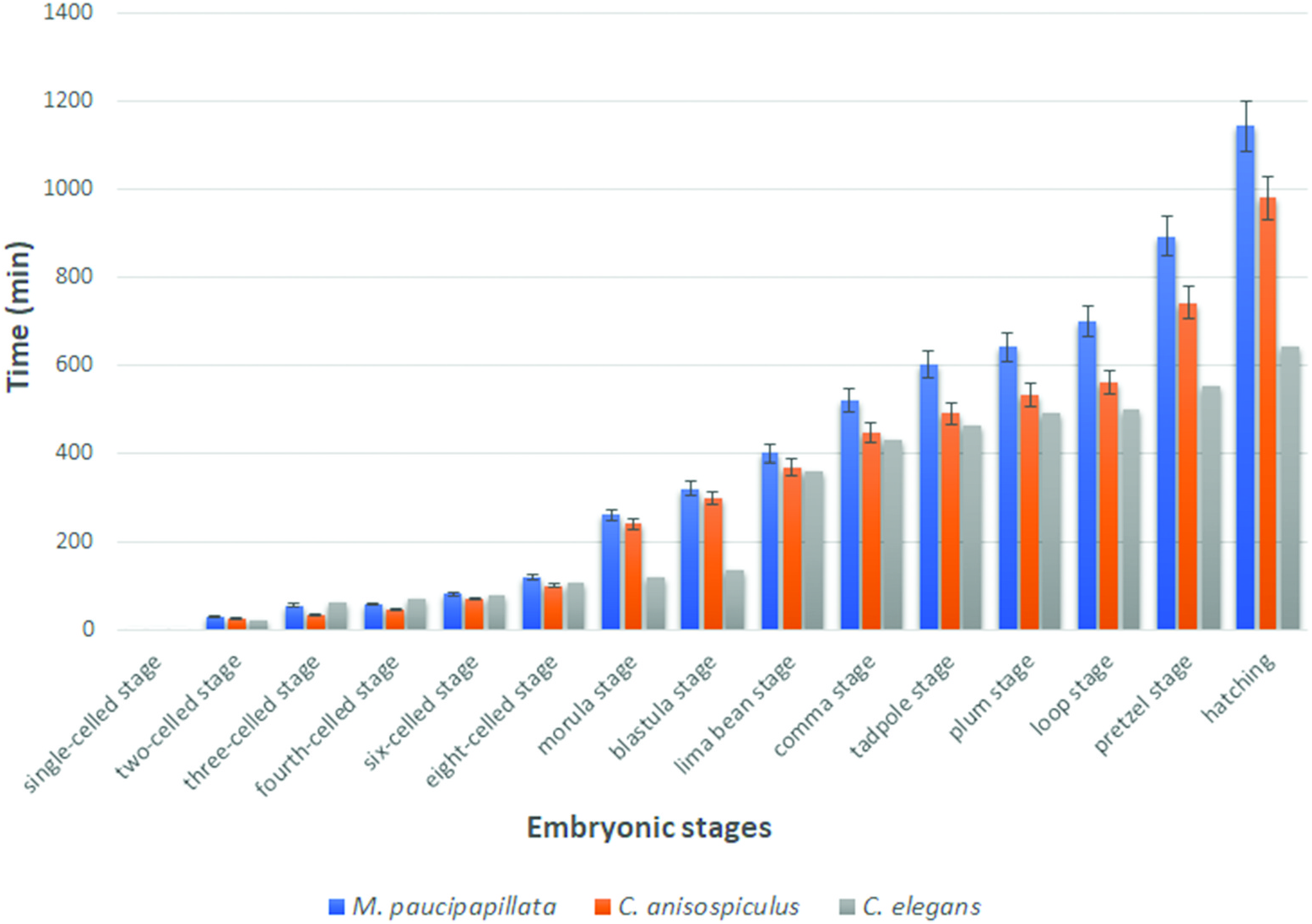
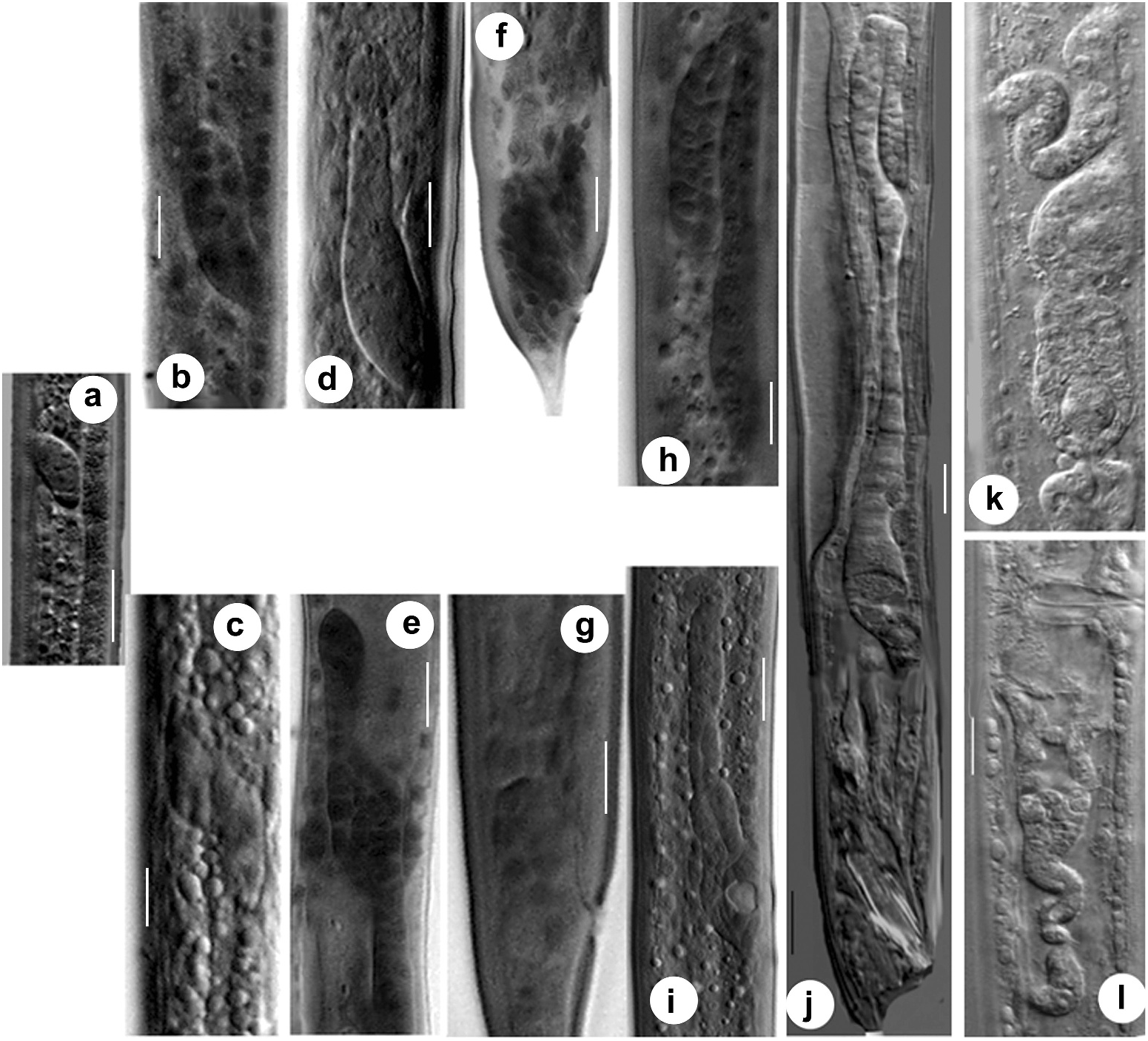
The eggs were smooth-shelled, elongated with rounded poles and measured 37– 42 × 20–24 µm. The pole of entry of the sperm marked the posterior end of the developing embryo. However, polarity could also be determined in the uterine tract in rare instances of delayed egg laying, where the pole pointing towards the vulva was the posterior pole. After sperm entry, cytoplasmic streaming led to pseudocleavage, which was followed by the migration of sperm and egg pronuclei to the centre of the egg. With the commencement of cleavage, the egg nucleus (Pο) first elongated along with the streaming movement in the egg cytoplasm and then the cleavage furrow was formed horizontally to the longitudinal embryonic axis, 20–25 min after the pronuclear fusion. The resulting blastomeres were unequal ( Figure 6b View Figure 6 ), with the anterior one (AB) slightly larger than the posterior (P1). Before the commencement of the second division, the cytoplasm of cell AB showed streaming. Thus, the second oblique cleavage after 10– 15 min gave rise to cells ABa (anterior) and ABp (posterior) blastomeres. The blastomere P1, after 5–10 min, divided obliquely to form EMS and P2, resulting in a four-celled stage ( Figure 7c View Figure 7 ). Simultaneously, within 10–15 min ABa longitudinally divided into ABal (left) and ABar (right) blastomeres ( Figure 6e View Figure 6 ) while within 5 min EMS divided into MS and E. ABp divided into ABpl (left) and ABpr (right) after 25–30 min followed by the division of P2 into C and P3 after 10–15 min to form the eight-celled stage. The latter stage was reached 1.5–2.0 h after egg laying. The 10-celled stage was formed 20–30 min after the eight-celled stage while the 12-celled stage developed subsequently, 25–35 min later. Morula formation occurred 40–45 min after the 12-celled stage. The blastula was formed ( Figure 6i View Figure 6 ) after 1.0–1.5 h of morula formation. Gastrulation started 50–60 min after the formation of the blastula or about 5.0–5.5 h after first cleavage, showing the ingression of endodermal cells and further led to the differentiation of anterior and posterior regions of the gut. The gastrula stage involved the differentiation of ectoderm as an outer layer covering the inner mass of blastomeres representing hyaline and granular halves. The embryo showed elongation along the longitudinal axis followed by a depression slightly away from the centre of the egg, in the granular zone. The invagination marked the ̍lima bean̾ stage ( Figure 6j View Figure 6 ) 30–40 min after the initiation of gastrulation, and further deepening of the cleft resulted in the ̍comma̾ stage ( Figure 6k View Figure 6 ) after another 35–45 min. The embryo then prepared for organogenesis and acquired a worm-like body in the ̍tadpole̾ stage after about 30–45 min of comma stage, with an anterior shallow depression representing the presumptive stoma ( Figure 6l View Figure 6 ). The plum stage ( Figure 6m View Figure 6 ) was formed about 30–40 min later and marked the commencement of embryonic movement. The loop stage ( Figure 6n View Figure 6 ) was attained after about 20–30 min of plum stage with a two egg-folds embryo that moved continuously in the antero-posterior direction. The pretzel stage ( Figure 6o, p View Figure 6 ) followed the loop stage after about 2.5–3.0 h, when stoma, pharynx, intestine, tail and rectum were differentiated.
The embryo attained a length of 2.5–3.5 times the egg length; with most structures formed, it now formed the first-stage juvenile. The juvenile exhibited antero-posterior body movements besides labial explorations and rotations on its own body axis. Due to continuous thrust and pressure exerted by the moving juvenile, the eggshell became very thin and finally ruptured in the form of a slit. However, it took several attempts for the first-stage juvenile to hatch out of the eggshell, almost 3 h to 4 h after completion of organogenesis. The total embryonation time from the single-celled stage to hatching ( Figure 3 View Figure 3 ) was 15.5–20.0 h at 25 ± 2°C.
Morphology of gonad development in the female ( Figures 5 View Figure 5 , 7 View Figure 7 )
Gonad development continued from J1 to J4 and it was difficult to differentiate juvenile stages exclusively oby morphometric values due to overlapping ranges. However, the growth patterns of the genital primordium were found to be good markers to differentiate these stages. The configuration of nuclei/cells including those in the ventral cord region at each developmental stage is given in Table 2.
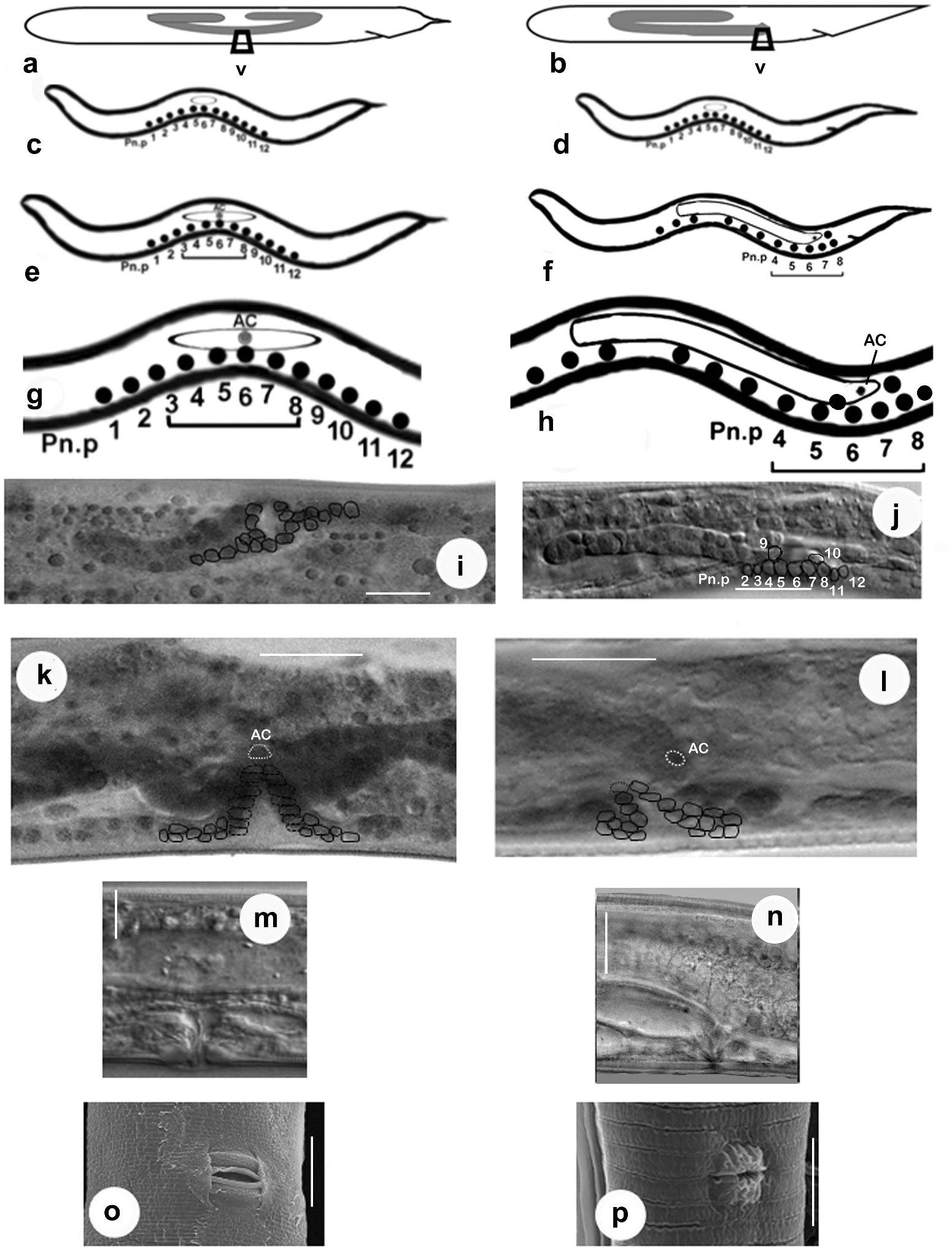
First-stage juvenile. The genital primordium of the first-stage juvenile of C. anisospiculus was located at 49.2–50.7% of the body from the anterior end. In ventral view, the genital primordium appeared spindle-shaped with an oblique orientation to the main body axis ( Figure 7a View Figure 7 ). It contained two large central germinal (Z2, Z3) and two relatively smaller polar somatic cells (Z1, Z4). The J1 primordium was similar in all juveniles, with a fixed number of primordial cells, and represented a sexually undifferentiated primordium. The presumptive vulva-forming cells existed in the form of a series of 12 ectoblasts – P(1–12).p, positioned at regular intervals along the mid-ventral line.
Second-stage juvenile. The spindle-shaped genital primordium was located at 51.65– 52.59% of the body length from the anterior end. Further proliferation of germinal cells (Z2, Z3) was not observed. The sexually undifferentiated second-stage juvenile possessed a primordium similar in shape to that of the first-stage juvenile. Initially, the primordium of J2 exhibited a mixed arrangement of somatic and germinal cells appearing as descendants of Z1/Z4 and Z2/Z3, respectively ( Figure 5c View Figure 5 ). However, during the moulting period, the localisation of cells and the resulting primordial growth indicated the future sex of the juvenile. By the end of moulting, two DTCs were positioned at the anterior and posterior distal ends that led to gonad elongation ( Figure 7c View Figure 7 ) and germ-line patterning; up to six somatic cells destined to form all future somatic gonad cells were gathered at the centre of the genital primordium; the germinal cells were placed in anterior and posterior primordial arms. Of the 12 ectoblasts – P(1–12).p, – the central five cells, P(4–8).p, appeared conspicuous, forming a vulval ̍equivalence group̾.
Third-stage female juvenile. The primordium of the third-stage female juvenile of C. anisospiculus was located at 41.0–49.4% of the body length from the anterior end. The primordium showed bidirectional growth and extended anteriorly as well as posteriorly due to cellular proliferation ( Figures 5e View Figure 5 , 7e View Figure 7 ). The genital primordium contained 4–6 germinal and 8–20 somatic cells including two DTCs at the anterior and posterior extremes. Some of the germinal cells were present at the ends of the elongating arms while the somatic cells largely remained in the centre along with a distinct AC in the middle indicating the site for vulva formation and facilitating vulval patterning. The moulting period marked the proliferation of somatic cells in the centre of the primordium, resulting in its extension. Five core precursor cells, P(4–8).p, for vulva formation were observed out of P(1–12).p ectoblasts at 55–60% of the body length from the anterior end on the ventral aspect of the primordium ( Figure 5e, g View Figure 5 ).
Fourth-stage female juvenile. The genital primordium, located at 43.2–45.1% from the anterior end, showed extensive elongation during this stage. It consisted of two arms directed anteriorly and posteriorly ( Figure 5i, k View Figure 5 , 7i View Figure 7 ). The arms represented the germ line while the central, broader part showed the somatic parts of the future reproductive system. The AC located in the centre and morphologically distinct from other somatic cells is supposed to play a significant role in vulva induction. The cell is also important for signalling to other ventral uterine cells for the connection between the uterus and the outside environment. Of the five vulva precursor cells, three central cells P(5–7).p acquired prominence and gave rise to descendants that actively participated in vulva formation with central cell P6.p, assuming the primary fate whereas P5.p and P7.p assuming secondary fate. The cells forming the vulva appear to be arranged in seven rings stacked one above the other.
In the fourth moulting stage, the two extreme ends of the primordium containing germinal cells reflexed, forming a flexure representing the germ line/ovary, while the remaining gonoduct formed the somatic gonad. The vulva formation along with the differentiation of the somatic gonad was completed with the termination of moulting.
Adult female. The female of C. anisospiculus possessed a didelphic–amphidelphic reproductive system with reflexed ovaries ( Figure 7k, l View Figure 7 ). The germ line of the reproductive system constituted of ovaries with germinal cells in different stages of development, while its somatic line included the oviducts, spermathecae, uteri, vagina and vulva besides the DTCs lodged in the germinal zone and the gonadal sheath. The genital tract of C. anisospiculus was found to have the somatic parts well separated by prominent sphincters ( Figure 7k, l View Figure 7 ). The posterior genital arm was relatively less developed and reduced in size compared to the anterior genital branch. The dorsally reflexed ovaries occasionally showed several small flexures instead of extending posteriorly to an appreciable distance. The anterior ovary was on the right side while the posterior ovary was on the left side of the intestine. Each slender ovary showed a distal cell at the apical end followed by 1–2 tiers of germinal cells in different stages of development. The proximal part of the oviduct was prominently dilated and separated from an ovoid spermatheca with a prominent sphincter. A cluster of round sperm was observed filling the spermatheca. The two spermathecae were separated with uteri by strong sphincters. The uteri were thick-walled. The vagina represented a well-developed and spacious tube at a right angle to the main body axis. The vulva was a transverse slit occupying about 2/3 of the corresponding body diameter, surrounded by lateral cuticular flaps ( Figure 5o View Figure 5 ).
Morphology of gonad development in the male ( Figure 7 View Figure 7 )
The genital primordium was similar in all first-stage juveniles with a fixed number of cells and represented a sexually undifferentiated primordium ( Figure 7a View Figure 7 ). Initially the primordium of J2 exhibited a mixed arrangement of somatic and germinal cells appearing as descendants of Z1/Z4 and Z2/Z3, respectively. However, during the moulting period, the anterior localisation of somatic cells and the resulting anterior primordial growth indicated the male sex of the juvenile ( Figure 7b View Figure 7 ). By the end of moulting, two DTCs were positioned at the posterior distal end and the somatic cells showed an anterior aggregation in the primordium, indicating its future direction of elongation.
Third-stage male juvenile. Third-stage male juveniles of C. anisospiculus possessed a genital primordium situated at 48.1–48.4% from the anterior end. It consisted of 4–6 germinal and 8–16 somatic cells including two DTCs at the distal end and a linker cell at the proximal end. The primordium showed anterior growth due to the proliferation of the somatic cells ( Figure 7d View Figure 7 ) and eventually developed a flexure during the moulting period. Two dense cellular aggregates were observed, each on one dorsolateral side of the rectum, representing the precursors of the spicules and associated structures.
Fourth-stage male juvenile. The fourth-stage male juveniles possessed a genital primordium at 47.9–49.5% of the body length from the anterior end. The genital primordium showed a small but relatively broader reflexed part showing the germ line of the future gonad constituted of germinal cells and a considerably longer tube elongating posteriorly ( Figure 7h View Figure 7 ). The tube contained proliferating somatic cells with the linker cell placed ahead to join with the rectum to form a cloaca during the late moulting stage. The number of germinal cells in the fourth-stage male juveniles ranged from 8 to 20 while the somatic ones were 20–38 in number. The spicular region ( Figure 7f View Figure 7 ) showed more compaction of approximately 28–40 cells that formed the spicules in the late fourth stage. The bursa, together with the genital papillae, was the last structure to develop. The latter was formed by special hypodermal cells.
Adult male. The males possessed a monorchic reproductive system as is customary for rhabditids, with a ventrally reflexed testis ( Figure 7j View Figure 7 ). The germ line constituted the testis with germinal cells in different stages of development while the somatic line comprised the seminal vesicle, vas deferens, ejaculatory duct and cloaca besides two DTCs at the distal end of the testis and the gonadal sheath. The proximal part of the testis containing spermatocytes gradually widened into a seminal vesicle. The latter was filled with spermatids. The seminal vesicle had a larger outer diameter than either the testis or the posteriorly located vas deferens. The vas deferens represented a long tube differentiated into an anterior narrower part that connected to the wider seminal vesicle; the middledistended part, presumably glandular in nature, comprising cuboidal cells; and the posterior part, often referred to as the ejaculatory duct, joined with rectum to form the cloaca. The accessory reproductive organs, spicules and gubernaculum were lodged in the pockets formed by the cloaca.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.