Nototriphyllus, Lawrence, John F., Escalona, Hermes E., Leschen, Richard A. B. & Ślipiński, Adam, 2014
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3826.1.6 |
|
publication LSID |
lsid:zoobank.org:pub:4C7900FD-656C-4180-80DA-449C310CD2B8 |
|
DOI |
https://doi.org/10.5281/zenodo.6141916 |
|
persistent identifier |
https://treatment.plazi.org/id/C73A879E-C4CF-4528-9E53-7F7EF5AA4F84 |
|
taxon LSID |
lsid:zoobank.org:act:C73A879E-C4CF-4528-9E53-7F7EF5AA4F84 |
|
treatment provided by |
Plazi |
|
scientific name |
Nototriphyllus |
| status |
gen. nov. |
Nototriphyllus gen. n.
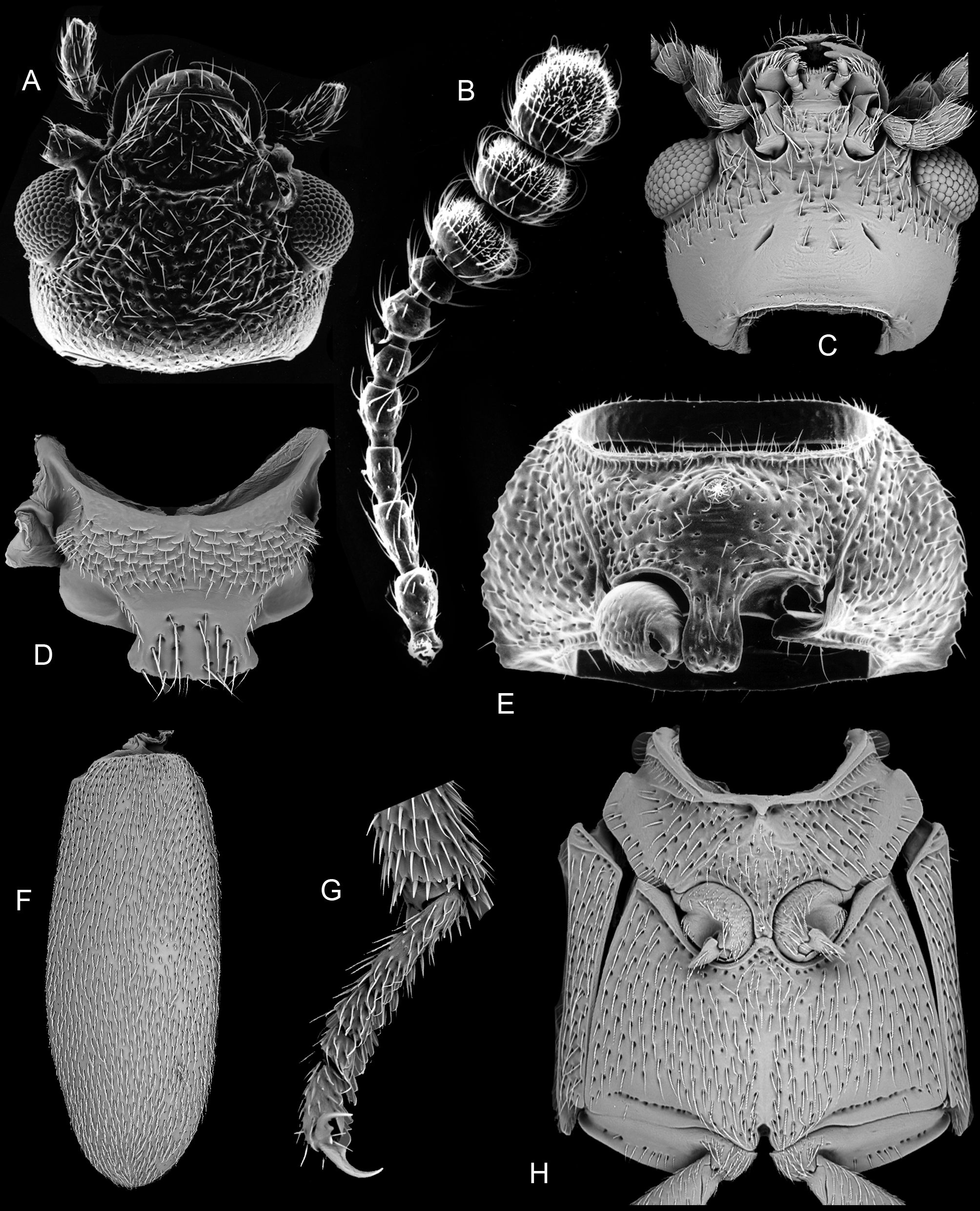
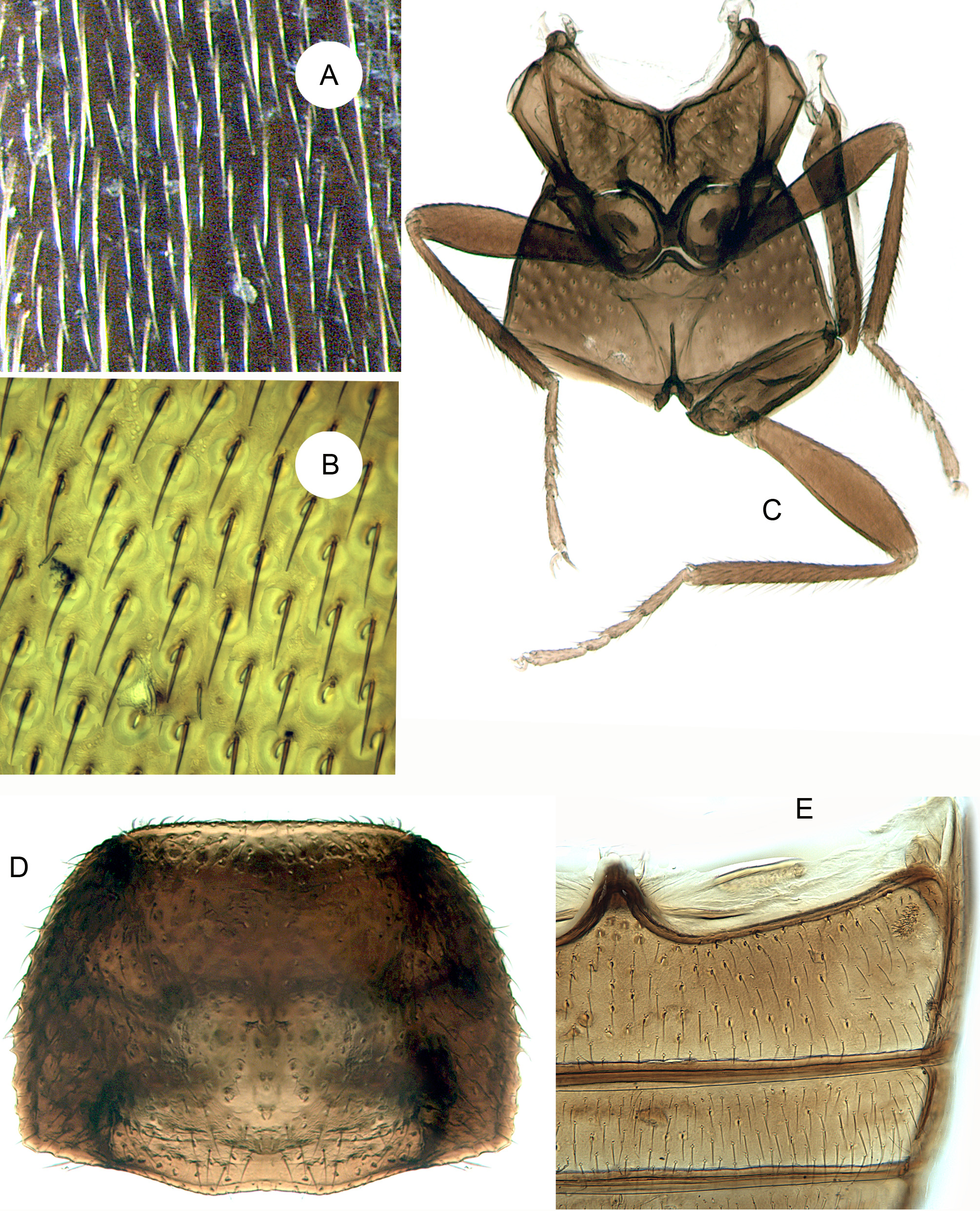
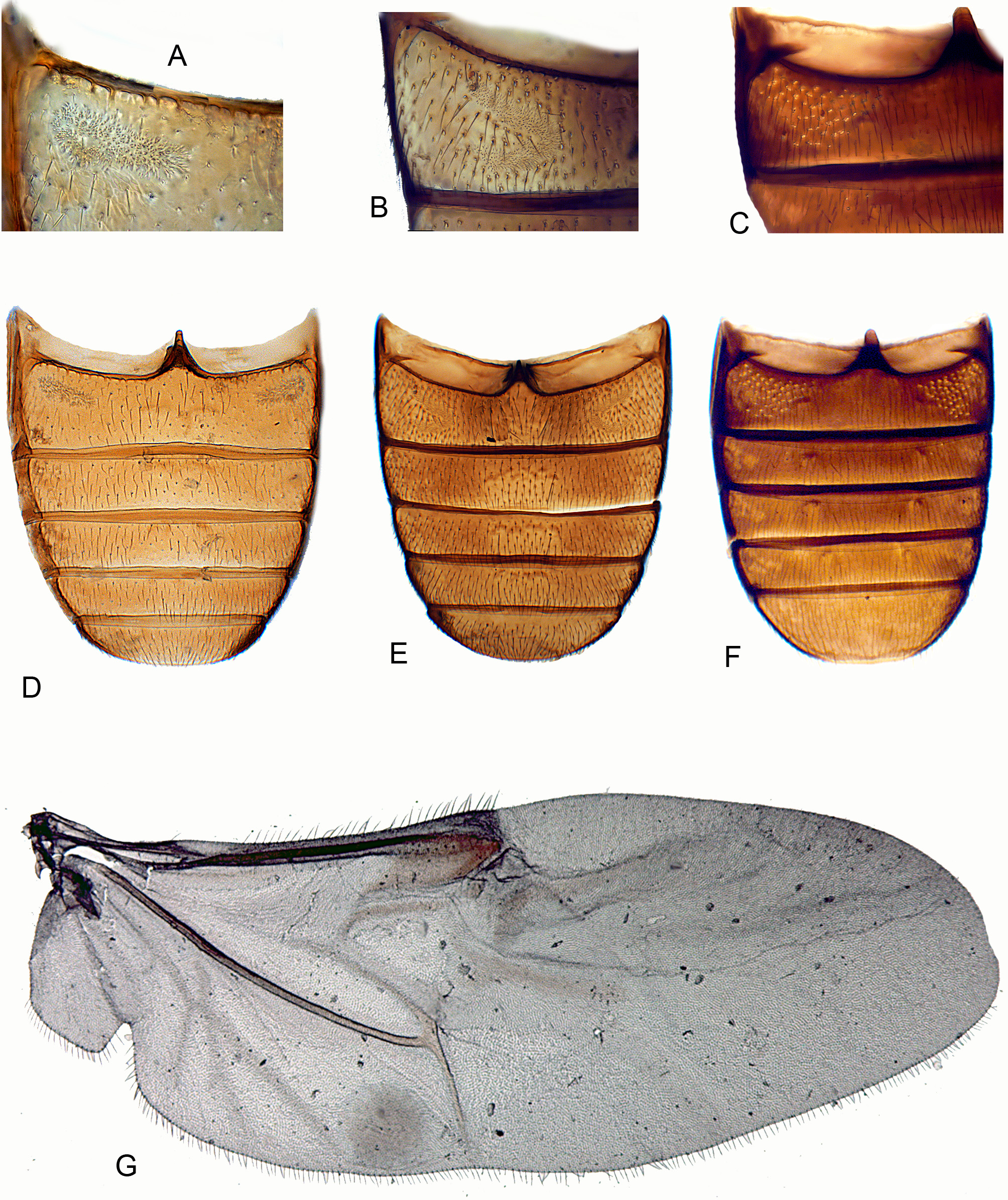
(3B, 3D, 4B, 4E, 4G, 5A–B, 5I, 5N, 14A–H)
Type species. N. araucania sp. n.
Diagnosis. This genus resembles Neotriphyllus , Thrimolus , Triphyllus and Pseudotriphyllus in having a distinct 3-segmented antennal club, more or less confused elytral punctation (occasionally with indistinct or incomplete puncture rows) and an unreduced metaventrite. It differs from the New World genus Thrimolus in the larger body size, lack of a large triangular prosternal rest on the mesoventrite, less rounded posterior pronotal angles, longer elytral epipleura, and shorter, broader pore fields on ventrite 1 in the male. From Neotriphyllus , it differs in having antennomeres 9 and 10 distinctly transverse with large sensory fields ( Fig. 14B View FIGURES 14 A – H ) and in the absence of a frontal setose fovea in the male. The presence of a setose fovea on the male prosternum ( Fig. 14E View FIGURES 14 A – H ) separates this genus from Neotriphyllus , Thrimolus and Triphyllus , whereas the equally broad antennomeres 9, 10 and 11 ( Fig. 14B View FIGURES 14 A – H ), the slit-like extension of the procoxal cavity and differently shaped prosternal process ( Fig. 14E View FIGURES 14 A – H ) and the very slender penis with long basal struts ( Figs 5A–B View FIGURES 5 A – H ) distinguish it from the Holarctic genus Pseudotriphyllus .
Description. Total length 1.4–3.3 mm. Body ( Figs 5 View FIGURES 5 A – H I, N) about 1.75–2.5 times as long as wide, moderately convex. Color usually yellowish-brown to reddish-brown, occasionally dark brown to black or with dark elytral maculae, occasionally black with small yellow maculae on elytra. Surface clothed with fine decumbent hairs.
Head ( Figs 14A, C View FIGURES 14 A – H ) slightly declined, not constricted posteriorly; posterior portion of head capsule without median endocarina but with large, shallow concavity on each side of midline. Eyes moderately well developed and protuberant, very slightly emarginate anteriorly, coarsely facetted. Antennal insertions barely exposed or concealed from above by weak frontal ridges. Frontoclypeal suture distinctly impressed, at least slightly curved; clypeus about half as long as wide at base; sides converging anteriorly; anterior edge truncate. Labrum about half as long as wide; sides strongly rounded; anterior edge broadly rounded or subtruncate. Antennae ( Fig. 14B View FIGURES 14 A – H ) 11-segmented with distinct, 3-segmented club; scape not or only slightly longer than pedicel; antennomeres 3–6 longer than wide, 7 and 8 usually about as long as wide; antennomeres 9–11 of equal width; sensory areas on club extensive, occupying between one-third to one half of antennomeres 9 and 10 and more than half of antennomere 11. Mandibles about 1.25 times as long as wide, strongly but evenly curved apically, with two apical, acute teeth, differing slightly in length and aligned perpendicular to plane of movement of mandible. Maxilla with galea expanded, broadly rounded and setose and lacinia slender and apically subacute, without uncus; apical maxillary palpomere ( Fig. 14C View FIGURES 14 A – H ) subparallel, fusiform or slightly expanded apically, with obliquely truncate apex. Mentum strongly transverse, sides subparallel and apex truncate; ligula truncate; apical labial palpomere cylindrical ( Fig. 14C View FIGURES 14 A – H ). Corpotentorium weakly arched, with anterior median process. Cervical sclerites small and slender.
Pronotum ( Figs 3 View FIGURES 3 A – B D, 5I, 5N) about 0.53–0.7 times as long as wide, widest at base, which is not or only slightly narrower than elytral bases; sides weakly rounded; lateral carinae complete and smooth or denticulate; anterior angles not produced forward, posterior angles right or slightly rounded; posterior edge weakly trisinuate; disc moderately convex with paired moderately large circular subbasal pits. Prosternum in front of coxae slightly longer than mid length of coxal cavity, strongly convex, with median pubescent fovea in male ( Fig. 14E View FIGURES 14 A – H ); anterior edge truncate or concave. Prosternal process slightly expanded apically, distinctly curved dorsally behind coxae, slightly overlapping mesoventrite; apex broadly rounded or subtruncate in ventral view. Notosternal suture complete. Procoxae not projecting below prosternum, without long concealed lateral extension. Trochantins usually slightly exposed. Procoxal cavities slightly transverse, with short, narrow lateral extensions; externally broadly open (postcoxal process short and acute); internally barely closed by slender bridge.
Scutellar shield ( Fig. 14D View FIGURES 14 A – H ) well developed, not abruptly elevated, about half as long as wide; sides apically diverging; posterior edge broadly rounded or truncate. Elytra about 1.28–1.92 times as long as combined width and 2.62–3.92 times as long as pronotum, irregularly punctate ( Fig. 14F View FIGURES 14 A – H ) or with an incomplete set of puncture rows; epipleuron narrow and incomplete; internal surface of each elytron with elongate, sutural, binding patch at apical third and two lateral binding patches at anterior fourth and about middle. Mesoventrite ( Fig. 14H View FIGURES 14 A – H ) separated by complete sutures from mesanepisterna, with slender, median subtriangular prosternal rest, sometimes reduced to short carina. Mesocoxae slightly, obliquely transverse and weakly projecting, with slightly exposed trochantins. Mesocoxal cavities at middle narrowly separated (distance between them about a fifth as great as shortest diameter of one cavity); open laterally (partly closed by mesepimera). Mesometaventral junction apparently simple, with overlapping ventrites. Metaventrite ( Fig. 14H View FIGURES 14 A – H ) moderately convex; discrimen about 0.7 times as long as ventrite excluding anterior process. Exposed portion of metanepisternum about 3.7 times as long as wide; metepimeron visible. Metacoxae narrowly separated, not extending laterally to meet elytra. Metendosternite with moderately long, broad stalk, long lateral arms, well-developed laminae and anterior tendons located on arms at about apical third. Hind wing ( Fig. 4 View FIGURES 4 A – C G) almost 3 times as long as wide; apical field more than half total wing length, with two vaguely indicated radial extensions; radial cell without base; cross-veins r3 and r4 not apparent, but longitudinally oblique rod-like sclerite present; basal portion of RP moderately long to very short; medial spur almost perpendicular to long wing axis and not reaching wing margin; medial field with three free veins, no wedge cell and subcircular, undivided medial binding patch; medial embayment very weak; anal embayment deep and notchlike. Trochanterofemoral joint strongly oblique with femur narrowly reaching coxa. Tibiae ( Figs 5 View FIGURES 5 A – H I, N, 14G) relatively slender with small, simple spurs; tarsi 4-4- 4 in female and 3-4- 4 in male; tarsomere 1 slightly longer than 2; pretarsal claws slender and simple.
Abdomen about as long as wide or slightly longer than wide; ventrite 1 not or only slightly longer than 2, with acute intercoxal process; ventrite 1 in male with paired linear or C-shaped pore fields ( Figs 4B View FIGURES 4 A – C , E); ventrite 5 strongly transverse and broadly rounded at apex. Abdominal tergites III–VI relatively lightly sclerotized, each with pair of large, circular wing-folding spicule patches; tergite VII more heavily sclerotized, a little more than half as long as wide and broadly rounded at apex. Sternite VIII in male without ( N. araucania ) or with short anterior strut (spiculum relictum), truncate or slightly emarginate at apex; tergite VIII lightly sclerotized and broadly rounded. Segment IX in male with long, slender, slightly curved spiculum gastrale and moderately sclerotized laterotergites; segment X membranous. Aedeagus ( Fig. 5A View FIGURES 5 A – H ) with phallobase about 1.3 to more than 2 times as long as apicale. Penis ( Fig. 5B View FIGURES 5 A – H ) about as long as to slightly shorter than phallobase and apicale combined, very slender and parallelsided, with basal struts more than half as long as body of penis. Sternite VIII in female with long, fixed, slender, slightly curved anterior strut (spiculum ventrale). Ovipositor slightly longer than wide, lightly sclerotized. Proctiger elongate with distinct baculi. Paraprocts subequal in length to gonocoxites, with distinct longitudinal baculi. Each coxite transversely divided into short, broad, proximal lobe with a curved transverse baculum and a longer distal lobe, narrowing apically and bearing long, slender, subcylindrical gonostyli slightly before apex.
Etymology. From the Greek, notos, “south”, referring to Notogean distribution, and Triphyllus (a mycetophagid genus); gender masculine.
Distribution. New Zealand, Madagascar, Chile and Argentina.
Biology. Adults of New Zealand Nototriphyllus are found commonly on the fruiting bodies of saproxylic Basidiomycota, have been taken from various plants ( Kuschel 1990), and do not appear to be host specific (R. Leschen, pers. obs.). Nototriphyllus hispidellus ( Broun, 1880) ( Fig. 5 View FIGURES 5 A – H I), however, is a common sooty mould specialist and adults and larvae may be very abundant on various host plants, especially tea (Leptosepermum ericoides) and beech trees (Nothophagus).
Included species: N. aciculatus ( Broun, 1880) (Cryptophagus) comb. n., N. adspersus ( Broun, 1880) (Cryptophagus) comb. n. (= Triphyllus maculosus Sharp, 1886 ), N. araucania sp. n., N. constans ( Broun, 1914) comb. n., N. fuliginosus ( Broun, 1880) (Cryptophagus) comb. n. (= Triphyllus huttoni Sharp, 1886 ), N. hispidellus ( Broun, 1880) (Cryptophagus) comb. n. (= Triphyllus confertus Sharp, 1886 ), N. integritus ( Broun, 1893) comb. n., N. pubescens ( Broun, 1909) comb. n., N. madagascariensis ( Fairmaire, 1898) (Triphyllus) comb. n., N. punctulatus ( Broun, 1880) (Cryptophagus) comb. n. (= Triphyllus concolor Sharp, 1886 ), N. rubicundus ( Sharp, 1886) (Triphyllus) comb. n., N. serratus ( Broun, 1880) (Cryptophagus) comb. n., N. substriatus ( Broun, 1880) (Cryptophagus) comb. n. (= Triphyllus zealandicus Sharp, 1886 ). A number of these species were originally placed by Broun (1880) in the genus Cryptophagus Herbst , as noted by Leschen & Gimmel (2012). The placement of Triphyllus madagascariensis Fairmaire is based on an examination of two specimens from Manjakatompo Forest Reserve, near Antananarivo, Madagascar (FMNH) which matched Fairmaire’s description.
Notes. This Notogean genus is similar in many respects to the Holarctic genus Pseudotriphyllus . The distinct narrowing of antennomere 11 with respect to the transverse antennomeres 9 and 10 was seen in specimens of P. suturalis (Fabricius) and P. lewisianus (Wollaston) and in illustrations of P. d ef oe i ( Carlton & Leschen 2009), P. colchicus Reitter ( Nikitsky 1993) and P. lewisianus ( Miyatake 1959; Nikitsky 1993). This character appears to break down in P. nepalensis Nikitsky (2003) , where all three club segments appear from the illustration to be subequal in length and width; these club segments are also unusual in being more or less quadrate and laterally rounded.
The largest number of Nototriphyllus species occur in New Zealand, and one has been described from Madagascar. Nototriphyllus araucania is known in Chile from the Araucanía region (IX) south to Chiloé, with a single record from Tierra del Fuego in Argentina, but a few doubtful specimens of N. araucania and at least one smaller Chilean species with a distinctive shape and coloration have been seen from the more northern provinces of Ñuble and Talca. Further collecting is needed to assess the distributional limits of the genus in South America.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.