Prunum apicinum, (MENKE 1828)
|
publication ID |
https://doi.org/10.1046/j.1096-3642.2003.00058.x |
|
DOI |
https://doi.org/10.5281/zenodo.5490938 |
|
persistent identifier |
https://treatment.plazi.org/id/03B6B923-EE28-FFC3-8CDE-601C392D6F5A |
|
treatment provided by |
Carolina |
|
scientific name |
Prunum apicinum |
| status |
|
PRUNUM APICINUM (MENKE 1828) View in CoL
Material examined
Missouri Key, Florida ( USNM 890944 About USNM ) .
External anatomy and mantle cavity
Smooth mantle covering shell. Anterior pedal gland opening to shallow lip under propodium. Ventral pedal gland present in females, opening to elongate slit near front of foot sole. Long, slender siphon lying between cephalic tentacles, left margin fused to side of head ( Fig. 7D View Figure 7 , si). Root of siphon lacking basal flaps. Extensive hypobranchial gland composed of smooth, unfolded epithelium with two distinct regions. Operculum absent.
Reproductive system
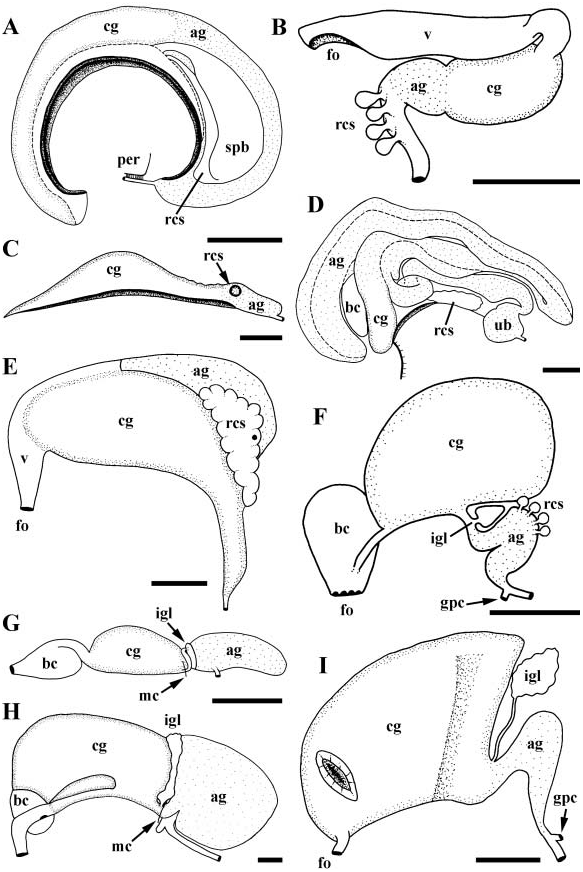
Gonad lying along right side of body, not penetrating tubules of digestive gland. Gonopericardial canal absent. Oviduct opening to anterior end of large albumen gland ( Fig. 2G View Figure 2 , ag); albumen gland visceral in position, closely appressed to side of kidney. Albumen gland opening anteriorly to single ingesting gland (igl) containing unorientated sperm. Ciliated, S-shaped duct near base of mantle cavity connecting ingesting gland and capsule gland (cg). Capsule gland bearing distinctly regionated glandular epithelium with anterior and posterior zones of mucus secreting cells. Capsule gland opening to highly folded vestibule. Vestibule curving around base of muscular bursa copulatrix (bc) and opening dorsally to bursa. Bursa narrowing to short vagina, terminating in simple female aperture (fo).
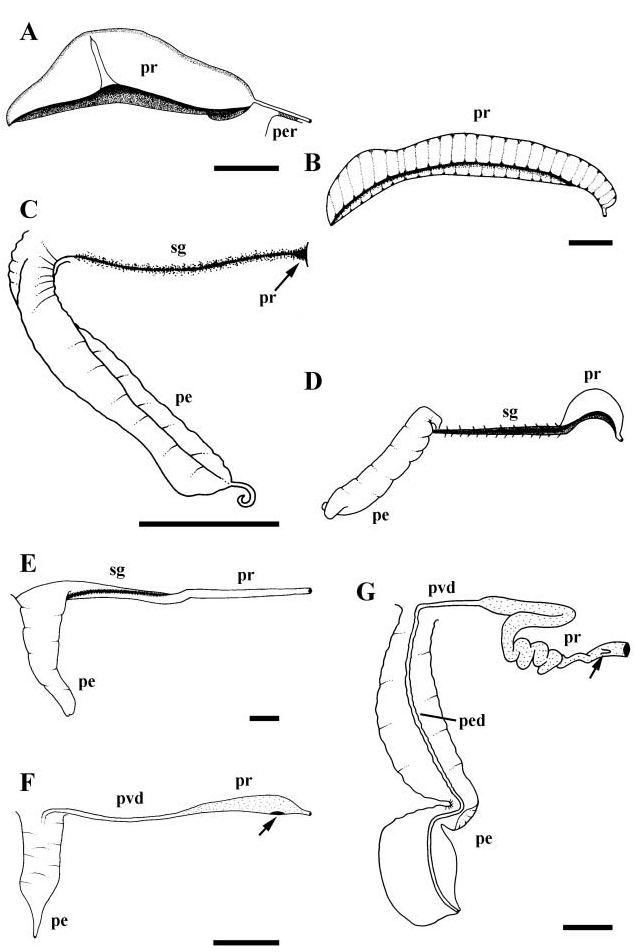
Broad, straight, ciliated vas deferens functioning as seminal vesicle. Gonopericardial canal absent. Vas deferens narrowing before entering long, glandular prostate ( Fig. 3F View Figure 3 , pr). Prostate lying at base of mantle cavity, across exhalent margin. Proximal prostate bearing slit-like opening to pallial cavity. Pallial vas deferens (pvd) extending anteriorly along side of head to base of large penis (pe) lying short distance behind right cephalic tentacle. Penial duct extending straight down centre of penis to open at tip. Penis broad, muscular, dorso-ventrally flattened, unilobed, narrowing from broad base to fine tip.
Alimentary system
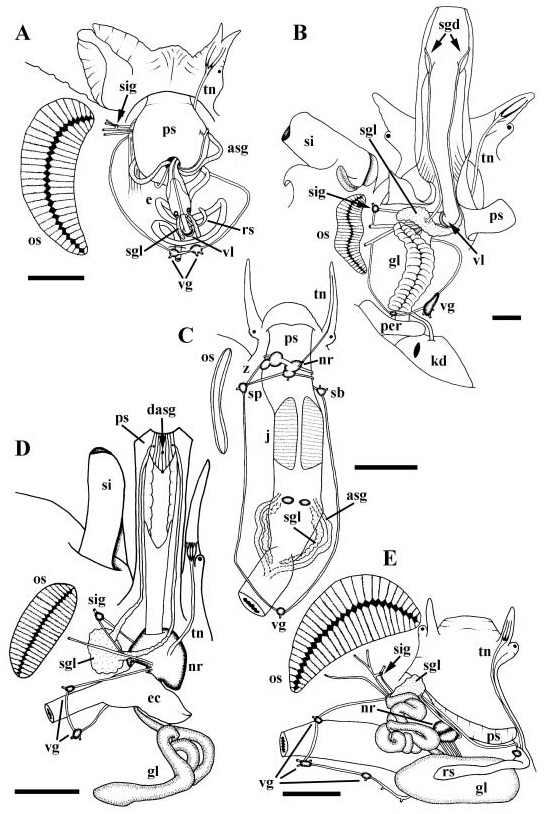
Foregut. Mouth lying at tip of large pleurembolic proboscis ( Fig. 7D View Figure 7 , ps), opening to short, strongly folded buccal cavity. Paired salivary glands (sgl) opening laterally, single accessory salivary gland (dasg) opening ventrally to buccal cavity. Jaw absent. Buccal pouch opening ventrally to posterior end of buccal cavity. Buccal pouch housing modified buccal mass, supported by thin, flexible radular cartilage fused anteriorly and posteriorly. Anterior end of cartilage forming gutter bearing uniserial, multicusped radula. Duct of poison gland (gland of Leiblein, gl) opening to floor of oesophagus at junction of buccal pouch and buccal cavity. Glandular ventral folds absent ( Fig. 10D View Figure 10 ). Salivary gland ducts lying free within proboscis, becoming attached anteriorly to lateral walls of buccal cavity. Paired salivary glands comprising large mass lying to left of proboscis; ducts not penetrating nerve ring. Single, tubular accessory salivary gland embedded within ventral wall of proboscis. Poison gland duct extending posteriorly attached to floor of oesophagus. Oesophagus and poison gland duct penetrating nerve ring. Narrow poison gland duct expanding into large, glandular tube upon passing through nerve ring. Poison gland terminating in pointed, muscular bulb. Oesophagus widening abruptly into large, saculate oesophageal caecum upon passing through nerve ring. Valve of Leiblein absent.
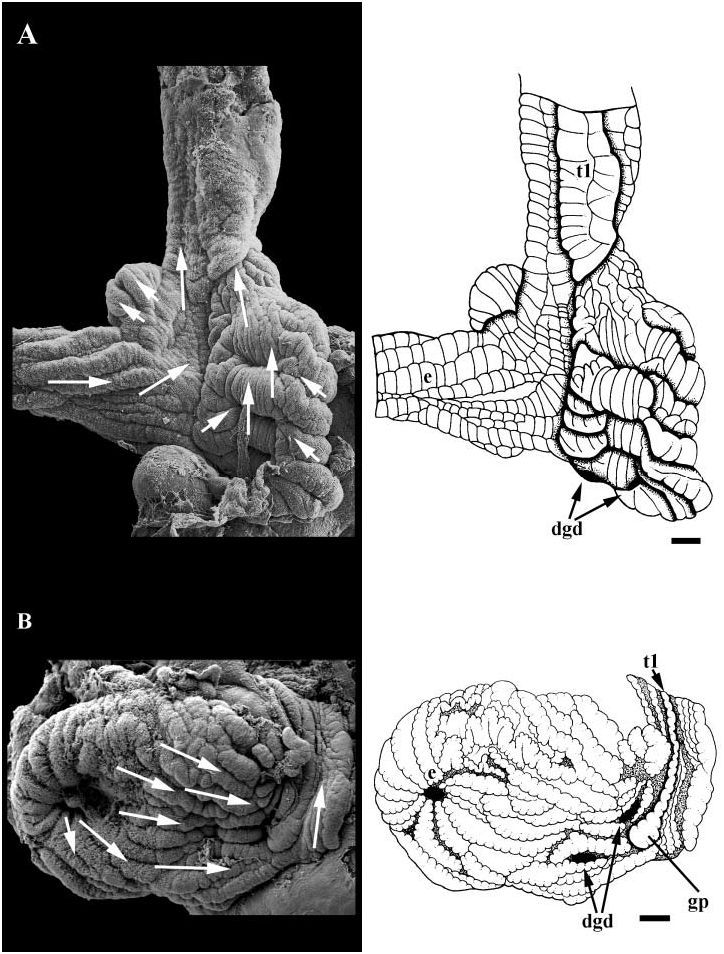
Midgut. Simple, sac-like midgut present behind pericardium ( Fig. 18A View Figure 18 ). Paired digestive gland ducts (dgd) and oesophageal aperture (e) opening on left. Walls of midgut simple and lined with uniformly ciliated folds. Finely grooved tract running along anterior wall, continuous with intestinal groove. Two typhlosoles present in style sac region. Major typhlosole (t1) continuous with folds forming posterior boundary of finely grooved tract. Style sac region longitudinally folded with uniform and undifferentiated cilia. Ciliary currents flowing clockwise within midgut lumen.
Hindgut. Straight, short intestine. Small anal gland forming simple branched tubule under hypobranchial gland. Anal gland opening directly to mantle cavity just in front of anus.
Reno-pericardial system
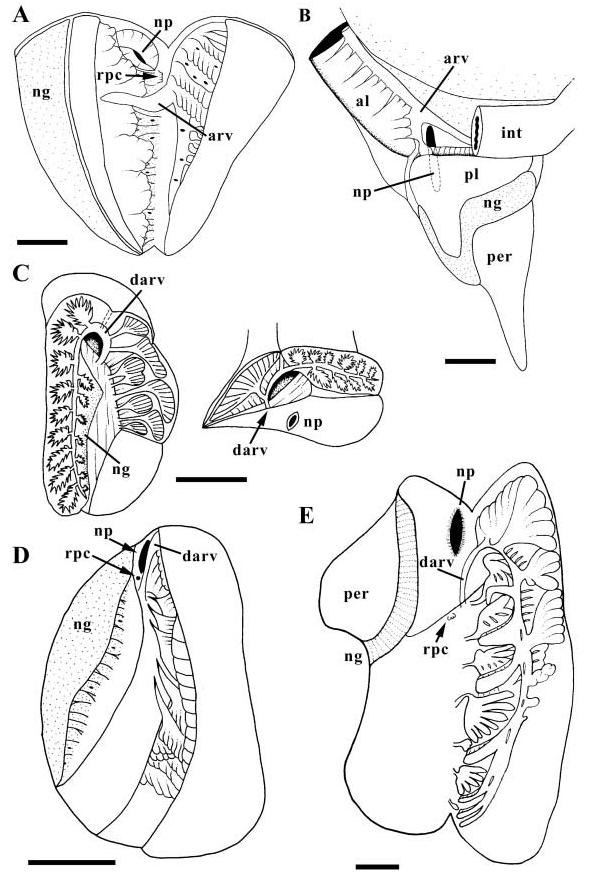
Kidney large ( Fig. 23D View Figure 23 ). Afferent renal vessel entering kidney floor anteriorly behind reno-pericardial canal (rpc). VARV extending back within floor, supplying numerous clusters of primary tubules lining right wall and roof. DARV curving dorsally behind nephropore (np), attached to front wall of kidney. Secondary tubules reduced to small region at front of kidney roof. Primary and secondary tubules not interdigitating. Nephridial gland present.
Nervous system and sensory structures
Nervous system epiathroid, right zygoneurous and left dialyneurous. All ganglia except visceral ganglia concentrated in large nerve ring ( Fig. 24F View Figure 24 ). Tentacular nerve continuously bifurcating, upon entering tentacle, into numerous small nerves of equal size ( Fig. 7D View Figure 7 , tn). Buccal ganglia ( Fig. 24F View Figure 24 , bg) fused to ventral surface of cerebral ganglia (ceg). Large siphonal ganglion ( Fig. 7D View Figure 7 , sig) present near base of siphon (si), at point of dialyneury between pallial nerve and branch from osphradial nerve. Propodial ganglia ( Fig. 24F View Figure 24 , ppg) present at anterior ends of pedal ganglia. Paired visceral ganglia ( Fig. 7D View Figure 7 , vg) present straddling oesophagus. Statocysts ( Fig. 24F View Figure 24 , sc) lying short distance below and behind pedal ganglia, each containing single, large, ovoid statolith. Osphradium ( Fig. 7D View Figure 7 , os) large, bipectinate, lying behind base of siphon. Eyes at outer bases of slender cephalic tentacles.
Remarks
Little has been published on the anatomy of Prunum apicinum . The description of the external anatomy and radula presented here are congruent with those provided by Coovert (1987) and Coovert & Coovert (1990), respectively.
Discussion
Reproductive anatomy of marginellid species remains poorly known compared to the extensive studies of radulae, external anatomy and, to a lesser degree, foregut anatomy discussed below. The oviduct typically joins the albumen gland near its anterior extent ( Ponder, 1970; Harasewych & Kantor, 1991). Similar to other neogastropods ( Fretter, 1941), the capsule gland has a distinctly regionated glandular epithelium. There may be an anterior and posterior zone of mucus secreting cells ( Ponder, 1970; present study) or only a posterior zone (Ponder). There are usually two, rarely one or three, glandular structures for the storage of sperm between the albumen and capsule glands ( Ponder, 1970; Fretter, 1976; Harasewych & Kantor, 1991). However, the presence of orientated or unorientated sperm is not always specified. Thus, it is not clear if these comprise ingesting glands, receptacles, or both. From those descriptions that do note sperm orientation, it appears that some taxa possess only ingesting glands ( Ponder, 1970), with orientated sperm within the ingesting gland ducts. It should be noted that Ponder (1970, 1973) inferred these structures to be receptacles despite the presence of unorientated sperm, based on the fact that ingestion was not observed. Other marginellids possess both receptacles and ingesting glands (e.g. Fretter, 1976).
Graham (1966) observed an unusual connection between the genital duct and kidney in Marginella desjardini , as well as an ingesting gland and a seminal receptacle proximal to the combined albumen/capsule gland; he found no evidence of a bursa copulatrix. Ponder (1970) offered a reinterpretation of Graham’s description and suggested that the posterior-most glandular structure Graham described as a seminal receptacle was in fact homologous to the albumen gland. Ponder further suggested that the structure described as an ingesting gland, with its numerous vesicles was homologous to the seminal receptacles of other marginellids.
However, based on the reproductive anatomy of Prunum apicinum an alternative interpretation of Graham’s findings is possible. Of particular importance is the nature of the connection between the albumen and capsule glands, as well as the close disposition of albumen gland and kidney. Because Graham’s ‘unusual’ connection between oviduct and kidney in Marginella desjardini strongly resembles the sinuous, S-shaped duct connecting the ingesting gland and capsule gland in P. apicinum , this suggests that Graham did not differentiate the kidney from the underlying albumen gland. Thus, the capsule/albumen gland of Marginella desjardini is homologous to the capsule gland of other marginellids. This reinterpretation renders the configuration of sperm storage structures in M. desjardini more easily interpretable. As is common within the family, M. desjardini , in fact, possesses two storage structures between the capsule and albumen glands. This interpretation of M. desjardini also renders the configuration in Prunum apicinum that much more unique, the latter being one of two described marginellids to possess a single sac ( Granula sp. ; Ponder, 1970).
Male reproductive anatomy is somewhat less variable than females. The vas deferens connecting testis with prostate may be straight (present study) or convoluted ( Graham, 1966; Ponder, 1970; Harasewych & Kantor, 1991). Pallial connections of the prostate gland may comprise a slit ( Marcus & Marcus, 1968; Ponder, 1970) or a tubular duct ( Ponder, 1970; Fretter, 1976). The prostate may lie across the exhalent margin ( Marcus & Marcus, 1968; Ponder, 1970; Harasewych & Kantor, 1991) or within the penis ( Ponder, 1970; Fretter, 1976). The penis is oval to spatulate, uni- or bilobed, with the vas deferens opening at its tip ( Eales, 1923; Graham, 1966; Marcus & Marcus, 1968; Ponder, 1970; Fretter, 1976).
Of all the organ systems, foregut morphology is best known among marginellid taxa. All marginellids share a large or small ( Eales, 1923) pleurembolic proboscis that commonly contains a unique buccal pouch. The pouch has been shown to be derived from the radular sac and houses a highly modified odontophore supported by cartilages that are fused anteriorly, posteriorly, or both ( Graham, 1966; Ponder, 1970; Ponder & Taylor, 1992; Coovert & Coovert, 1995). The buccal mass may be lacking ( Eales, 1923; Graham, 1966; Ponder, 1970).
The foregut glandular apparatus is highly variable. Salivary glands may be ascinous or tubular, the salivary gland ducts may be free or attached to the oesophageal walls, the accessory salivary gland may be absent or present, the poison gland (gland of Leiblein) may have a pointed or blunt muscular, terminal bulb, and the poison gland duct may open midventrally or laterally to the buccal cavity, or to the mid-oesophagus ( Eales, 1923; Graham, 1966; Marcus & Marcus, 1968; Ponder, 1970; Fretter, 1976; Ponder & Taylor, 1992; Coovert & Coovert, 1995). In some species, the poison gland lacks a terminal bulb ( Ponder, 1970). Alternatively, as in Marginellona gigas , the poison gland may be broad and saculate rather than narrow and tubular as in the remaining marginellids ( Harasewych & Kantor, 1991). Both the oesophageal caecum and the valve of Leiblein may be present or absent ( Graham, 1966; Marcus & Marcus, 1968; Ponder, 1970; Fretter, 1976; Ponder & Taylor, 1992; Coovert & Coovert, 1995). It is interesting to note that the oesophageal caecum and valve of Leiblein lie in a similar position, at the junction of the anterior and mid-oesophagus. While both may be lacking, they are never present simultaneously (except in the disputed description of Marcus & Marcus, 1968), suggesting that they may be homologues. The valve of Leiblein, when present, differs from other neogastropods in that a duct ventrally by-passes the valve ( Ponder, 1970; Ponder & Taylor, 1992).
Typically, the midgut is small and sac-like with two digestive gland ducts ( Graham, 1966; Ponder, 1970; Fretter, 1976); a single duct or caecum-like duct may be present ( Ponder, 1970; Fretter, 1976). Finely grooved tracts commonly connect the intestinal, oesophageal and digestive gland apertures ( Ponder, 1970; Fretter, 1976). The intestine is rather straight and no style sac region is apparent ( Graham, 1966). The anal gland may be present or absent, often opening to the rectum via a single duct ( Ponder, 1970; Harasewych & Kantor, 1991), and rarely to the pallial cavity ( Ponder, 1970; Fretter, 1976).
The kidney is a large, elongate organ along the left margin of the viscera. The secondary tubules are restricted to an antero-dorsal region ( Ponder, 1970). Ponder was not explicit about the size of the mass of secondary tubules, thus, it is unknown whether the extremely small mass in Prunum apicinum is unique. Ponder likened marginellid kidney morphology to that of olivids ( Marcus & Marcus, 1959). However, the latter description indicates a long strip of ‘villous’ secondary tubules extending the length of the kidney roof, and is not comparable to the conditions in P. apicinum .
The circum-oesophageal nerve ring is highly concentrated ( Eales, 1923; Marcus & Marcus, 1968; Ponder, 1970); paired visceral ganglia are present near the left side of the asymmetrical cephalic haemocoel ( Bouvier, 1887; Graham, 1966; Fretter, 1976).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.