Collohmannia johnstoni, Norton & Sidorchuk, 2014
|
publication ID |
https://doi.org/ 10.1051/acarologia/20142134 |
|
persistent identifier |
https://treatment.plazi.org/id/03B5A03F-BE61-FFBB-C02F-F9AE6970F594 |
|
treatment provided by |
Marcus |
|
scientific name |
Collohmannia johnstoni |
| status |
sp. nov. |
Collohmannia johnstoni View in CoL n. sp. ( Figs. 1-19 View FIGURE View FIGURE View FIGURE View FIGURE View FIGURE View FIGURE View FIGURE View FIGURE View FIGURE View FIGURE View FIGURE View FIGURE View FIGURE View FIGURE View FIGURE View FIGURE View FIGURE View FIGURE View FIGURE )
Diagnosis — With characters of Collohmannia (see Sellnick 1960 and below).
Adult. Total length 1435-1945 µm. Hysterosoma slightly to significantly compressed dorsoventrally, 1.2-1.5 times broader than high. Bothridial seta filiform, gradually tapered in distal third; with minute, rather sparse barbs; dorsolaterally directed with gentle sigmoid curve in middle third. Notogaster with five pairs of subflagellate setae (e 1, e 2, h 1, h 2, p 1), noticeably longer than others. Leg tarsus I with four solenidia; neotrichy limited to several (lateral) setae; tarsus of both sexes similar, not noticeably swollen relative to other tarsi, male without ribbon-like setae. Tarsus II with setal pair (pl). Seta v" of male genu IV hypertrophied, in form of large, flattened, asymmetrical diamond, with low crest across widest part and distally tapered to point that extends well beyond end of segment; tibia IV not modified.
Ontogeny. Setal formula of protonymphal leg IV 0-0-0-0-7; tectal pair forms in deutonymph. Iteral setae form in tritonymph on tarsi I-III, absent from IV; accessory pair (v 3) forms in tritonymph on tarsus IV.
Adult
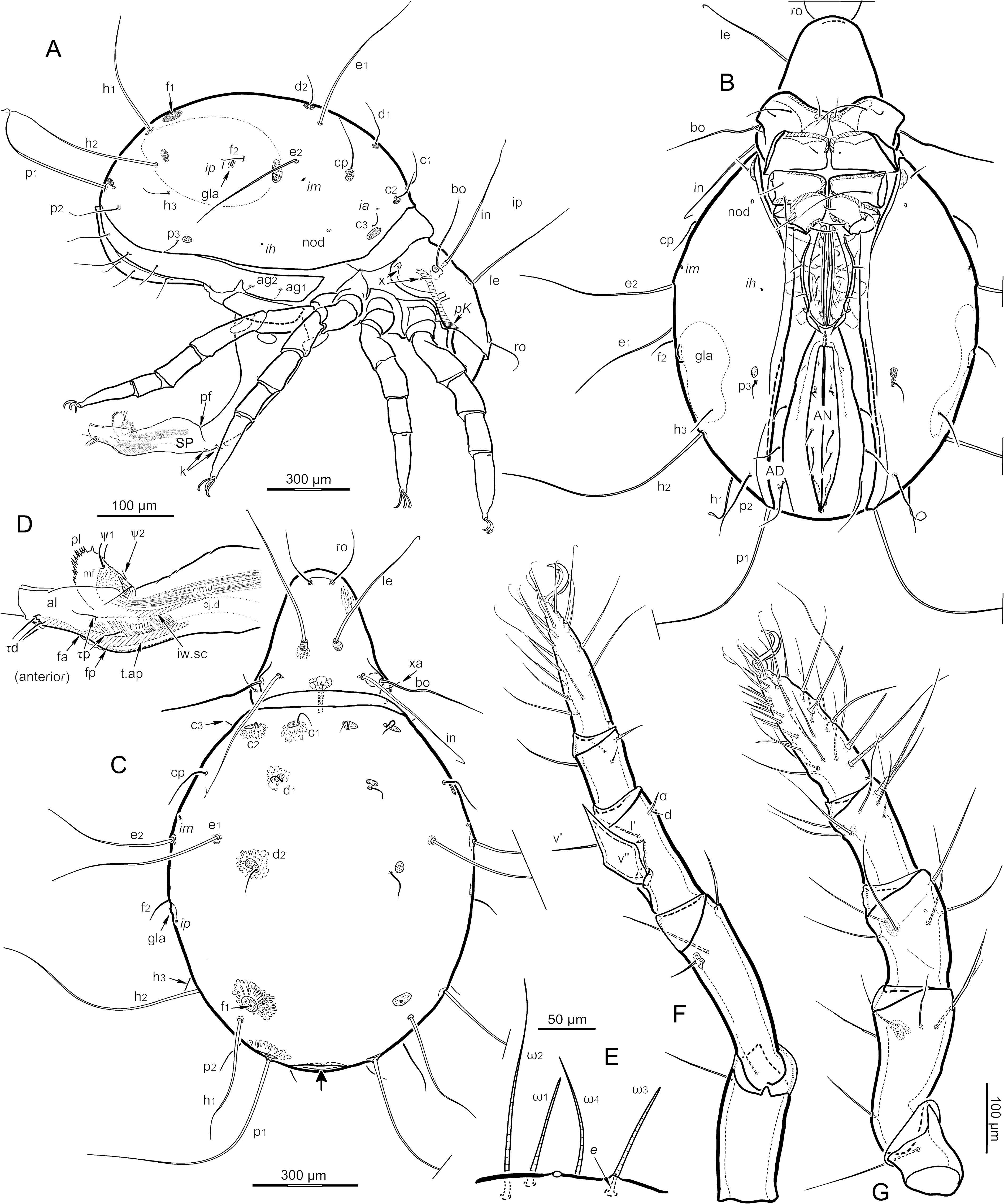
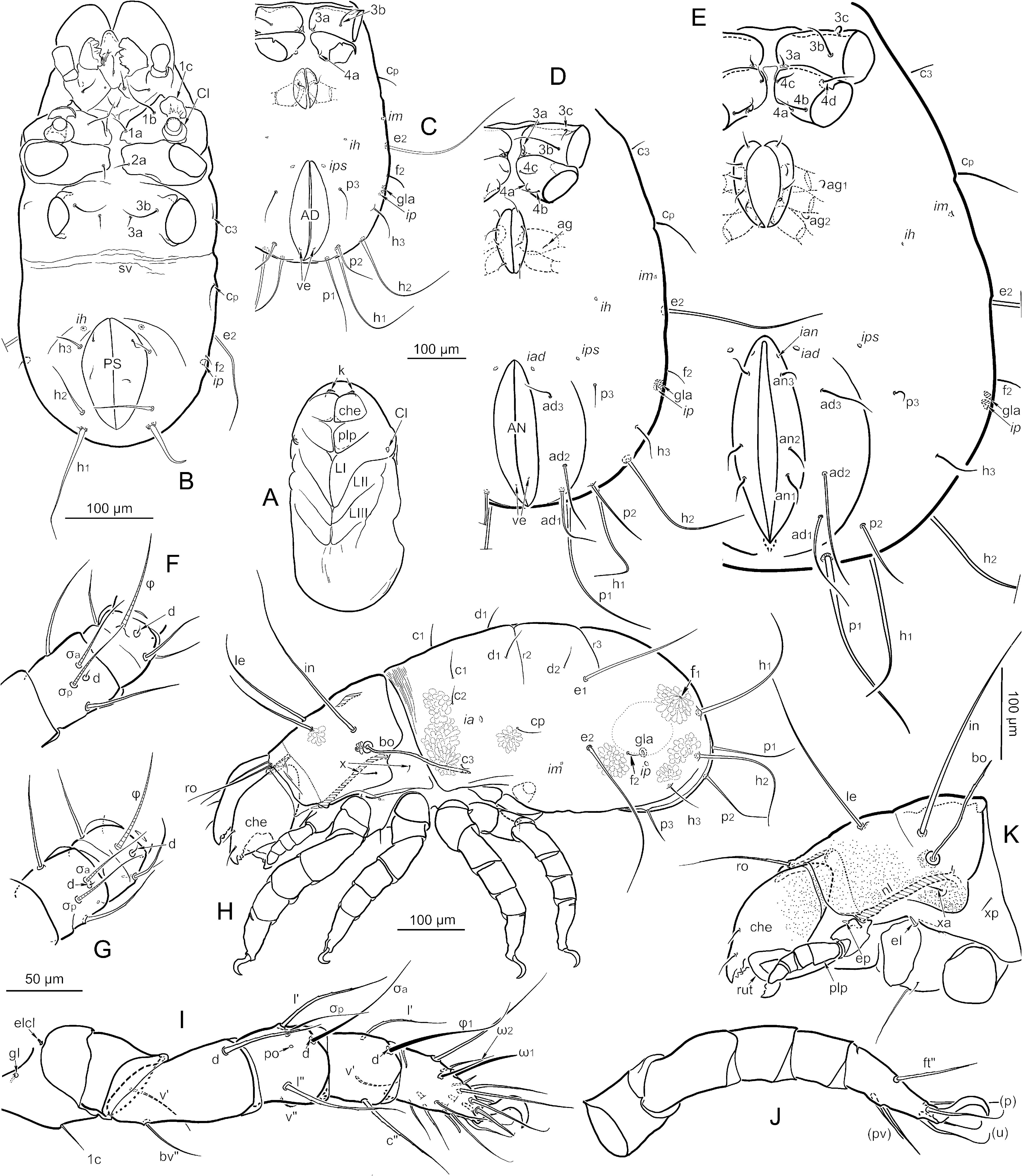
Dimensions, color and general form ( Figs. 1-4 View FIGURE View FIGURE View FIGURE View FIGURE ) — Adults are large, dichoid mites having a sejugal articulation that is broad dorsally but narrow ventrally; relative movement of proterosoma and hysterosoma therefore is restricted mostly to modest dorsoventral flexing, with the base of the prodorsum capable of slight telescoping under the notogaster, particularly in males. The total length of females (n = 49) ranges from 1542-1950 µm (mean 1767) and that of males (n = 35) 1435-1688 µm (mean 1555). Maximum width is respectively 970- 1333 µm (mean 1148) and 800-1058 µm (mean 946), slightly less than 2/3 the total length. In both sexes the hysterosoma is usually about 1.4 times wider than high at the level of the genital plates (mean 1.36; range 1.23-1.55), and is sub-elliptical in crosssection ( Figs. 1D View FIGURE , 10B View FIGURE ); the under-turned part of the notogaster can occupy ¾ of the width in ventral aspect. Non-gravid, contracted females can have a rather flattened venter. In dorsoventral aspect, outlines are well-rounded ( Figs. 1A, B View FIGURE ; 2A, B View FIGURE ): that of the prodorsum is somewhat pear-shaped in both sexes and the notogaster is usually elliptical or (in females) sometimes slightly ovate, wider anteriorly (see below).
In reflected light, living mature adults are opaque and nearly black ( Fig. 19D View FIGURE ). In living teneral adults and in adults long-preserved in alcohol there is more heterogeneity of color, as follows. The notogaster is medium to dark reddish-brown ( Fig. 2 View FIGURE ), with a darker, narrow collar along the anterior border. The mid-region usually appears somewhat darker, due at least in part to internal objects that show vaguely through the slightly translucent cuticle; these can include food and fecal boli, but most noticeably the region around the pair of large opisthonotal glands usually darkens in alcohol (’autocoloration’ of Raspotnig et al. 2001). One anomalous male had a variegated pattern ( Fig. 2D View FIGURE ). In females, eggs nearest the surface can be discerned through the cuticle. Ventral sclerites and legs are medium brown, mostly lacking the reddish tint. The prodorsum is the lightest major sclerite, mostly yellowish brown. It appears darker anteriorly due to the doubled cuticle of the rostrum and to the underlying mouthparts showing through the slightly translucent cuticle; the posterolateral edges are also darker, as described below. Articulating cuticle is cream-colored and setae are hyaline and shine in reflected light, contrasting strongly with the pigmented sclerites ( Figs. 2 View FIGURE , 9A View FIGURE , 10 View FIGURE ).
The genders tend to differ in several body measurements and proportions, but these overlap and therefore are not definitive. For example, adults longer than 1700 µm are invariably female, and those shorter than 1500 µm are invariably male. Females usually have a proportionally larger hysterosoma, sometimes much larger: measured in dorsal view, the projected surface area of the notogaster is 8-17 times larger than that of the prodorsum, while in males it is less variable and usually smaller, 6-9 times. In ventral view, the prosoma (measured along the midline from tip of rostrum to posterior end of coxisternum) usually occupies a slightly greater proportion of the body length in males (0.36-0.44) than in females (0.33-0.39). Another difference is that usually the notogaster of females is proportionally broader anteriorly; as a result, in the humeral region a line drawn from the intersection of prodorsal and notogastral outlines to the insertion of seta cp on the same side forms an angle with the body axis that is 36-48º in males ( Fig. 2D View FIGURE ) but 48-57º in females.
Other than the highly modified genu IV seta v" of males (see below), two traits easily distinguish sexes ( Fig. 2A, B View FIGURE ). Measured along the sagittal plane, the genital plates of females are slightly longer than the coxisternum, while in males they are slightly shorter than the coxisternum. Second, males have proportionally larger legs. Specifically, when leg IV is directed posteriorly, in females the femur reaches only to the posterior end of the genital plate, whereas in males it reaches further, to a level about one-fifth of the distance along the anal plate.
Integument ( Figs. 5 View FIGURE , 7 View FIGURE , 9 View FIGURE ) — Sclerotized cuticle is shiny in reflected light, and lacks apparent cerotegument or adherent debris. The notogaster and plates of the anogenital region – but not the prodorsum or coxisternum – have imbricate surface sculpture, giving a ’shagreened’ appearance that is discernible in transmitted light ( Fig. 5 View FIGURE A-C, G), but most apparent in reflected light and under SEM ( Fig. 6C View FIGURE ). Articulating cuticle is simple and cream-colored in preserved specimens, but some broad articulations are bordered with cuticle having both the color of articulations and the imbricate surface sculpture of sclerites (t.cu, Fig. 5A, G View FIGURE ). This type of cuticle is referred to below as transitional; its appearance and location is consistent with having elasticity that is intermediate between that of the harder sclerite and the soft articulating cuticle, but this is supposition. Seen in transmitted light, nearly all sclerotized cuticle has dense, fine porosity, but small circumscribed areas of more luminous and slightly larger pores (porose areas) exist and are conspicuous in transmitted light ( Figs. 3-5 View FIGURE View FIGURE View FIGURE ). The alveolus of most body setae is surrounded by a small area or single ring of such broader pores ( Fig. 5 View FIGURE D-G), and often a larger porose area (po in the figures) is adjacent to the seta or a short distance away. These porose areas have different forms and sizes, as described below, but each seems to be underlain by a cluster of gland-like cells (gl, Fig. 5B View FIGURE ; see Remark 1).
Certain regions of several structures, including the labrum, spermatopositor and chelicera, have special cuticle in which sclerotized, pigmented layers are overlain by sharply contrasting layers that are hyaline and flexible. We refer to these as ’embedded’ sclerites.
Prodorsum ( Figs. 1 View FIGURE , 3 View FIGURE , 4 View FIGURE , 7-9 View FIGURE View FIGURE View FIGURE ) — The sclerotized cuticle of this region forms an isolated aspis, i.e. it is separated laterally from epimeres by soft cuticle ( Figs. 7A View FIGURE , 9A View FIGURE ). Its outline in dorsal aspect is broadest near the level of the bothridia, abruptly narrowing anterior to setae in, then narrowing slightly and gradually to the broadly rounded, smooth-edged rostrum. In lateral aspect it curves only slightly from its base to the rostral setae, then slightly more strongly to the smoothly rounded rostral margin. The length, comprising about 25-35 % of the total body length, is about equal to the maximum prodorsal width in dorsal aspect. The cuticle of the aspis is uniform in having dense fine pores, which are absent only from the pale, thin edge of the rostrum. Prodorsal setae generally have no conspicuous basal ring of larger pores, and only one (paired) porose area exists, usually positioned posterior and adjacent to the lamellar seta (po, Fig. 7A View FIGURE ); rarely the seta inserts eccentrically within the area. This round area (ca. 30-50 µm diameter) shows as a sharply defined macula in transmitted light, made pale due to the thinness of its internally excavated cuticle ( Fig. 7E View FIGURE ); it looks superficially similar to a muscle sigillum, but is underlain by gland-like cells, not muscles (gl, Fig. 7F View FIGURE ).
With minor exceptions, the aspis surface is smooth and without imbricate sculpturing or obvious relief features. The exceptions include a slight medial bulge on the rostrum (rb, Figs. 1C View FIGURE , 7 View FIGURE A-D) and two indistinct transverse grooves – one linear, just anterior to the interlamellar setae (sz, Fig. 1A View FIGURE ) and a second, broader groove anterior to the lamellar setae. There is also a small longitudinal groove posteromedially, above the sagittal apodeme ( Fig. 1A View FIGURE , arrow).
Seen in sagittal section, the rostral tectum occupies the distal 40 % of the aspis ( Fig. 7B View FIGURE ) and has distinct regions. The distal edge comprises a very narrow, uncolored tectal limb (tl), ca. 20-30 µm wide. Throughout the rest of the tectum the dorsal and ventral (rostrophragma, rp) cuticles are clearly distinguishable. These cuticles are well separated in the region of the rostral bulge ( Fig. 7B, D View FIGURE ), then come together at mid-length, and then separate in the posterior region of the tectum where the sclerotized rostrophragma diverges from the surface cuticle. At its posterior end the rostrophragma attaches (at *) to the soft cheliceral frame (c.fr, Fig. 7C View FIGURE ). A pair of paler regions lie posterolateral to the rostral bulge; here the two cuticular layers nearly touch (large black arrow in Fig. 7A, D View FIGURE ), and the internal surface is excavated to form irregular striae, seen in transmitted light ( Figs. 3A View FIGURE , 4C View FIGURE , 7A View FIGURE )
Two regions of the aspis margin are distinguishable in lateral view, with the inward-facing prodorsal condyle (pK) marking their boundary ( Figs. 4A View FIGURE , 9D View FIGURE ). The simple, thin edge of the rostral tectum occupies the anterior half, with a weakly concave outline. Running proximally from pK, the aspal margin appears doubled by a distinct submarginal carina (car, Fig. 9D View FIGURE ); it runs almost straight above leg I, but turns ventrally in the exobothridial region to form the border of a large exobothridial lobe (ex.l; Fig. 1C View FIGURE ). This lobe is a simple extension of the prodorsal aspis, not an overhanging ’prodorsal tectum’ (see Grandjean 1970). Condyle pK ( Figs. 4A View FIGURE , 9D View FIGURE ; see also Fig. 17A View FIGURE ), which articulates with the posterodorsal corner of the subcapitulum, is the enlarged anterior end of an oblique, straight rib-like thickening (’nervure latØrale’) on the inner face of the sclerite (nl, Figs. 3D View FIGURE , 4A View FIGURE , 7E, F View FIGURE , 9D View FIGURE ). Posteriorly from the condyle, the rib follows the straight margin above leg I, then continues to traverse the base of the exobothridial lobe between the bothridium and seta xa, before curving ventrally and effacing in the middle of the lobe ( Fig. 9D View FIGURE ). No muscles were seen attaching at the rib; its function seems to be lateral stabilization and support of the distal condyle.
The posterior margin of the aspis projects ventrally at a right-angle from its attachment (*) with the soft sejugal cuticle; it forms a thick, vertical occipital phragma (op, Fig. 7B, H View FIGURE ), an apodematal wall that connects laterally to the exobothridial lobes. A portion of the large extrinsic muscles of the chelicera (mu, Fig. 7 View FIGURE F-H) originate on the anterior face of the phragma; others originate from the thin, shallow sagittal apodeme (sa, Fig. 7A, G View FIGURE ), ca. 65-85 µm long, and from sigilla on either side and immediately anterior to it; yet other cheliceral muscles originate from patches of sigilla on the exobothridial lobe and anterior to the bothridium (sg, Fig. 7A View FIGURE ). On the posterior (hysterosomal) side of the occipital phragma, prodorsum-retractor muscles that originate in the hysterosoma insert via numerous tendons (te, Fig. 7H View FIGURE ). The insertions are immediately below the attachment of the sejugal cuticle (not shown, see Fig. 7B View FIGURE , sj.c), side-by-side in a row behind each bothridial region. Several other tendons from probable retractor or adjustor muscles insert together at a small sigillum on the posterior edge of the exobothridial lobe (one muscle, mu, is visible on Fig. 7H View FIGURE ).
Prodorsal setation is normal ( Figs. 3 View FIGURE , 4 View FIGURE ). Interlamellar (in) and lamellar (le) setae are smooth, erect, subflagellate, ca. 550 and 450 µm long, respectively; in inserts medial to the bothridia (mutual distance of pair ca. 280 µm); seta le inserts at about mid-length of the aspis (mutual distance of pair ca. 90 µm). The finely attenuated rostral seta (ro) is directed anteriorly and distally curved (ca. 160 µm long); the pair is inserted on the rostral bulge (mutual distance ca. 60 µm). Both exobothridial setae insert on the exobothridial lobe and are short, simple; xa (ca. 50 µm) is transversely aligned with seta in, whereas xp (ca. 25 µm) inserts near the posterior margin of sclerotization.
The bothridium ( Fig. 8 View FIGURE ) opens on a slight bulge, about equidistant between setae in and xa. It has a small, inconspicuous circular opening (arrows, Fig. 8A, D View FIGURE ), less than three times the width of the bothridial seta, with a narrowly reinforced rim and a complex internal structure that surrounds the tight sigmoid bend at the base of the seta. Moving inward from the opening, three distinct regions can be recognized by the form of their cuticular wall. The first (br.1, Fig. 8A, B, D View FIGURE ) is a cup-like chamber, lined with dense spicules and twice as wide as the opening, through which the bothridial seta first descends. The next (br.2, Fig. 8 View FIGURE B-D), surrounding the U-shaped bottom curvature of the seta, comprises several dozen contiguous locules (lo, Fig. 8C View FIGURE ) – sausage-shaped outpockets from the central tube that collectively appear like the drupelets of a raspberry; the locules are thin-walled but not porose, and none is extended as a trachea or brachytrachea. The third region (br.3, Fig. 8A, B, D View FIGURE ) lies at the top of the sigmoid bend; its broad outpockets form irregular lobes with very thick walls having an intricate pattern of narrow slits, somewhat resembling a brain-surface when viewed flat. The slits resemble broad pore canals when viewed in optical section ( Fig. 8A, D View FIGURE ), but they open into the chamber, rather than into the body as pore canals do. This innermost region constricts abruptly to form a narrow collar that surrounds the short, descending terminal bend just prior to the actual setal insertion (*, Fig. 8D View FIGURE ). External to the bothridium, the bothridial seta (bo, Fig. 3E; M View FIGURE 370, F440 µm long) is filiform, with rather sparse, inconspicuous barbs; it is directed dorsolaterally with a gentle sigmoid curve in its middle third. It is isodiametric for most of its length (basal two-thirds to more than nine-tenths), then tapers to a narrow but stiff tip. A hyaline, isotropic external layer covers the entire seta and includes the isotropic barbs, but the layer is most easily distinguished from the birefringent core at the setal tip (iso, Fig. 8E View FIGURE ).
Notogaster ( Figs. 1-6 View FIGURE View FIGURE View FIGURE View FIGURE View FIGURE View FIGURE , 10 View FIGURE ) — The notogaster comprises about 65-75 % of the total length, generally a higher percentage in females than in males (see above). Seen in dorsal aspect, it is 1.1-1.5 times as long as wide: while there is great overlap, males have the largest ratios and gravid females the smallest. The cuticle is imbricate dorsally and lateroventrally, but the pattern is weak or absent in the pygidial region and in a wide pleural band that reaches anteriorly to the level of lyrifissure im. The ’cells’ of the imbrication vary in shape (from diamond to rhomboid, Figs. 5A, G View FIGURE , 6C View FIGURE ), but are similar in height, mostly between 10-15 µm. The cell length varies greatly, from about 10 to more than 100 µm; cells are aligned end-to-end, in a pattern that generally parallels the notogastral margin (i.e., transverse in the sejugal region, longitudinal in the lateroventral region). The anterior margin of the notogaster is slightly constricted, forming a collar (col, Figs. 1A, C View FIGURE , 5A View FIGURE ) that appears darker than the rest of the notogaster in preserved specimens. The juncture of this collar with the broad sejugal articulation (sej) is distinct, but it comprises one of the transitional articulations noted above; i.e. the dark, well-sclerotized imbricate collar is bordered by more flexible, pale, imbricate transitional cuticle (t.cu, Fig. 5A View FIGURE ), which then blends into soft, pale cuticle that lacks surface pattern. In lateral aspect the three types of progressively more flexible cuticle often appear tiered, but when the prodorsum is retracted most of the soft cuticle can be hidden ( Fig. 5A View FIGURE ). Usually there is a slight incursion of paler imbricate cuticle into a crease or undulation in the collar, above seta c 3 ( Fig. 5A View FIGURE ).
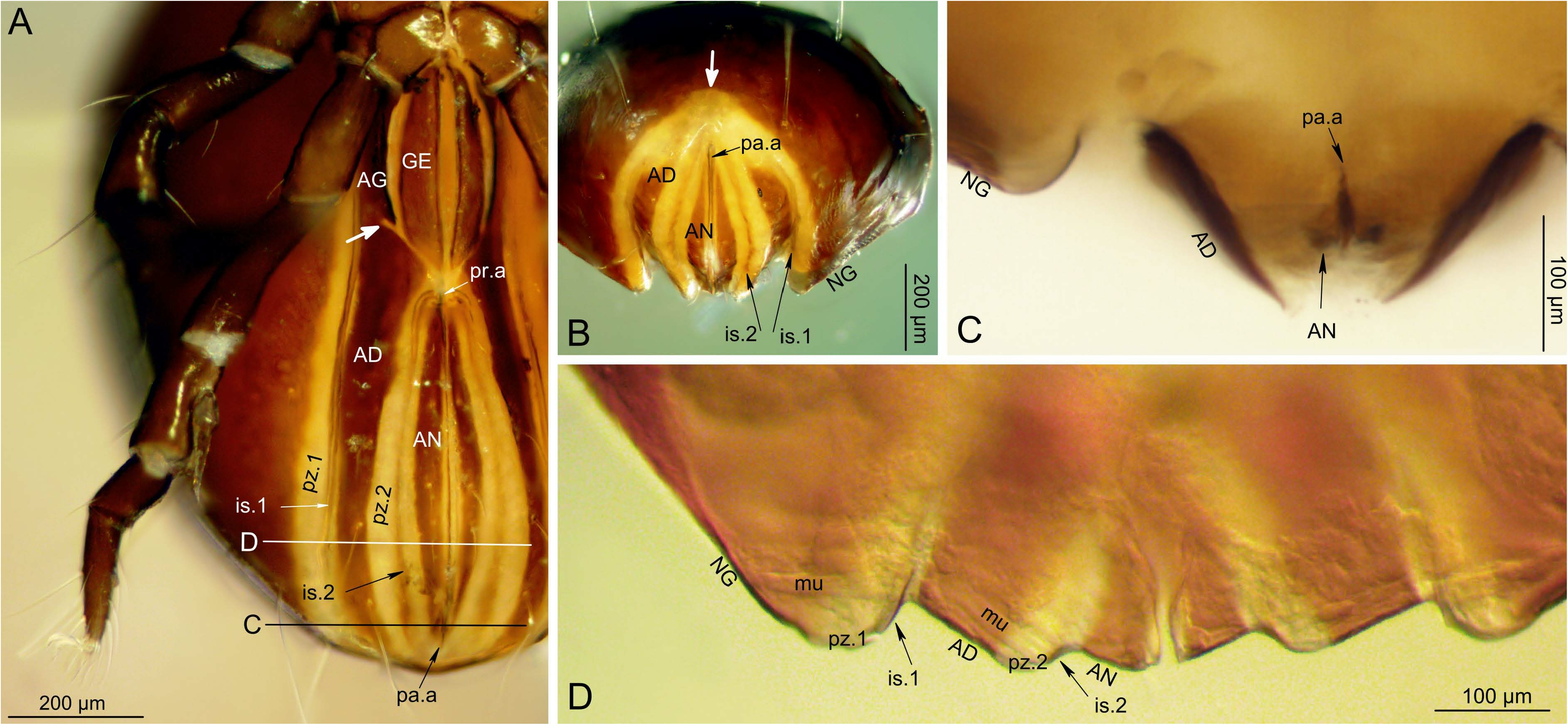
The body form is considered dichoid, since a sejugal articulation runs completely around the mite, but the width of the articulation is not uniform. The broad dorsal part continues around the notogaster as a circumgastric band (in the fashion of holoid taxa), such that the articulation of the notogaster with ventral plates has a similar structure and range of flexibility. In the anogenital region the articulation forms a plicature zone (pz.1, Fig. 10A, D View FIGURE ), folded or exposed according to degree of body distension. Posterior to the anal region the paired zones flatten and merge to form a terminal sinus, a permanently exposed pale region of transitional cuticle; in posterior view the sinus appears rounded or triangular (arrow, Fig. 10B View FIGURE ), and it is visible even in dorsal view as a pale terminus of the body (arrow, Fig. 4C View FIGURE ). Just lateral to the top of the plicature angle, a thin intercalary sclerite (is.1, Fig. 10A, B, D View FIGURE ) supports the insertions of the transverse compressor muscle band originating on the notogaster.
Notogastral setae comprise 15 pairs, plus a distinct alveolar vestige of seta f 1. All setae are smooth, simple, but they vary greatly in size. Five pairs are long, flagellate and conspicuous: e 1 (M580, F660 µm); e 2 (M580, F550 µm); h 1 (M490, F440 µm); h 2 (M640, F590 µm); and p 1 (M490, F540 µm). These large setae insert on low tubercles and are slightly flattened at their base, which is also expressed in the shape of their insertion in the alveolar membrane (p 1, Fig. 5E View FIGURE ). Other setae are shorter and finely attenuate; in approximately decreasing order of size, they include: p 2 (M220, F230 µm); cp (M150, F230); c 1 (M170, F200 µm); d 1 (M170, F200 µm); c 2 (M170, F170); h 3 (M150, F 100); p 3 (M120, F120 µm); d 2 (M110, F100 µm); f 2 (M90, F110 µm); and c 3 (M55, F65 µm). Most notogastral setae have at least a small ring of porosity at their base that stands out from the general cuticular porosity by having pores that appear slightly larger and more luminous in transmitted light ( Fig. 5E, F View FIGURE ). This ring is similar in diameter for all notogastral setae (except vestige f 1), such that it is narrower around the five flagellate setae, which have a relatively large alveolus. The porose rings around medium to small setae are each closely adjacent to, or merged with, a larger porose area that contrasts with the background porosity also by having cuticle of darker color ( Fig. 5 View FIGURE D-G). Most of these larger areas are slightly oblong, 20- 90 µm at their widest dimension. If they merge with the porose ring around a seta, the appearance is similar to that of the excentrosclerites of juvenile oripodoid mites (e.g. Grandjean 1959b); alveolar vestige f 1 differs in being always well within a relatively large and often circular porose area (50-90 µm). The position of the large porose areas varies considerably among and within individuals: not only can they be attached or slightly removed from the ring (with a few exceptions noted below), but the diameter of a given area can vary by 100 %. Porose areas in the anterior half of the notogaster – those associated with c -row setae, d 1 and e 1 – are generally lateral or anterolateral to the seta. The area near f 2 is usually removed anterodorsally from the seta, evenly spaced and aligned with ip and gla ( Figs. 3D View FIGURE , 5D View FIGURE ), or is shifted further anteriorly ( Fig. 4A View FIGURE ); it is often the largest individual porose area (75- 95 µm). Another porose area (40-60 µm) is either dorsal to seta h 3 ( Fig. 3D View FIGURE ) or slightly anterior to h 2 ( Fig. 4A View FIGURE ). An elongated porose area dorsal to large seta p 1 can be 100-130 µm long, but it may be broken into smaller sections ( Fig. 5E View FIGURE ); a small porose area is closely anterodorsal to p 3, but no separate area was seen near seta p 2.
These porose areas appear to be dermal glands (see Remark 1). On lightly cleared specimens with soft tissues somewhat preserved, each area (including that near prodorsal seta le) is underlain by an umbrella-shaped cluster of drop-like, sometimes elongated cells. While similar in color to surrounding tissue, the cells are clearly defined under brightfield illumination, and the cluster is always centered on the porose area ( Figs. 4C View FIGURE , 5B View FIGURE ). Seen perpendicular to the surface, the diameter of each cluster is about three times that of the respective porose area, and its depth is about the diameter of the area. Isolated, individual large pores are present in the region posterior to setae h 2 and h 3 (por, Fig. 5F View FIGURE ), but we could not determine their nature.
The typical five pairs of lyrifissures are present, but not uniform: ia, im and ip are ca. 20-25 µm long and slit-like, i.e. formed as typical lyrifissures ( Fig. 5A, D View FIGURE ); ia is located between setae c 2 and c 3, im is anteroventral to seta e 2, and ip is closely posterior to the opisthonotal gland opening (gla). By contrast, lyrifissure ih is small (ca. 10 µm) and almost pore-like, inconspicuous among the imbricate pattern near the ventral margin of the notogaster, at about mid-length ( Figs. 3C View FIGURE , 4B View FIGURE ). Lyrifissure ips is similar, but is on the transitional cuticle (pale but imbricate) of the plicature fold, at a level just anterior to seta p 3 ( Figs. 3B View FIGURE , 5G View FIGURE ), and is difficult to see without dissection. In transmitted light, a small, dark internal nodule is visible approximately aligned between ih and seta c 3 (nod, Figs. 3C, D View FIGURE , 4A, B View FIGURE , 5B, C View FIGURE ); tendons from the dorsum attach here. The opisthonotal gland is flat, lens-like, with a diameter of ca. 450 µm.
Coxisternum ( Figs. 1B View FIGURE , 2A, B View FIGURE , 3C View FIGURE , 4B View FIGURE , 9 View FIGURE ) — The four epimeres are progressively narrower, such that the coxisternum tapers significantly from anterior to posterior; epimere I is 1.4-1.5 times wider than IV. The coxisternum is divided into four parts by a cross of soft cuticle, of which the central intersection is relatively large ( Fig. 9B View FIGURE ). The soft ventrosejugal furrow (or scissure) is narrow but deeply indented (vsj.f, Fig. 1B View FIGURE , 9B View FIGURE ), providing a transverse axis for dorsoventral flexing. The shallow sagittal (or sternal) scissure (st.s, Fig. 9B View FIGURE ) separates the epimeral plate pairs, except for a very narrow connection at the anterior margin of epimere I (often broken during preparation). Each plate of epimere II has a transverse band of weakness (w, Fig. 9B View FIGURE ) at the level of seta 2a, vaguely indicated by lighter color in transmitted light. The medial edges of the paired plates are not uniform: those of epimere I merge in a simple fashion with the sagittal scissure for most of their length; in epimere II the anterior half of each medial edge (stopping at w) turns inward along the sagittal scissure, forming a thick, dark margin in transmitted light (sm.2, Fig. 9B View FIGURE ); in epimere III a similar margin reaches posteriorly past seta 3a; and in epimere IV a much weaker inturned margin (sm.4) reaches posteriorly to seta 4c. The sagittal scissure widens posteriorly to form a triangular patch of cuticle behind the plates of epimere IV. While it appears soft relative to the epimeres, the central region of this triangle is lightly sclerotized and porose, and its posterior edge is slightly concave, forming a saddle (sd, Fig. 9B View FIGURE ) that accommodates the anterior margin of the genital plates. The unsclerotized lateral parts of the triangle merge with the soft postpedal furrow that isolates epimere IV from the plates of the genital region. By contrast, the broadly triangular region of cuticle between epimere I and the subcapitulum is entirely soft.
On each side of the sagittal scissure, the fusion between plates of epimeres I and II is complete, as is that between III and IV. However, the lines of fusion are well marked externally by shallow grooves ( Fig. 1B View FIGURE ) and in transmitted or reflected light by dark, slightly oblique epimeral borders ( Fig. 2A, B View FIGURE ). Border III (bo.3, Fig. 9B View FIGURE ) appears to fork near the medial margin to encompass seta 3a. The epimeral plates are strongly and evenly porose, and generally lack circumscribed areas of larger pores (i.e. porose areas), but there are two locations that might represent the porose area of dermal glands. One surrounds seta 3a, filling the forked medial part of bo.3; the pores within this small circular area are more luminous, and the cuticle is thinner. A similar small area underlies seta 1a. On each side of epimere IV, a strong internal ridge (r) runs posteriorly from bo.3 to the insertion of seta 4d, and may extend further to merge with the small marginal condyle (con) that articulates with trochanter IV. In some specimens, a vague analogous internal ridge runs obliquely across the lateral half of epimere III from bo.sj to the respective marginal condyle, passing near seta 3c.
Apodemes I and II, extending internally from the anterior margin of their respective epimeral plate, are roughly triangular in form, reaching a peak near mid-width of their plate; apodeme I (ap.1, Fig. 9B View FIGURE ) has its medial limit near seta 1a, while apodeme II starts closer to the midline and rises more steeply. The sejugal apodeme is smaller and less conspicuous; it rises from the sejugal border of epimere III, starting near the medial margin, rising to a peak at about the middle of the border, then dropping quickly away to disappear well medial to the leg insertion. Apodeme III is highest near the leg insertion, then gradually diminishes to end medially at the fork in bo.3. There is no apodeme IV.
The small supracoxal region of each epimere, above the leg insertions ( Fig. 9A View FIGURE ), serves as the origin for muscles; most of these appear to insert on the endosternum as suspensors, though they were not studied thoroughly. On epimeres II and IV the supracoxal region is simply constructed – it is the dorsal part of a short, subcylindrical extension of the epimere to which the trochanter articulates – but on I and III there are other features. Above leg I the small supracoxal seta (eI) inserts just proximal to the articulation with the trochanter; it is ca. 12 µm long, spiniform but with a rounded tip ( Fig. 9D, E View FIGURE ). The sclerotized supracoxal cuticle expands anteriorly, rising dorsally from its general curvature, to form a broad flange along which the epimere meets the soft pleural cuticle; the flange ends in a short beak-like extension ( Fig. 9D View FIGURE ) that is isolated by a narrow but distinct notch or groove. At the posterior end of this flange is the typical gland opening (g), from which products of coxal and lateral accessory glands presumably emerge (see Woodring 1973 for a detailed description of the glands of C. gigantea ). The gland opening and ducts are well visible, but no clearly defined podocephalic canal was observed in this region, even though it is distinct where it crosses to the subcapitulum (see below). The supracoxal region of epimere III rises in a large triangular extension (tr.e, Fig. 9 View FIGURE A-C) from which large muscles run to the endosternum. The extension ends in an internal apophysis on which insert thin tendons or fasciae of several groups of muscles that appear to originate on the notogaster. Near the base of the triangle two long tendons insert at a small, but clearly marked sigillum (sg, Fig. 9C View FIGURE ).
The epimeral setation (I to IV) is 3-1-3-4. Coxisternal setae all are smooth and finely attenuate, but they differ widely in size: 3a is shortest (ca. 40 µm); 1b and 3b are conspicuously long (240, 270 µm), about twice the length of the next longest seta, 4b (130 µm); setae of epimere I are about 60-70 µm; others are about 90-100 µm long.
Anogenital region ( Figs. 1B View FIGURE , 2A, B View FIGURE , 3 View FIGURE B-D, 4A, B, 6C, 10) — The venter comprises three pairs of rather narrow plates – genital, anal and fused aggenitoadanal plates – that collectively occupy the area behind the coxisternum. In highly distended specimens ( Fig. 3B View FIGURE ) their collective width does not vary much longitudinally, but in more laterally contracted specimens ( Fig. 3C View FIGURE ) the venter assumes a rather elongated pear-shaped outline. All sclerotized plates are porose and have imbricate cuticular sculpturing, although the latter can be quite faint on the anal plates. In cross section all plates are oblique and connected to adjacent plates by softer cuticle that is angled in the opposite direction; thus, each pair forms a ’V’ that becomes flatter with greater hysterosomal distension ( Fig. 10 View FIGURE ). Including the notogaster, the folded effect gives the cross section a flattened W form on each side ( Fig. 10D View FIGURE ).
The genital plates comprise ca. 1/3 the length of the anogenital region in both sexes. Together, they occupy a narrow elliptical space in ventral aspect that is usually more elongated in females: ca. 2.5- 3.5 times longer than broad (F) vs. ca. 2.0-2.5 (M), though proportions vary with plate orientation and degree of hysterosomal distension. Seen flat, each genital plate is at least twice as broad at the oblique posterior margin as at the tapered, rounded anterior end. In lateral view, the outline is distinctly convex, bulging more strongly outward in the anterior half, and the anterior margin projects internally as a small apophysis for muscle attachment. In the anterior half, a narrow strip of the medial margin of each genital plate is delineated by a low carina from the row of genital setae (see below); in the posterior half a separate low carina parallels the margin but this is medial to the setal row. The actual edge of the valve comprises a narrow band of pale cuticle; this is the limit of the unsclerotized cuticle that forms the wall of the genital vestibule (pregenital chamber). Genital setae are aligned in a single row near the medial margin, and they spread along almost the entire length of the plate with spacing that is slightly closer anteriorly ( Figs. 3C View FIGURE , 6C View FIGURE ); rarely, a posterior seta may be inserted away from the margin. Genital setae are simple, finely attenuate, with lengths that decrease gradually from anterior (usually 60-80 µm) to posterior (30-40 µm). Usually, there is no noticeable ring of larger, more luminous pores around the alveolus, and no separate porose areas were seen. There are 7, 8 or 9 setae on each plate; respectively, these numbers were on 2, 7 and 15 of 24 plates examined, and 5 of the 12 studied individuals were asymmetrical in setal count.
Together, the anal valves have an outline in ventral view that is lens-like: narrowly elliptical, broadest near the middle and tapered at each end ( Figs. 1B View FIGURE , 3C View FIGURE ). They are convex in lateral view, particularly in the middle of their length ( Figs. 3D View FIGURE , 4A View FIGURE ). The plates themselves are strap-like, ca. 35-50 µm wide in the middle, tapering at both ends ( Fig. 10A View FIGURE ). Along most of their length, the medial edge attaches to soft, pale cuticle lining the anal vestibule (rectal chamber), but anteriorly the plate edges invaginate and fuse to form the linear preanal apodeme, with much of its approximately 120 µm length extending forward from the plates. This apodeme, which is visible through the cuticle in intact mites as a dark line (pr.a, Figs. 2A View FIGURE , 10A View FIGURE ), serves as the origin for part of the long series of transverse ventral compressor muscles that insert at the lateral margin of the adanal plate, as well as oblique muscles leading anteriorly to the genital plates. At the posterior end of the anal vestibule, an unpaired, conical cluster of suspensor muscles from the notogaster tapers to insert on an indistinct postanal apodeme (pa.a, Fig. 10 View FIGURE A-C). Lyrifissure ian is consistently present near the anterior end of each anal plate, longitudinally aligned, and ca. 15-30 µm long; it can have a typical, slit-like form or be slightly oval. The three pairs of anal setae are longitudinally aligned at midwidth; an 1 (100-190 µm) is well anterior to the others, such that ian is about midway between the seta and the anterior end of the plate; an 2 (100-110 µm) is at mid-length, and an 3 (60-100 µm) is less than a setal-length behind it. The setae are simple, finely attenuate, and usually have a narrow ring of luminous pores around the alveolus, A small, inconspicuous, separate porose area is usually present immediately anterior to ad 1.
The two components of the long aggenitaladanal plate are distinguishable by a deep notch of unsclerotized cuticle that separates them along the medial margin, opposite the posterior part of the genital plate; depending on orientation, it can appear linear (large arrow, Fig. 10A View FIGURE ) or V-shaped ( Fig. 4A View FIGURE ). Seen flat, the aggenital portion is subrhomboid, about 100-110 µm wide. It bears two simple, attenuate aggenital setae (ca. 30-45 µm) that are longitudinally aligned in the posterior half of the plate (ag 1, ag 2; Fig. 4A View FIGURE ). These insert eccentrically in small porose areas that are several times as wide as the setal alveolus; no separate porose areas were noticed. Anteriorly, the adanal portion of the compound plate has the same width and direction as the aggenital portion, but it twists toward the side and narrows posteriorly to less than half its anterior width, ending at the same level as the anal plate and intercalary sclerite. The posteriorlydecreasing adanal plate width is complemented by an increasing width of transitional cuticle (pale but porose and imbricate) along its medial side, such that the entire width of the adanal cuticle is similar throughout. The transitional cuticle forms a second plicature zone (pz.2; Fig. 10A, D View FIGURE ) that leads dorsomedially from the medial edge of each adanal plate to form a plicature angle with the anal valve. All along the line of juncture, just lateral to the top of the plicature angle, lies a second intercalary sclerite (is.2, Fig. 10A, D View FIGURE ); this thin, brown band of sclerotization probably helps maintain the form of the fold and serves for insertion of a band of transverse compressor muscles that originate on the lateral edge of the adanal plate. Lyrifissure iad lies anteriorly in the plicature zone, which indicates that pz.2 is part of the adanal segment. Lyrifissure iad is similar to ian in size and form and aligned at the same transverse level, but is slightly oblique in orientation. The three pairs of adanal setae are almost evenly spaced in the posterior half of the adanal portion of the plate, and insert on its margin, just lateral to the beginning of the pale transitional cuticle ( Fig. 3C View FIGURE , 4B View FIGURE ). The setae are simple and finely attenuate; an 2 and an 3 are similar (ca. 200-230 µm), while an 1 is shorter (ca. 100-125 µm); each has a narrow ring of luminous pores around its base. A small porose area lies just anterior to an 3 or may merge with the porose area at its base. Rarely, a fourth adanal seta exists unilaterally, aligned with the others, which may represent a duplication of an 3. When four are present, each of the two most anterior setae can have a separate porose area near its base, and in typical specimens only an 3 has such an area.
Genital vestibule and genitalia — The genital papillae are large, the posterior papilla (ca. 95 µm) being slightly shorter than the other two (ca. 110-120 µm). All have similar form, with a single discrete basal annulus and a larger, slightly flattened ovate distal portion that respectively comprise about … and ¾ of the total length.
The female ovipositor is a thick, densely plicate double-walled tube, typical of oribatid mites in its general form and setation ( Figs. 3D, F View FIGURE , 13 A View FIGURE ; cf. Grandjean 1956; Ermilov 2010, 2011a). When fully extended it is directed anteroventrally (in the descriptions below, one should imagine it projects straight ventrally) and is slightly shorter than the height of the hysterosoma. There are three pairs of short (ca. 30 µm), simple, acuminate coronal setae (k) around the mid-length constriction. The three distal lobes – unpaired posterior (or ventral) and paired anterior (or dorsal) lobes – are relatively short, comprising about one-third the total length of the distal section (i.e. distal to setae k); each is broadly rounded, about as wide as long. The outer face of each lobe is smooth in its distal half, but without evidence of a sclerite (neither pigmented nor porose). The inner faces, which meet when the ovipositor is retracted, seem less deformable than outer faces. That of the posterior lobe (pl, Fig. 13A View FIGURE , insert) is convex and darkly pigmented, possibly sclerotized. In contrast, the inner face of each anterior lobe (al) is colorless and concave, accommodating the convex posterior lobe when the ovipositor is retracted. Each lobe has the usual complement of four setae. Seta Τ 1 (ca. 90 µm) on each ventral lobe, and pair ψ 1 (ca. 80 µm) on the dorsal lobe are longest and are finely attenuate; they insert on distinct, sclerotized tubercles in the smooth distal half of the respective lobe, well proximal to its tip. Other setae are shorter (Τ 2, ψ 2 ca. 30; Τ 3-4 ca. 40 µm), tapered but blunt-ended; they insert on smaller tubercles and more proximally, at the limit of the plicate cuticle. All setae are eupathidial.
By contrast, the male spermatopositor (’penis’) is highly atypical of oribatid mites (cf. Woodring 1970). As Grandjean (1966) noted for C. gigantea , the length and direction of the spermatopositor are consistent with mating behavior (see below, and Remark 2). When fully extended (SP, Fig. 4A View FIGURE ) it is longer than the ovipositor and has the same general facies, but lacks the dense plication and bends postero- rather than anteroventrally (discussed below as if ventrally directed). Like the ovipositor, it is a double-walled tube; when retracted, the distal part is pulled inside the proximal part ( Fig. 6A, B View FIGURE ), and the doubly-folded structure is pulled into the genital vestibule. The softest, most deformable region is where the primary, most distal fold occurs (pf, Fig. 4A View FIGURE ) but there is no distinct constriction, as exists in the ovipositor. The cuticle of the proximal (dorsal) part is smooth and featureless, except for two pairs of eupathidial coronal setae (k; Fig. 4A View FIGURE ). These insert dorsal to the primary fold, therefore are between primary and secondary (sf, Fig. 6A, B View FIGURE ) folds when the spermatopositor is fully retracted. They are not arranged in a ring, as in the ovipositor, but rather are all on the anterior face, with one pair proximal to the other.
The part distal to the primary fold is ca. 250 µm long, of which the terminal lobes comprise slightly less than half. The paired anterior lobes (al, Fig. 4D View FIGURE ) are large, laterally-flattened, and mostly immovable with respect to each other and to the main tube. They form the distal region of a tongue-shaped terminal structure ( Figs. 4D View FIGURE , 6A, B View FIGURE ), with a distinct bulge in its anterior outline. Externally, the anterior half of each lobe is noticeably sclerotized and porose. Where the lobe joins the main tube, about in the middle of the bulge, the sclerotized cuticle abruptly thins, and then changes to soft cuticle proximal to the bulge. The posterior half of each lobe has flexible, unpigmented cuticle. The lobes are separate distally, but they cannot spread far apart because they fuse basally in the anterior midline (point marked fa in Fig. 4D View FIGURE ); here the sclerites turn internally as a single, laterally-flattened medial apodeme, which Woodring (1970) called the tongue (t.ap). The attached portion of the tongue (from fa to fp) is rather short, and for this distance the sclerite is covered externally with a hyaline cuticular layer. Proximal to fp the apodeme leaves the surface cuticle and extends freely as a narrow blade-like arm, almost three times the length of the attached portion. This long, freely projecting apodeme is unlike the antero-posteriorly flattened apodeme of C. gigantea , and more typical of that of other oribatid mites ( Woodring 1970). Posteriorly, the mostly-soft inner surfaces of the opposing anterior lobes form a deep cleft (cl; Fig. 6A View FIGURE ) into which the posterior lobe is pulled during retraction (see below).
Each anterior lobe bears two large, distal eupathidial setae (Τ d, Figs. 4D View FIGURE , 6A, B View FIGURE ; ca. 50 µm). They are tapered, but distally rounded, and insert close together near the distal limit of sclerotization. Two similar, but shorter proximal setae (Τp, ca. 20 µm) insert near the dorsal limit of each sclerite, one more proximal than the other. This complement is homologous with the four setae (Τ 1-4) of the paired ovipositor lobes, but specific notations are uncertain.
The unpaired posterior lobe (pl, Fig. 4D View FIGURE ) is also highly compressed laterally, but is much differently structured. It is movable, swinging outward when the spermatopositor is fully extended. But when the spermatopositor is retracted ( Fig. 6A, B View FIGURE ), the lobe is rotated inward and fully enveloped in the cleft formed between the supple, free posterior halves of the anterior lobes (this creates the ’central pleat’ of Woodring 1970; see Remark 2). Basally, the surface of the lobe comprises a thick, embedded (hyalinecovered) sclerite, ca. 50 µm long. The sclerite has a very narrowly U-shaped cross-section, like a hairpin, such that most of it is parallel with the walls of the anterior lobes. The sclerite is well pigmented and conspicuously, but irregularly porose. On each side, the lobe is extended distally beyond the sclerite, about an equal length, by a broad flap of very thin, membranous cuticle (mf, Fig. 4D View FIGURE ); the flaps are rounded and the pair meet medially at the distal edge of the sclerite. They bear some minute cilia scattered on their proximal surface (not shown) and terminate in a fringe of much longer (ca. 15 µm) setules. These membranous flaps are easily overlooked, making the posterior lobe appear much shorter than the anterior lobes. Proximal to the sclerite, a region of transversely striate soft cuticle attaches the lobe to the main tube. Two pairs of eupathidial setae insert on the posterior lobe: pair ψ 1 (ca. 35 µm) insert close to the midline at the distal edge of the sclerite, and pair ψ 2 (ca. 15 µm) insert at its base, directly aligned with ψ 1 ( Fig. 4D View FIGURE ).
In addition to the sclerites of the three lobes, which are on the outer wall of the spermatopositor, the inner wall (’inner cup’ of Woodring 1970) has a pair of elongated sclerites (iw.sc, Fig. 4D View FIGURE ) that are conspicuous in transmitted light. They are broad and weakly porose on the inner face of each anterior lobe (in the region lateral to the posterior lobe sclerite), where they may add structural support to the cleft; then they narrow abruptly and gradually taper to end at a level slightly proximal to the end of the tongue-apodeme.
Our observations of musculature are superficial and require confirmation by histology, but the descriptions of Woodring (1970) seem to apply also to C. johnstoni . The largest muscles seem to be retractors that insert at various locations, including around the primary fold. A thick, seemingly unpaired and strongly banded muscle (r.mu, Fig. 4D View FIGURE ) inserts at the base of the posterior lobe, running between the inner-wall sclerites. An oblique band of muscles runs laterodistally from each side of the tongue; the more proximal members attach directly on the outer wall and the more distal members are directed toward the broad part of sclerite iw.sc, but we are uncertain if they attach there.
Gnathosoma ( Figs. 6D View FIGURE , 11 View FIGURE , 12 View FIGURE ) — Many traits of the mouthparts are typical of middle-derivative oribatid mites, and shared by most members of Nothrina (cf. Grandjean, 1957a, van der Hammen 1968). The subcapitulum is stenarthric and approximately as wide as long. All sclerotized ventral cuticle is strongly porose, like most of the body cuticle, but there are no circumscribed areas of larger, more luminous pores, not even around the base of setae. The mentum (men, Fig. 11D View FIGURE ) is triangular, about twice as wide as long. A coarse, dark sclerotized region on the ventral surface of the pharynx (ph.s, Figs. 11D View FIGURE , 12G View FIGURE ) shows by transparency; it is narrow at the mid-ventral commissure, then broadens posteriorly. The pattern is reticulate, but edges are strongest, giving its outline a ’wish-bone’ appearance. At its narrow anterior end, the structure appears to attach the pharynx to the subcapitulum, but it stays with the pharynx during dissection. The robust gena (gen) blends smoothly into a porose manubrial zone (between dorsolateral fissure f and line l.br on Fig. 11D View FIGURE ), beyond which is the strong, colorless, birefringent rutellum (rut). The single hypostomal (h) and two genal (a, m, Fig. 11D View FIGURE ) setae are finely acuminate, inconspicuously barbed, and similar in size, usually 70-80 µm.
The dorsal surface has the usual paired cheliceral groove (’mandibular fossa’), with relatively widely spaced pore canals (not illustrated). The posterior region is dominated by a large capitular apodeme that has a broadly curved margin in dorsal aspect and an inverted-V shape in crosssection (c.ap; Fig. 12H, I View FIGURE ); the two halves are fully connected, articulating at a weak scissure along the sagittal peak (*). At its projecting posterolateral corner, each gena has a deep vertical groove where the prodorsal condyle (pK) articulates; the podocephalic canal (cp; Fig. 12H, J View FIGURE ) passes across the soft articulating cuticle attached to this groove, on its way to the cervical region. The post-palpal (laterocoxal) seta (ep, Figs. 11D View FIGURE , 12H, J View FIGURE ) inserts in a small, strongly bulging, tubercle-like patch of soft cuticle on the dorsal margin of the gena, just anterior to the groove; it is a short (usually 11-13 µm) thick, hollow spine, varying from acute to nearly truncate, and minutely roughened distally.
The rutellum (rut, Fig. 11D View FIGURE ) has the common mixonomatan form: it is directed obliquely, exposing the lateral lips medially (i.e. it is atelobasic), and of similar width throughout. The distal edge is truncated, with four obvious teeth that increase in size from ventral to dorsal; a fifth, most dorsal tooth is seen in ventral view mostly by transparency. The dorsal surface ( Fig. 12K View FIGURE ) is concave, but has two sharply defined carinae (car), an oblique one, directed anteroventrally from the rutellar base to the middle tooth, and a smaller carina that extends from the middle of the oblique carina to the base of the most dorsal tooth. The position and direction of these carinae resembles those of the rutellar brush of many Nothrina and Brachypylina , but they have no cilia. However, immediately ventral to the position where these carinae meet are 1-3 spines. The spines are smooth, hyaline, tapered and rounded distally, and commonly broken near midlength; they are isotropic in polarized light, contrasting strongly with the highly birefringent rutellum. Usually there are two such spines, similar in size and slightly diverging but with adjacent bases (r.sp, Figs. 11D View FIGURE right side, 12L), but of 14 rutella examined, three ( Fig. 12K View FIGURE ) had a single spine, and one ( Fig. 11D View FIGURE left side) had three small spines.
The mouth lacks a ventral lip. The paired, adjacent lateral lips (ll, Fig. 11D View FIGURE ) each has a distinct, porose, distally tapering sclerite, covering about half the ventral surface; the medial edge of each sclerite is aligned along the medial edge of the lip, and is distinctly thickened. The sclerite bears the usual three adoral setae, all with minute barbs: or 1 (ca. 40 µm), inserted at the narrow tip of the sclerite, has a smooth base but a distal, dorsally curving fork with dense small barbs; the slightly longer or 2 and or 3 (ca. 60-70 µm), aligned transversely or obliquely across the middle of the sclerite, are simple, and barbed for most of their length. The distal parts of adoral setae are frequently broken. Dorsally, each lip has a row of posteriorly curved cilia along the lateral edge only. The long labrum (ls) is transverse at its base and tapers to a rounded tip that is similar in shape to the lateral lips, and extends slightly beyond them. A weak embedded sclerite occupies much of the dorsal surface, starting at the base of the labrum and narrowing gradually to efface in the distal quarter; the hyaline coating strongly bulges up, ridge-like, along most of the midline. The lateral margins of the sclerite are thickened to form narrow supporting ribs (sr, Fig. 12H View FIGURE ); proximally, each rib turns medially a short distance to form an attachment point for the pair of typical levator muscles. Three parallel U-shaped rows of distallydirected cilia encircle the soft tip ( Fig. 11D View FIGURE ); the dorsal row is short, reaching proximally on each side to only about the end of the sclerite, while the middle and ventral rows reach about one-third the distance to the labral base. No separate transverse rows of cilia were noticed on the dorsal surface. The ventral surface has about a dozen transverse grooves distributed along its whole length; overhanging each groove is a row of minute, proximally-directed cilia.
The palp ( Fig. 11C View FIGURE ) is five-segmented, about 150-200 µm long; measured along the lateral midline, segments of a 200 µm palp (trochanter to tarsus) were 14-73-25-33-55 µm. The respective setal counts (solenidion in parentheses) are 0-2-1-3-9(1). Four distal tarsal setae are eupathidial: setae ul", ul’ and su insert in a distal pad of soft cuticle, with the latter two being fused in their basal third (but counted separately); acm inserts just proximodorsal to them in a separate large alveolus. Solenidion ω inserts high on the abaxial face, at a level slightly distal to seta cm and well proximal to acm; it is about 1.5 times the length of the tarsus, tapers evenly to a thin, rounded tip (ceratiform), and curves gently laterad. The tarsal lyrifissure is well formed.
The chelicera has a typical chelate-dentate form ( Figs. 11A, B View FIGURE , 12A View FIGURE ). The soft cuticle of the cheliceral sheath (c.sh) attaches to the dorsal half of the principal segment along a strongly oblique line (en) positioned so that the principal cheliceral segment is ’inserted’ into the flexible cheliceral sheaths, i.e., about the basal fifth of it is internalized. The cuticle of the internal portion retains the same porosity as that external to the sheath (see Remark 3). Near the ventral midline of the principal segment, a thick apophysis projects internally (r.ap, Fig. 11A View FIGURE ), and serves as the insertion for one of the large retractor muscles (mu). The chelicera is emarginated proximally on the adaxial (anterior) face, where the principal segment meets the trochanter at nearly a right angle. On its abaxial face, the principal cheliceral segment is essentially smooth, but the adaxial face has numerous hard spicules of various sizes ( Fig. 11A View FIGURE ); most of these are in the middle third of the external portion, but usually a more distal one is largest. The two abaxial setae of the principal segment are acute to acuminate and relatively short: cha is near the dorsal midline, ca. 40 µm long, and is weakly barbed in the distal half; chb is on the abaxial face, near the base of the fixed digit, only slightly longer (ca. 50 µm) than cha but noticeably thicker and almost smooth. On each digit, the distal extent of porosity is sharply defined, beyond which is dense birefringent cuticle bearing the teeth: on the principal digit porosity stops just distal to seta chb, while on the fixed digit it occupies the basal third adaxially and the basal half abaxially. The fixed digit has four well-aligned teeth, plus a fifth small tooth that is subterminal on the adaxial side; the movable digit has four teeth, the distal two are offset to receive the terminal and subterminal teeth of the fixed digit ( Fig. 12F View FIGURE ).
In approximately the middle of the adaxial face are Trägårdh’s organ (Tg, Figs. 11A View FIGURE , 12C, D View FIGURE ) and the lamellated organ (l.or). Trägårdh’s organ – essentially an elongated oncophysis ( Alberti et al. 2011) – extends distally from the soft cuticle at the juncture of the trochanter, principal segment and cheliceral sheath. It is well developed, tapering gradually and extending distally to just beyond the base of the movable digit. A thin, lightly sclerotized central area (an embedded sclerite) is surrounded by membranous cuticle with a fine fringe of cilia; near its base a small scale-like flap (T.sc, Figs. 11A View FIGURE , 12D View FIGURE ) also is fringed with cilia. The organ is delicate: the fringed cuticle is often torn away or even the entire organ is lost when chelicerae are dissected from the gnathosoma. The lamellated organ (’fenestrate area’ fe 1 of van der Hammen 1968) lies just dorsal to the base of Trägårdh’s organ, near line en. It is a proprioceptor associated with a portion of the large levator muscle of the movable digit ( Alberti et al. 2011) and is covered with thin, but porose cuticle ( Fig. 12D View FIGURE , left).
The cheliceral trochanter has the general form of a deeply convex scale that underlies the principal segment, most noticeably on the adaxial face; it is subtriangular in adaxial view, and the ventral half of the cheliceral sheath attaches along its vertical proximal edge. Its cuticle is sclerotized but mostly rather thin in the proximal 2/3, where it is excavated by large regions of muscle insertions; narrow places where no muscles attach (i.e. cuticle of normal thickness) show in transparency as darker lines, analogous to the dark epimeral borders of the coxisternum. The ventral cuticle is thin proximally but abruptly thickens in the mid-region (tr, Figs. 11A View FIGURE , 12B View FIGURE ), then gradually becomes thinner distally. The articulation between trochanter and the principal cheliceral segment is inconspicuous and we did not study its full path; it is most noticeable by transparency in the ventral midline (art, Figs. 11A View FIGURE , 12B View FIGURE ), at the thickest point of the trochanteral cuticle. The part of the trochanter that extends distal to this articulation is a tectum, a large protective scale. The tectum gradually thins distally and near its margin it is hyaline and membranous, fringed with distally directed, inconspicuous cilia (tr.m, Figs. 11A View FIGURE , 12B, C View FIGURE ). This U-shaped, hyaline region of the tectumcalled the ’ventral process’ by Grandjean (1947a; pr.v)-covers the broad arthrodial membrane of the movable digit from below.
The complement of oncophyses associated with the articulation of the movable digit is similar to that described by van der Hammen (1968) and Alberti et al. (2011) for the nothrine genera Hermannia and Archegozetes , respectively. Oncophysis op’ is a soft, hyaline lobe projecting over the base of the movable digit on the adaxial face ( Figs. 11A View FIGURE , 12E, F View FIGURE ). Oncophysis op.v ( Figs. 11A View FIGURE , 12A View FIGURE ) does not project as a lobe, but extends from the articulation onto the ventral region of the fixed digit where it attaches; its distal extent coincides exactly with the end of porosity and the beginning of birefringence in the digit cuticle. Under the hyaline coating, pore canals are clearly visible in the underlying sclerotized layers of the fixed digit, but they are noticeably less dense than in most other porose cuticle, as with the embedded sclerite on the posterior lobe of the spermatopositor (see above).
Both oncophyses give the impression of being deformable and fluid-filled, consistent with observations of Hermannia by Bäumler (1970) that they inflate under pressure. The membranous distal margin of the trochanteral tectum (tr.m) has been considered an oncophysis in the three studies mentioned above, the so-called ’coxal oncophysis’ (their op.x), but the term is probably inappropriate, since it derives from a sclerite, not soft arthrodial cuticle.
Abaxially, just dorsal to op.v, a thin, stiff scale projects horizontally from the movable digit (md.s, Fig. 12E View FIGURE ). Most of the scale is hyaline, but its base has porose, sclerotized cuticle, such that in lateral aspect the movable digit seems to have a short ridge along its ventral adaxial edge ( Fig. 11A View FIGURE ).
Legs ( Figs. 4F, G View FIGURE , 13-16 View FIGURE View FIGURE View FIGURE View FIGURE ) — Overall, legs are rather simple, tubular in form ( Fig. 14 View FIGURE ). Leg IV is longest, ca. 0.6 times as long as the body in both sexes. Relative to leg IV, other legs have approximately the following proportional length (males tend toward the higher fractions): I (0.80-0.83), II (0.74-0.76), III (0.83-0.92). Width of most segments is slightly greater on I-II than on III-IV. At its base, tarsus I is ca. 1.3 times thicker than tarsus IV, but all tarsi have similar thickness distally; tarsi I and II taper mostly in their distal third, but tarsi III and IV taper gradually, throughout their length.
Sclerotized leg cuticle is generally porose, like that of the body, but there are isolated lines of more luminous pores that form no discernible larger pattern ( Fig. 13B, F View FIGURE ); these are most abundant and conspicuous on tibiae and tarsi. There are no differentiated areas of larger pores that could be compared to the discrete adaxial respiratory areas on femora and trochanters III and IV of most Brachypylina . However, on femora, genua and tibiae, setae of the anterior ventral rows (v’, ev’) insert eccentrically in a small, rounded, luminous porose area similar to those of the notogaster ( Fig. 13 View FIGURE , cf. H, I); these were not seen associated with any other leg seta. Several types of cuticle are present distally on the tarsus. The normal, porose sclerotized cuticle (Fig, 13B, F) stops at the level of proral and unguinal setae insertions ( Fig. 14E View FIGURE ). The tarsus continues laterally and ventrally as a U-shaped extension that is mostly pale but seems stiff, not deformable; only along the upper edge is sclerotization retained, where it forms a rigid, elongated condylar rib that supports the ambulacrum (co, Fig. 14E View FIGURE ). Superficially this pigmented rib resembles the condylophores of most desmonomatan taxa, but unlike them it is not an isolated, cylindrical structure. Most distally is the soft, deformable ambulacral pad from which the claws emerge. The pad extends proximally in the dorsal region to the level of the proral setae, thereby ’capping’ the U-shaped extension and allowing the ambulacrum to flex backward.
The pretarsal ambulacrum of each leg is tridactylous, with the empodial claw being similar to laterals, except very slightly shorter. Each claw has minute, inconspicuous barbs along its dorsal curvature, except near the base and tip. The globular basilar piece – generally similar to that described by Grandjean (1941) for Camisia – is isotropic and slightly pigmented, with a pair of small cotyloid cavities that articulate with the end of the respective condylar rib. There is no clear channel in the ventral tarsal cuticle to guide the tendon of the ambulacral depressor muscle, but a vague proximal groove accommodates the distal part of the muscle entering from the tibia.
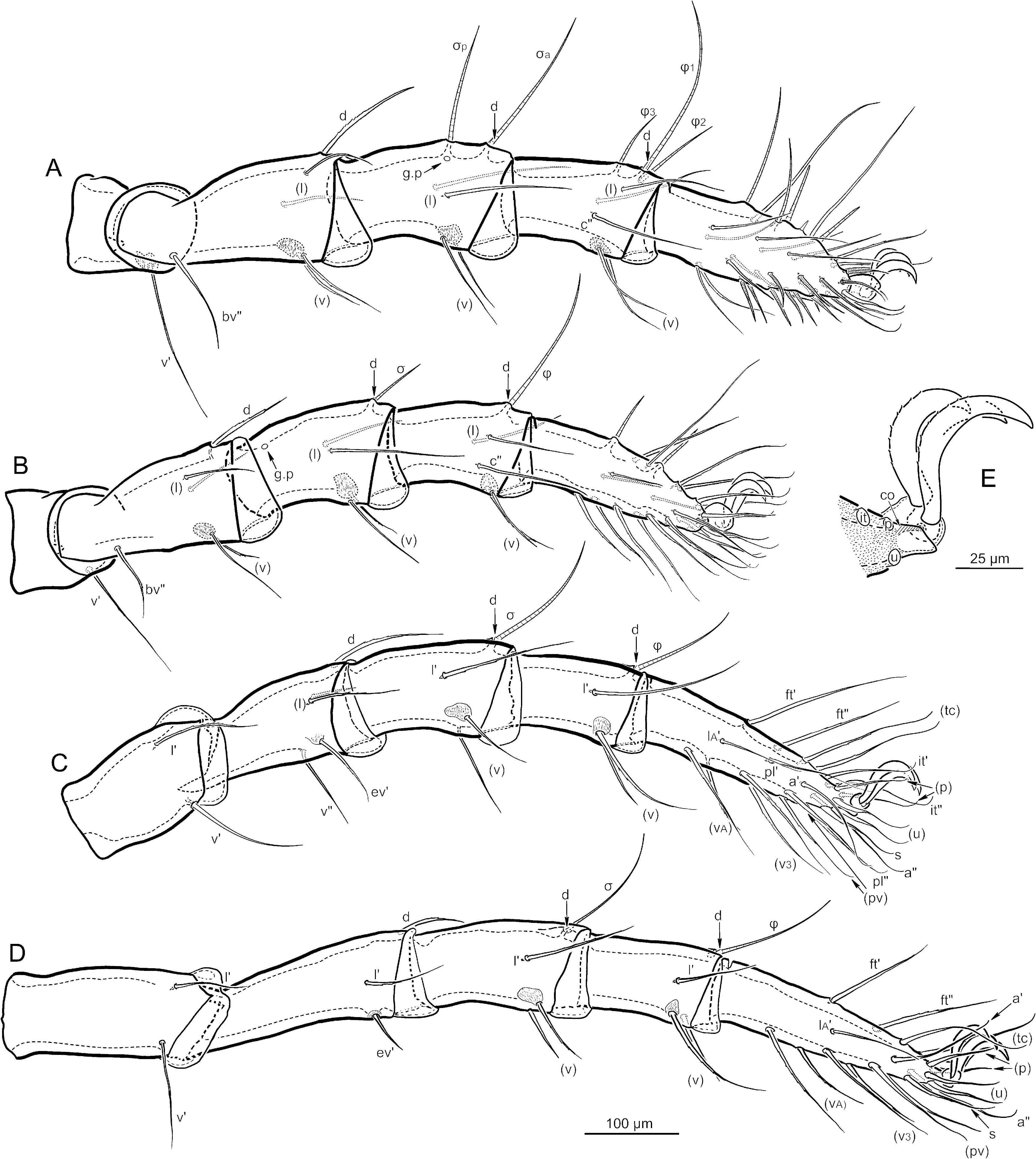
Except for the rare absence of tibia II seta v", the setation of adult legs varies only on tarsi (Table 2). Numerical formulas (counts on legs I-IV) of the proximal segments are as follows: trochanters (1-1-2-2); femora (6-6-5-3); genua (5*-5*-4*-4*); tibiae (6*-6*-4*-4*). An asterix indicates that this count includes minute seta d (see below), which is easily missed so that the count appears to be one fewer. The tarsal setation is rich in accessory setae of the l and v rows, as well as an intermediate c row on tarsus I only. The total count of tarsal setae varies considerably, both within and among individuals, and this is due almost entirely to variation in the l and v rows (Tables 1, 2; see below, Development and variation of leg setation). The following represents the most common count for tarsi I-IV (famulus not included on I), preceded in parentheses by the minimum observed and followed by the maximum observed: (35)37(39)-(23)25(26)-(21)22(23)-(17)18(19). The relative size, position and identity of setae are shown in Figs. 14 View FIGURE , 15 View FIGURE . Except for d, setae of the basal four segments are thin, finely attenuate, and have inconspicuous, minute barbs (mostly not illustrated) in approximately the middle third. Seta d of the femora is similar, but slightly thicker and distally acicular. Seta d of all genua and tibiae is minute and coupled to a solenidion on the respective segment; for leg I, this is σ a on the genu and ’ 1 on the tibia. The seta is appressed to the anterior (’) side of the solenidion and inserted with it in the same alveolus; it is seen best when the solenidion is detached, as often happens after long treatment in a clearing agent ( Fig. 13D, E View FIGURE ). The seta lacks barbs and ranges in length from about 3-15 µm, with longer setae (i.e. that of genu I) being attenuate and shorter ones spiniform; on a given leg the genual seta d is usually longer than that of the tibia.
Tarsal setae have a variety of forms. On tarsi II- IV the more proximal setae are similar to those of basal segments, i.e., finely attenuate and minutely barbed. More distal setae – tectals (tc), iterals (it), prorals (p), unguinals (u), antelaterals (a), primiventrals (pv) and the unpaired subunginal, s – are somewhat flattened, ribbon-like, in their distal third and usually curl strongly dorsad near the tip. Alveoli of most setae in lateral and ventral regions are tear-shaped ( Fig. 13F View FIGURE ), rather than circular, with the point directed distally. On tarsus I, setae (it), (p), s, (pv), (c 1), (c 2), (v 1, if present), (v 2), and (v 3) are eupathidial, i.e. hollow, shorter than other setae, lacking barbs and slightly curving ventrally near the tip. The famulus (e, ca. 15 µm) is erect, bluntly spiniform and roughened by numerous microtubercles or callosities ( Fig. 13C View FIGURE ); it lies approximately in the dorsal midline, usually closest to seta ft" and solenidion ω 3, but the three do not form a tight tarsalcluster.
Solenidial counts (legs I-IV) are as follows: genua (2-1-1-1); tibiae (3-1-1-1); tarsi (4-2-0-0). Relative sizes and positions are shown in Figs. 14 View FIGURE , 15 View FIGURE . Most are finely attenuate (piliform in the terminology of Grandjean 1935) to sub-flagellate, but ω 1 and ω 3 of tarsus I, and ω 1 and ω 2 of tarsus II taper only to a thick, rounded tip (ceratiform).
Two other cuticular features are present on legs. Each tarsus has a relatively large transverse lyrifissure (ca. 25-30 µm long) immediately distal to its base, almost centered in the dorsal midline. On genua I-III (but not IV), a so-called genual pore (po) is present high on the posterior (") side; each comprises a simple canal through the procuticle that is covered with thin, hyaline cuticle. On genu I it is in
ABLE
2 Parentheses indicate that both setae of the pseudosymmetrical pair (ʹ, ʺ) appear together; a dash (‐) indicates there is no addition in that instar; an asterisk (*) indicates that seta d is present only as a minute vestige and coupled with the respective solenidion, usually in the same alveolus. Abbreviations: Pn (protonymph; Dn (deutonymph); Tn (tritonymph). Subscripts of eustasic tarsal setae of rows (l), (v) and (c) denote the instar in which they form (1, 2, 3, A = Pn, Dn, Tn, adult, respectively). Studied adults were female.
ABLE
mid-segment, approximately aligned between seta l" and solenidion σ p ( Fig. 14A View FIGURE ); on one leg examined, two tandem pores were present on genu I (as observed by Grandjean 1958a in Perlohmannia ). On genua II and III the pore is far proximal, close to the articulation with the femur ( Figs. 14B View FIGURE ).
The legs of males are similar to those of females, with the following exceptions. The solenidia on tarsus I are more evenly spaced and each is more proximally positioned (cf. Fig. 4E, G View FIGURE with Figs. 14A View FIGURE , 15N View FIGURE ). More striking are the modifications of leg IV relating to the transfer of materials that are interpreted as nuptial food (See below: Courtship behavior). The expanse of articulating cuticle between genu and tibia seems larger than in the female, such that the tibia can flex dorsally to an unusual extent ( Fig. 2C View FIGURE , arrow); presumably this prevents interference between distal segments when legs IV come together during courtship. The genu is thicker than that of females, with a strong adaxial depression accommodating a strikingly hypertrophied seta v" ( Figs. 2C View FIGURE , 4F View FIGURE , 6B View FIGURE ). This seta is inserted unusually high on the posterior face, and has the form of an asymmetrical diamond. Its thick base is separated from the flattened distal region by a well-defined, curved drop-off across its broadest part; the distal region is tapered and elongated to extend well beyond the end of the segment. The entire seta is strongly birefringent in polarized light ( Fig. 13G View FIGURE ). Solenidion σ is smaller than that of females but has the same piliform shape and coupled minute seta d. The tibia is unmodified, except for being slightly shorter than in females, and having a smaller solenidion ’.
Active juvenile instars ( Figs. 17-19 View FIGURE View FIGURE View FIGURE )
Dimensions, facies, integument — The total length range (mean in parentheses) for 10 specimens of each juvenile instar is as follows: larva 382-607 (473); protonymph (Pn) 723-843 (760); deutonymph (Dn) 856-1021 (952); tritonymph (Tn) 1100-1478 (1286). Body proportions are similar among the instars, with maximum width being about half the length (0.40-0.56). Larvae are most variable in length: expressed as a percentage of the mean, the range is 48 % (225/473). We attribute this to the fact that each individual larva expands significantly from the time it ecloses from the egg to when it attains its final length after the commencement of feeding. This aspect of variation, which is not applicable to studied nymphs, was not controlled in sampling of larvae, so individual differences in time-since-eclosion probably magnified the observed range. Among nymphs, the percentage is much lower, viz.: Pn (16 %), Dn (17 %); Tn (29 %). The near doubling of the range between Dn and Tn, with the latter approaching the figure for adults (31 %), suggests that the size difference between males and females is attained largely in the Tn.
Nymphs are strikingly two-toned, with both types of cuticle being shiny in reflected light. Lightto medium-brown sclerotized cuticle is found on the prodorsum (aspis), epimeral plates, legs and gnathosomal components, and appears darkest on the cheliceral digits and the lateral rib of the prodorsum. As in the adult, this cuticle is porose and mostly smooth, lacking an imbricate surface. By contrast, most cuticle of the hysterosoma is colorless and nearly transparent in life, so that dark food boli, internal organs and the oily, yellow contents of the opisthonotal gland are well visible externally ( Fig. 19A, C View FIGURE ). In preserved specimens this cuticle is creamy white to slightly yellowish, and is weakly translucent ( Fig. 17C, D View FIGURE ); it is deformable but seems more elastic than that of the soft, flexible articulations. In transmitted light it appears thick and uniform, and glows conspicuously under DIC or polarized illumination ( Fig. 17F View FIGURE ; see Remark 4). This part of the cuticle, which lacks apparent porosity or surface ornamentation in juveniles, becomes sclerotized, imbricate and porose in the adult. Small porose tubercles at the base of large setae and a narrow ring around the opisthonotal gland opening are lightly sclerotized and therefore are contrasting brown. The larva is colorless throughout when first eclosed from the egg, and sclerotized parts (the same structures as in nymphs) darken only slightly as it matures. Its structure is nearly identical to the larva of C. gigantea , which was described and illustrated by Grandjean (1966), and his figures are referred to below to illustrate certain features of C. johnstoni .
As in the adult, there are porose areas and associated dermal glands positioned near setae; their distribution is noted below.
Prodorsum — The general form of the aspis is similar to that of adults except for the limbless rostral tectum, which occupies only a narrow distal portion (ca. 15 %) and is short enough to leave all but the base of each chelicera exposed ( Figs. 17A, D View FIGURE , 18H, K View FIGURE ). The aspis is therefore broadly truncate anteriorly, except for a medial projection that bears the rostral setae (ro). Seen flat in dorsal aspect, the projection is broadly convex in the larva, hardly long enough to contain the alveoli of ro; it becomes somewhat triangular in the Pn, but is a larger and nearly an equilateral triangle in the Dn and Tn, with the alveoli of ro near its base. As in C. gigantea ( Grandjean 1966, his Figs. 4C, D View FIGURE ), the lower wall of the tectum (rostrophragma) projects ventrally between the chelicerae to form a purse-like pouch, narrowly V-shaped in cross section, that he considered a naso. In nymphs, the presence of a larger medial projection alters the shape of the optical section (cf. Fig. 17B, E View FIGURE ), and the width of the ’naso’ increases, until in the Tn its form is more that of an inverted dome or swelling. In the medial region of the rostral tectum both surfaces are pigmented and distinctly porose ( Fig. 17A View FIGURE ), but in lateral regions the distal margin becomes hyaline and lacks distinct porosity. In all immature instars the lateral rib (nl) and exobothridial lobe are similar to those of the adult, except that the ventral marginal thickening is less distinct than in the adult, and the lobe is less extensive posteriorly, such that seta xp is not on the aspis ( Fig. 18K View FIGURE ). The cuticle in which xp inserts is not sclerotized, but it seems somewhat stiff, lacking the fine striation of the soft cuticle that otherwise surrounds the aspis and forms the sejugal groove. There is no occipital phragma; the posterior margin of the aspis curves slightly downward before attaching to the sejugal cuticle but otherwise is simple. The thin, blade-like sagittal apodeme (sa, Fig. 17A View FIGURE ) is present in all immature instars, similar in form and relative size to that of the adult (see Remark 5). Except for the absence of the phragma, muscle insertions seem similar to those of the adult, with sigilla in approximately the same locations; the latter are more clearly marked in nymphs than in the larva. The macula lying immediately posterior to each lamellar seta, which is conspicuous in the adult, was not discerned in the larva or Pn; it is weak, but present in the Dn and Tn ( Fig. 17E View FIGURE ; see Remark 1). Except for the bothridial seta, prodorsal setae of all immature instars are similar in form to those of the adult, but some have slightly different relative lengths. Most noticeable is that the rostral seta is about twice as long in immatures as in the adult, relative to the other prodorsal setae. The bothridial seta (bo) is similar in length to ro in immatures (becoming 2-3 times as long as ro in adults), and its shape changes significantly during ontogeny. In the larva bo is narrowly lanceolate, usually with a single strong tip but subterminal large barbs can make it appear bifid or trifid. Barbs are relatively numerous in the distal half, being minute on the shaft but several times larger on the head; as in the adult they are formed in the hyaline, isotropic external layer. The birefringent core appears hollow throughout, but conspicuously so only in the expanded head region. In the Pn the head is narrower, sometimes indistinguishable, and has fewer barbs ( Fig. 18H View FIGURE ); in the Dn and Tn seta bo is filiform, tapering only very close to the tip, as in many adults ( Fig. 18K View FIGURE ). The gentle sigmoid curve immediately distal to the bothridium may be less distinct than in the adult, especially in the larva. The bothridium is similar in structure to that of the adult, but the outpockets in the middle two portions are few in the larva, becoming more numerous in successive instars.
Gastronotic region — This region accounts for about two-thirds of the total length in nymphs but slightly less (about 60 %) in well-expanded larvae. Anteriorly, in the region of the sejugal groove, the cuticle is flexible and finely, transversely striate in all immature instars ( Fig. 18H View FIGURE ); this allows a limited degree of retraction of the prodorsum, probably similar in extent to that of the adult. Its posterior extent is probably what Grandjean (1966) designated line as (his Figs. 1A View FIGURE , 2 View FIGURE ), though he did not mention cuticular striations. This dorsosejugal band of soft, striate cuticle continues into the podosomal region above the coxisternum. Two transverse grooves are present dorsally in all immature instars, but efface mid-laterally ( Fig. 18H View FIGURE ). The anterior groove (r2 of Grandjean 1966) passes between setal pairs d 1 and d 2, and is usually inconspicuous. The other (r3) lies posterior to setal pair e 1 and is usually conspicuous even at low magnification. In the more contracted preserved nymphs, the region anterior to r3 and above the legs is concave, and the region posterior to r3 is somewhat depressed in lateral view. Setal development is normal and holotrichous, except that f 1 is vestigial, appearing like a minute pit with distinct walls, in all immature instars. Setal pairs e 1, e 2, h 1 and h 2 are flagellate and notably larger than others in all instars; proportionally they are at least as long as in the adult. Seta p 1 is of medium length (similar to p 2) when first joining the gastronotic complement in the Pn ( Fig. 18H View FIGURE ), but it enlarges in the Dn to become one of the five long pairs. As in the adult, other setae are short and thin in all immature instars, with c 3 and f 2 being smallest. Other than the normal shifts in posterior setae from larva to Pn, as segment PS is incorporated into the gastronotum, the relative positions of gastronotic setae remain similar throughout ontogeny, with the exception that pair e 1 migrates anterolaterally: it is posterior to d 2 in the larva, posterolateral to d 2 in nymphs, and anterolateral to d 2 in the adult (see Remark 6). Lyrifissures are all cupular in form and have the normal ontogeny: ia, im and ip have similar positions in all instars; ih and ips lie off the anterolateral corner of the paraprocts in the larva and Pn, respectively, then shift anterolaterally as the next paraproctal segment appears ( Fig. 18 View FIGURE B- E). The opisthonotal gland opening (gla) lies closely dorsal to ip in all immature instars (becoming more nearly aligned between ip and seta f 2 in the adult).
No porose areas were seen in the gastronotic region of larva or nymphs. However, after short treatment (one day) in cold lactic acid, clusters of cells can be seen that – based on their shape and locations – appear to be homologues of the adult dermal glands. In the Dn and Tn, all the setae accompanied by porose areas in adults are underlain by similar umbrella-like clusters of elongated cells (including seta le of the prodorsum). This condition appears to develop gradually: in the larva the elongated cells are clustered near the setae, but do not form well-circumscribed umbrella-like formationsrather, they occupy broader expanses, including all the subcuticular space in the posterior part of the hysterosoma. A similar arrangement is seen in the Pn, but posterior clusters are more differentiated ( Fig. 18H View FIGURE ). Apart from these tight clusters, similar elongated cells were observed dispersed under the cuticle of body and legs in the prelarva, larva and Pn, but only of legs in the Dn and Tn. Whether any of these elongated cells are truly glandular could be determined only with appropriate histological study.
Coxisternum — The form of the coxisternum differs little from that of the adult. Moving posteriorly, each successive epimere is narrower and the coxisternum is divided into four sclerotized units by soft, striated cuticle by the combination of a deep ventrosejugal furrow and a sagittal scissure; the latter is broader than in the adult. The plates of epimere I do not fuse anteriorly in the larva or Pn, but a small bridge of sclerotized cuticle like that of the adult is present from the Dn. The triangular region posterior to epimeral plates IV seems unsclerotized in all nymphs. In all instars epimeral plates are porose, but their pigmentation and the distinctness of borders and apodemes increases during ontogeny. There is no vestige of epimere (or leg) IV in the larva. The epimeral setation (pairs on I to IV) usually develops as follows: larva (3-1-2, including seta 1c); Pn (3-1-2-1); Dn (3-1-3-3); Tn (3-1-3-4). Variations were mostly noted in the Tn: of 18 plates (nine individuals) studied, one plate III lacked 3c, and four plates IV lacked 4d; on one Tn, 4b was doubled and on another 3c was doubled, each unilaterally. In nymphs, the relative sizes of setae are as in the adult. In the larva, ClaparŁde’s organ (Cl, Fig. 18B View FIGURE ) and its scale-like protective seta 1c have the usual form, with no secondary annulation (identical to that of C. gigantea ; Grandjean 1966, his Figs. 1-3 View FIGURE View FIGURE View FIGURE ). The supracoxal seta and coxal gland opening on epimere I are like those of the adult, but no clear flange was observed on the dorsal edge. Epimere III does not extend dorsally as it does in the adult.
Anogenital region — This region lacks distinct sclerites in all immature instars. The larva has a noticeable transverse groove with soft, striated cuticle situated behind the coxisternum but it is well removed from the posterior margin of epimere III (sv, Fig. 18B View FIGURE ). There is no such isolated groove in nymphs, but the same relative position is occupied by a transverse depression between epimere IV and the genital valves. In nymphs the genital valves are small and well-removed (by more than their length) from the paraprocts ( Fig. 18 View FIGURE C-E). Their usual setation (Pn, Dn, Tn) is 1-4-7; variations were usually asymmetrical and included: Dn may have 3 setae (3 of 12 valves studied) and Tn may have 5 or 6 (1 each of 18 valves studied). The first aggenital seta forms in the Dn, and the second is added in the Tn; one anomalous individual had 3 on one side (ag 1 doubled) and 1 on the other (ag 2 absent). The paraproctal valves are elongated in all immature instars, with the pair collectively lens-shaped, tapered at each end ( Fig. 18 View FIGURE B-E). The larva lacks transcupular (inguinal) seta h 4 and there are only two setae on each paraproctal valve (segment PS). Because of their positions, we interpret the missing third seta as p 1, as did Grandjean (1966) for C. gigantea ; p 1 first forms in the Pn, as does cupule ips. In the Pn and Dn, paraproctal valves (segments AD and AN, respectively) are glabrous, so in the terminology of Grandjean (1949a) C. johnstoni exhibits paraproctal atrichosy at two levels. Marks in the paraproctal cuticle of some Pn and Dn specimens seem to represent setal vestiges; these may include vestiges (ve, Fig. 18C, D View FIGURE ) of only ad 1 (in Pn) or of both an 1 and an 2 (in Dn). Setal rows ad and an, as well as their respective cupules (iad, ian), appear fully developed in the subsequent instar (Dn and Tn, respectively). Segment AD retains a convex, valve-like, ’parenthetic’ appearance around the paraprocts in the Dn and Tn, being separated from the gastronotic region by a shallow groove. Genital papillae are similar in form to those of the adult.
Gnathosoma — Few changes occur to the gnathosoma during ontogeny. The larva lacks adoral seta or 3, which is present from the Pn in its adult form and location. The second seta of the palp-femur, inf, is added in the adult. In all immatures, palp-tarsus seta su forms as a small, noneupathidial branch from the ventral side of eupathidial seta ul’ (as illustrated for C. gigantea by Grandjean 1966, his Fig. 4B View FIGURE , or slightly longer and thinner); in the adult su enlarges to the size of ul’ and becomes eupathidial, still fused with ul’ proximally. Seta acm is normal until the Tn, when it becomes eupathidial but otherwise does not change shape or position. The dark pattern on the ventral surface of the adult pharynx, described above, develops gradually; it is narrow and without apparent reticulation in the larva, then increases in darkness, relative width and degree of reticulation in successive nymphs. No structural change in the chelicera during ontogeny was detected.
Legs — The structure of legs in immatures is generally like that of adults, except most segments become relatively longer and thinner with successive instars (cf. Fig. 18H View FIGURE , Fig. 14 View FIGURE ). The most conspicuous ontogenetic change is the steady increase in relative size of leg IV. When first formed in the Pn, it appears small and weak, with length ca. 0.8 times that of leg I; it is about equal to leg I in length (though thinner) in the Dn, and about 1.1 times leg I in the Tn (becoming ∼ 1.2 times leg I in adult). All pretarsi are monodactylous, and the ambulacrum articulates by means of a condylar rib at the distal extreme of the tarsus, as in the adult. Sclerotized cuticle is porose throughout ontogeny, but this porosity becomes more noticeable in successive instars, as the cuticle darkens. The lines of luminous pores are present in nymphs, though less obvious and less extensive than in the adult. No porose area (such as those associated with ventral setae in the adult) was observed in the larva or in early nymphs but they can be vaguely discernible in the Tn. All immature instars have genual pores on legs I-III, with the same distribution as in the adult.
Solenidial ontogeny (Table 1) does not vary. For legs II-III, the adult complement is present from the larva and for leg IV it appears abruptly in the Dn. The ontogeny of leg I solenidia is more complex, and the following formulas apply (genu-tibiatarsus): larva (2-1-1), Pn (2-1-2), Dn (2-2-3), Tn (2- 2-4); the third tibial solenidion, ’ 3, appears in the adult.
The ontogeny of leg setae is more complicated and variable (Table 1); it is discussed separately below, with reference to both immatures and adult, but salient traits include the following. Seta d is minute and coupled to the respective solenidion on all genua and tibiae, as in the adult, though in the larva the nature of the coupling is variable (see below). Iteral setae are added to tarsi I-III in the Tn, but are absent from tarsus IV. The setal formula for leg IV in the Pn is typical of oribatid mites: 0-0-0-0-7, with the tarsal setae being ft", (p), (u) and (pv). From the larva legs I and II bear the primilateral pair (pl), tarsus III bears only pl’ and tarsus IV lacks primilateral setae (see Remark 7). Only the proral pair (p) are eupathidial in the larva; for other eupathidia, transformation from a normal seta occurs in a specific nymph or adult (see below).
Prelarva
The prelarval instar is a typical calyptostase, remaining fully within the egg. It has neither setae nor projecting appendages, but has relatively wellformed vestiges of chelicerae, palps and all three pairs of legs, with the pairs meeting in the midline ( Fig. 18A View FIGURE ). The leg vestiges are strongly angled, forming chevrons. The chelicera has a clear egg-tooth (k), and a vestige of ClaparŁdes organ (Cl) is at the base of leg I. The general form is that commonly seen in middle-derivative oribatid mites, being most similar to those of Desmonomata , particularly Nothrina and early-derivative Brachypylina such as Neoliodidae ( Sitnikova 1960; Grandjean 1962b).
Material examined and distribution
The holotype male and allotype female of Collohmannia johnstoni were collected by R. A. Norton from the Monongahela National Forest in Randolph County, West Virginia, north of US Rt. 33 near the towns of Bowden and Alpena, on 2-XI-2013. The type locality (38°56.505’N, 79°40.084’W; elevation 930 m) is the trailhead that leads into the Otter Creek Wilderness, accessible from National Forest Rt. 91. The site is a riparian mixed forest with overstory of red maple ( Acer rubrum L.), yellow birch ( Betula alleghaniensis Britton ), white spruce ( Picea glauca (Moench) Voss ) and eastern hemlock ( Tsuga canadensis (L.) Carr.), with a dense understory of rhododendron (Rhododendron maximum L.); mites were collected from the thick layer of leaf litter. These and 10 paratypes (3 females, 3 males, 4 immatures) from the same collection are preserved in alcohol in the collection of the Acarology Laboratory, Museum of Biological Diversity, the Ohio State University (Columbus, Ohio). Thirty other adult paratypes were designated from material collected from the same site, on various dates (9-IX-1980, 22-X-1983, 30-V-1999, 13-VI-2003, 24-V-2005, 13-VI- 2006, 1-V-2009); the earliest collection was by M.O. Johnston, the others by R. A. Norton. Four of these paratypes (2 males, 2 females) along with several juveniles have been deposited in each of the following collections: the Canadian National Collection, Agriculture and Agri-food Canada (Ottawa, Canada) GoogleMaps ; the Senckenberg Museum of Natural History ( Görlitz , Germany); the Siberian Zoological Museum (Novosibirsk, Russia); and the collection of the Tyumen State University Museum of Zoology (Tyumen, Russia). The remainder of paratypes and several dozen non-type specimens are in the personal collections of the authors. Several additional specimens were collected from non-riparian hardwood forest litter, several hundred meters from the type locality .
The species has been collected from only two other sites, both also within the Monogahela National Forest. (1) West Virginia, Pocahantas Co., 19.3 km E. Richwood; col. S. O’Keefe, 23-VIII-1990, 5 females, 2 males from sifted hardwood litter by log. (2) Same, but Forest Trail 150, 23.7 km S. junction with Trail 55; col. S. O’Keefe, 17-V-1991, 1 female, 1 male from sifted litter, moss, and rotten wood at base of tree.
Etymology
The species epithet johnstoni is a double patronym. It is named with respect to honor the memory of the late Prof. Donald E. Johnston, long a principal figure at the Acarology Laboratory of the Ohio State University. Don had a major positive influence on the early career of R. A.N. and on the careers of many other acarologists who passed through that unique program over several decades. It is also named for his son, Prof. Mark O. Johnston (Dalhousie University), who as a student made the initial collection at the type locality .
| R |
Departamento de Geologia, Universidad de Chile |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |