Messelophis variatus, Scanferla & Smith & Schaal, 2016, Scanferla & Smith & Schaal, 2016
|
publication ID |
https://doi.org/ 10.1111/zoj.12300 |
|
persistent identifier |
https://treatment.plazi.org/id/03B8878E-FFD9-FFFA-92F2-27A6FB924891 |
|
treatment provided by |
Marcus |
|
scientific name |
Messelophis variatus |
| status |
gen. nov. |
Premaxilla
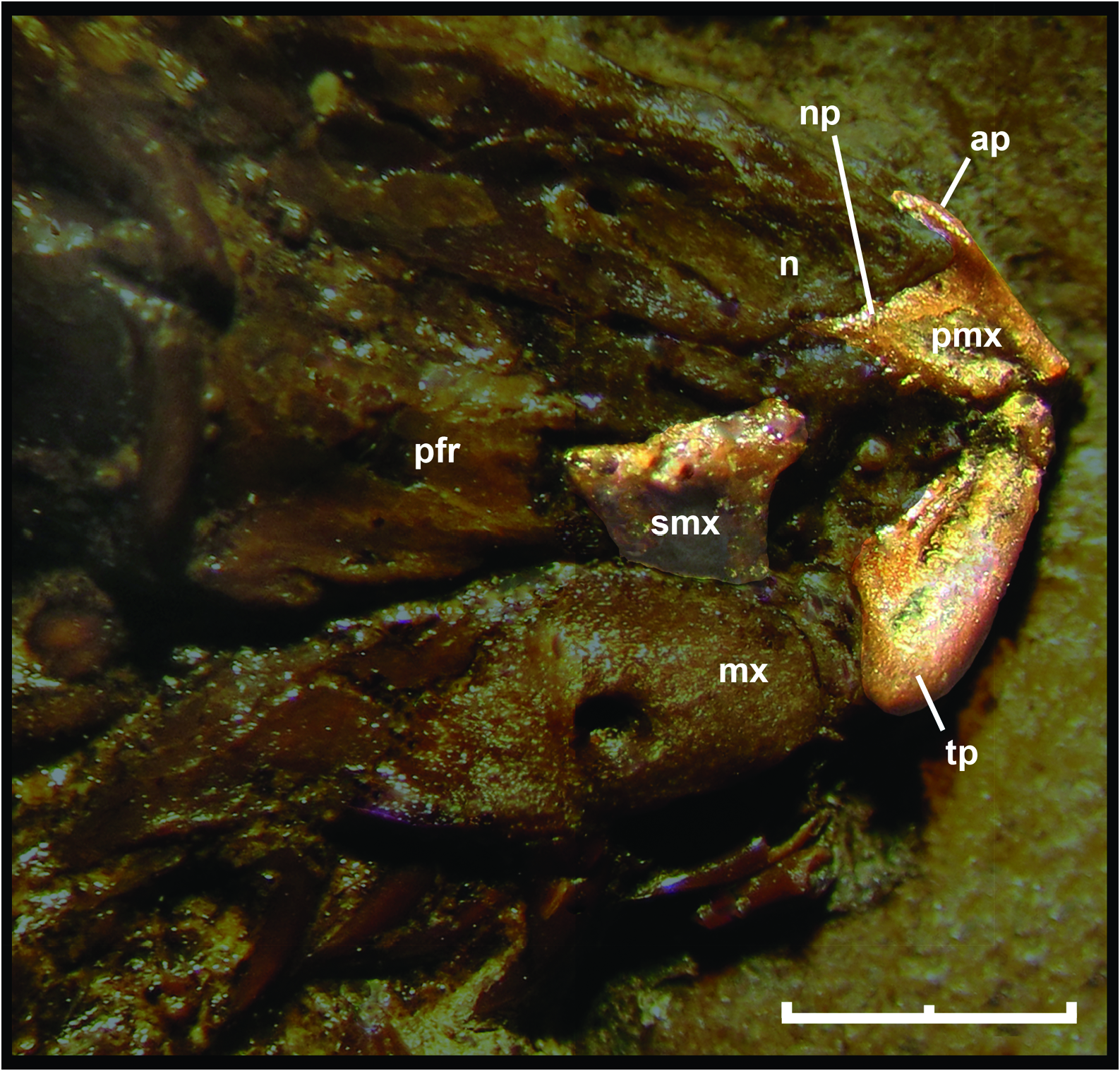
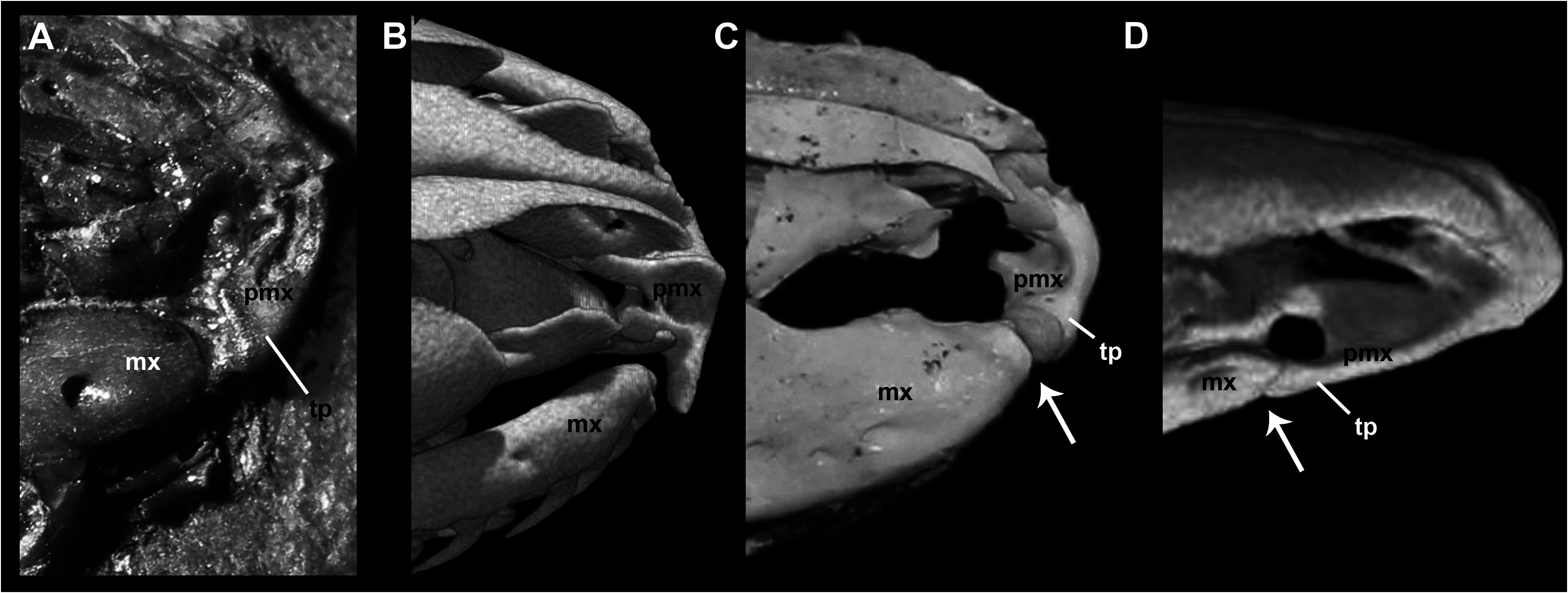
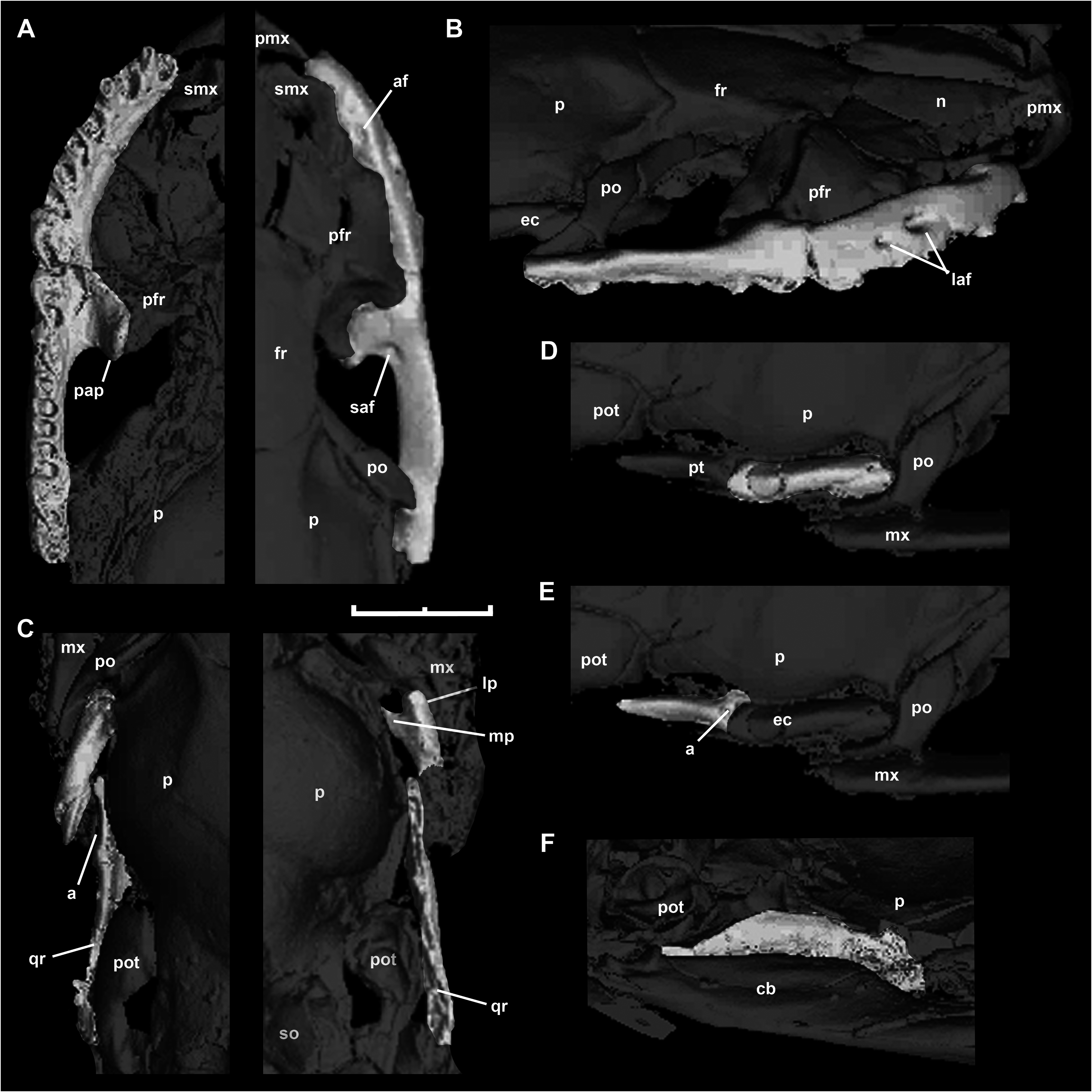
As was pointed out by Baszio (2004: fig. 11), specimen SMF ME 2379 of M. variatus exhibits a toothed premaxilla, a condition shared with Anilius and basal macrostomatans, such as Xenopeltis , Loxocemus , and pythonines ( Smith, Bellairs & Miles, 1953). The premaxilla of M. variatus also has a well-developed ascending process ( Fig. 2 View Figure 2 ). This structure, which is present in boine snakes Exiliboa and Ungaliophis but is absent in tropidophiids, is clearly visible in the snout region of the holotype specimen SMF ME 1828 a+b and in AMNH FARB 30650. The nasal bones clasp the dorsal tip of this process in the former specimen, in a way similar to the condition in boines such as Boa constrictor Linnaeus, 1758 . Also, Baszio (2004) briefly mentioned the presence of an articular facet on the premaxilla that contacts the anterior tip of the maxilla. Although he did not specify the exact position and morphology of the articular facet, we interpret that this structure would have to be located in the posterior edge or at the tip of the transverse process of the premaxilla, as occurs in lizards. An articular contact between premaxilla and maxilla is a rare condition among snakes, documented only in the fossil ‘madtsoiid’ Yurlunggur (Scanlon, 2006; Fig. 3C View Figure 3 ) and uropeltids ( Olori & Bell, 2012; Fig. 3D View Figure 3 ). In these snakes there is a welldefined facet at the tip of the transverse process of the premaxilla and a corresponding facet at the anterior tip of the maxilla ( Fig. 3 View Figure 3 ). On the right side of the holotype specimen ( Fig. 3A View Figure 3 ), it is possible to observe that the tip of the transverse process of the premaxilla Thus, we consider that M. variatus lacks an articular contact between the premaxilla and the maxilla (contra Baszio, 2004). The possible occurrence of vomerine processes cannot be evaluated because of poor preservation.
Septomaxilla
The holotype preserves a small fragment of the right septomaxilla in lateral view ( Fig. 2 View Figure 2 ), located above the anterior portion of the right maxilla. This fragment corresponds to the lateral vertical flange, which exhibits the typical alethinophidian condition of a dorsal laminar projection with a broad base. In its anterodorsal corner, this flange possesses a small anteriorly direct- ed projection as in a number of macrostomatans like Python . As a result of the fragmentary condition of the lateral vertical flange, it could not be determined whether the posterior dorsal process is broken or absent. is located close to the anterior tip of the maxilla, but is not in contact with it. Also, there are no signs of an articular facet either on the premaxilla or on the maxilla in any specimens of this species examined. In fact, the transverse process terminates in a somewhat rounded tip, as in most extant snakes ( Fig. 3B View Figure 3 ). Prefrontal
AMNH FARB 30650 and SMF ME 1828 a+b provide novel information about the morphology of this circumorbital bone ( Fig. 4A View Figure 4 ). Despite the incompleteness of the anterior region of preserved prefrontals in both specimens, it can be inferred that both lateral and dorsal laminae were expanded, as in most booids; however, the dorsal lappet of pythonines and boines is not present. As in most macrostomatans, the prefrontal of M. variatus seems to retain only a posterior contact with the dorsal surface of the maxilla through a tongue-like lateral foot process. Dorsal to this structure is a conspicuous outer orbital lobe that reaches the dorsal region of the prefrontal. This lobe is found also in boines and erycines in different degrees of development ( Fig. 4D View Figure 4 ). The medial foot process is a remarkably long finger-like structure, approaching the development observed in boines and erycines. Between these processes is a deep notch that received the lacrimal duct; in the fossil species, as in boines, erycines, Ungaliophis , and Exiliboa , it opens ventrally.
Postorbital
Although this bone is present in most specimens of M. variatus , the right postorbital of AMNH FARB 30650 is preserved practically undistorted, located close to its original anatomical position ( Fig. 4B View Figure 4 ). In dorsal view, this bone is characterized by a long unforked dorsal region that lies upon a narrow shelf of the parietal and makes contact with the posterior part of the supraorbital margin of the frontal. The dorsal portion, which contacts the postorbital process of the parietal, is strongly anteriorly expanded and forms a lamina. This expansion is also observed in boines and pythonines. In contrast, the ventral portion is slender. The length and orientation observed in examined specimens suggest that the postorbital probably contacted a ventral element (maxilla or ectopterygoid), as was pointed out by Baszio (2004). The inferred morphology is similar to that present in Eryx and Ungaliophis , but in those snakes the region that contacts with the postorbital process of the parietal is not expanded.
Ectopterygoid
The morphology of this bone in M. variatus is best seen in AMNH FARB 30650 ( Fig. 4C View Figure 4 ) and the embedded side of SMF ME 958b. Both ectopterygoids are present in AMNH FARB 30650 in articulation with maxillae. The exact length of the ectopterygoid cannot be determined because the posterior part seems to be broken; however, it is clear that this bone has a well-developed shaft without signs of reduction, unlike in small booids like Lichanura or Ungaliophis ( Fig. 4F View Figure 4 ). The ectopterygoid shaft exhibits a slightly curved lateral edge, as in numerous macrostomatans. The right ectopterygoid of AMNH FARB 30650 preserves the maxillary process, which bears a small anteromedial prong (partially covered by an unidentified fragment of bone) and an anterolateral prong, which between them bound a small notch. This morphology, which is also seen in boines, ungaliophiines, and pythonids, contrasts with that in erycines and tropidophiids, where the anteri- or end of the ectopterygoid is unforked.
Pterygoid
A small fragment identified as the palatine ramus of the pterygoid can be observed in the embedded side of SMF ME 1828 a+b. Because of its fragmentary nature, we only recognize the presence of the base of three small teeth. Additionally, AMNH FARB 30650 bears both quadrate rami of the pterygoid bones in dorsal view ( Fig. 1C View Figure 1 ). The preserved portion of the right pterygoid clearly exhibits a blade-like structure, although the state of preservation prohibits the identification of other important features like a dorsal keel, which is present in some booids.
Parietal
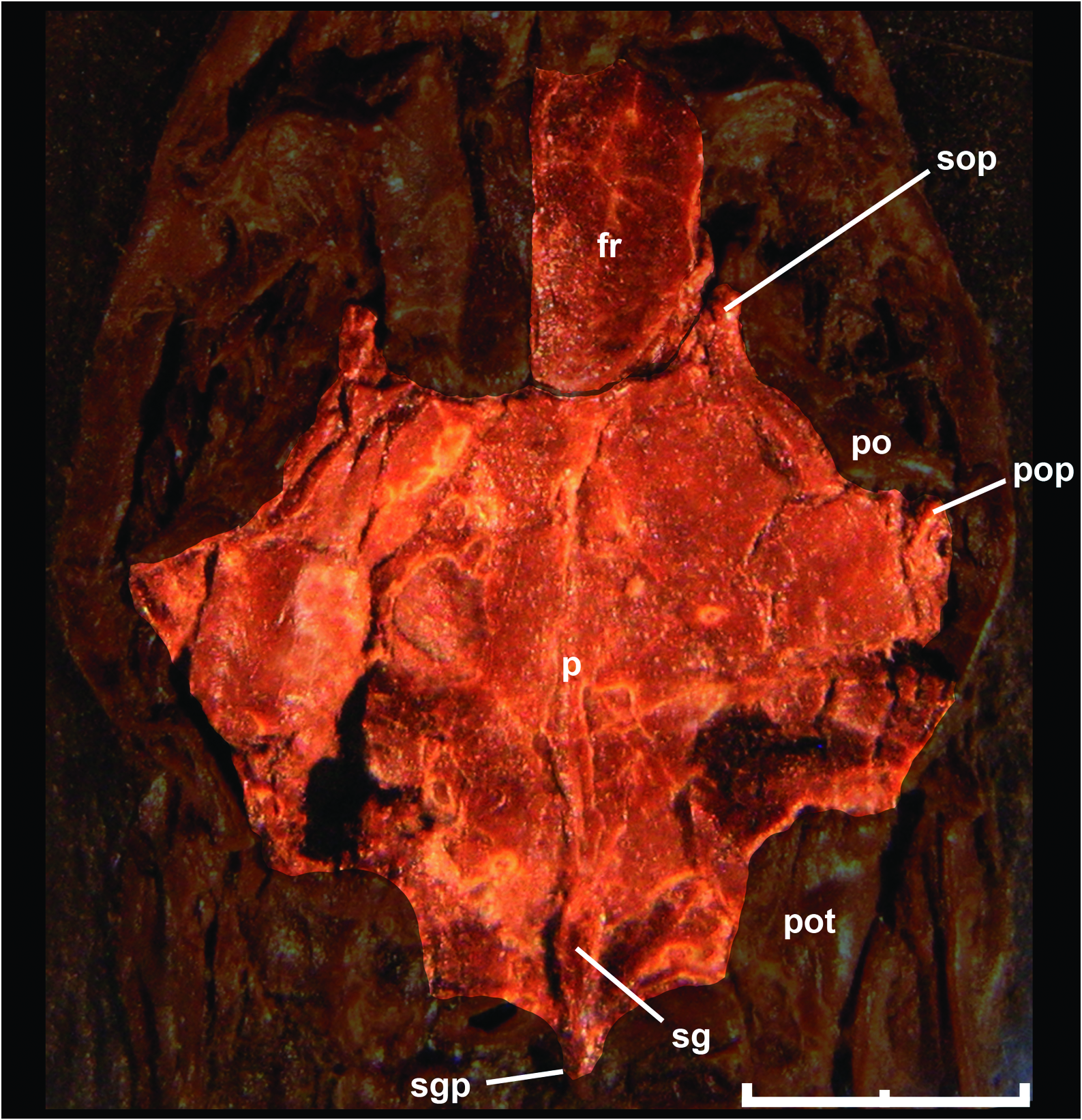
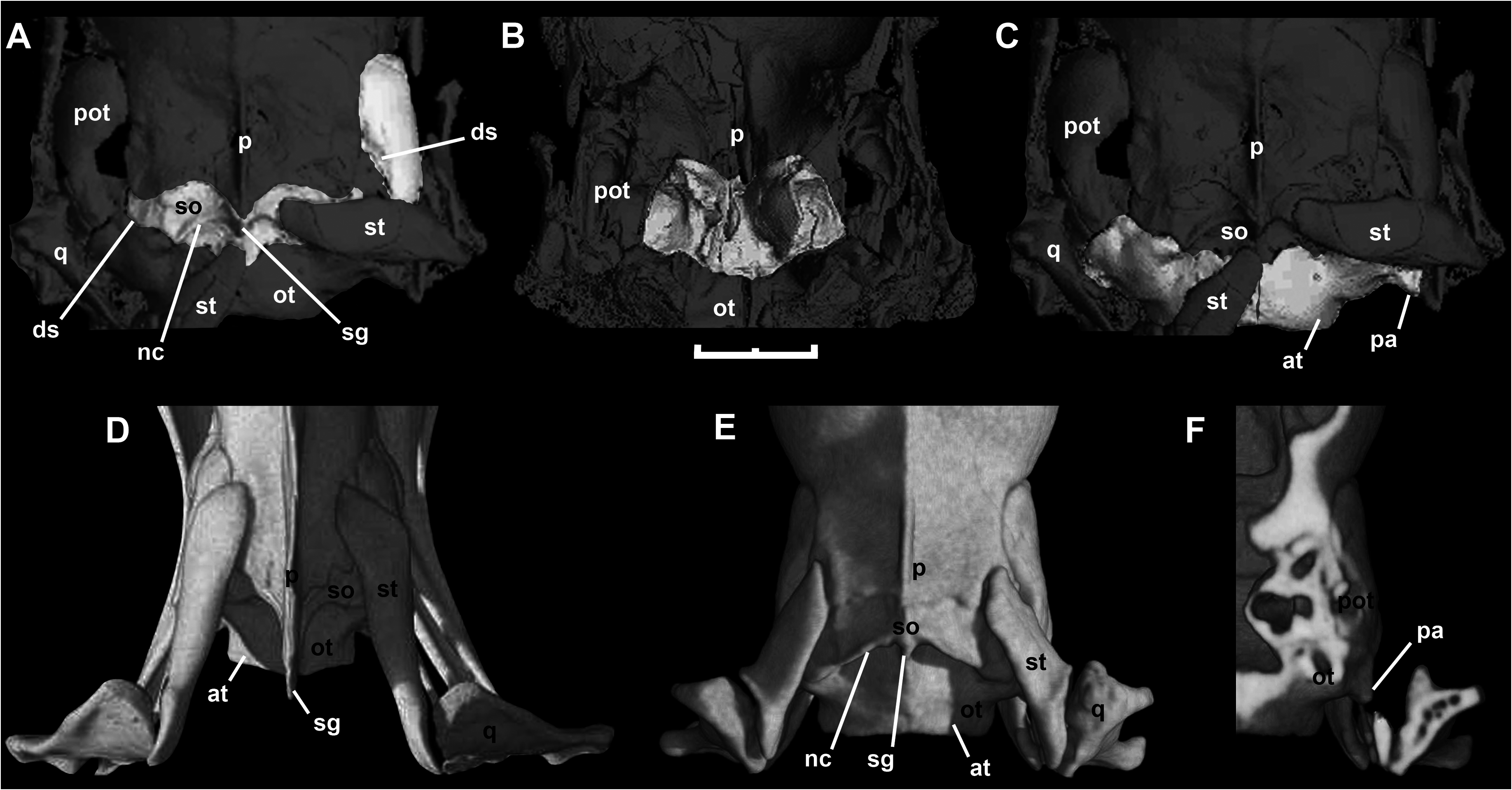
Like most braincase elements of M. variatus , the parietal is strongly crushed in all specimens examined; however, specimen AMNH FARB 30650 adds further anatomical information about the dorsal aspect of this bone ( Fig. 5 View Figure 5 ). The anterior margin that contacts with frontal bones is concave because of the presence of small, anteriorly directed supraorbital processes, a feature that is also observed in small booids like Ungaliophis . In dorsal view, it is possible to recognize a weakly defined parietal table, which is also present in small booids such as Exiliboa , Ungaliophis , erycines (except Eryx ), and derived macrostomatans. As pointed out by Baszio (2004), the parietal bears a sagittal crest on the posterior third. This crest continues backwards as a pointed posterior process, as in erycines and boines, conceal- ing the mid-sagittal part of the supraoccipital bone. As a result of the displacement of the postorbital from its articular position, it is possible to observe a small postorbital process in the right side of the parietal of AMNH FARB 30650 and SMF ME 1828 a+b. Also, the parietal develops a shelf that extends from the anterior end of the supraorbital process to the postorbital process.
Prootic
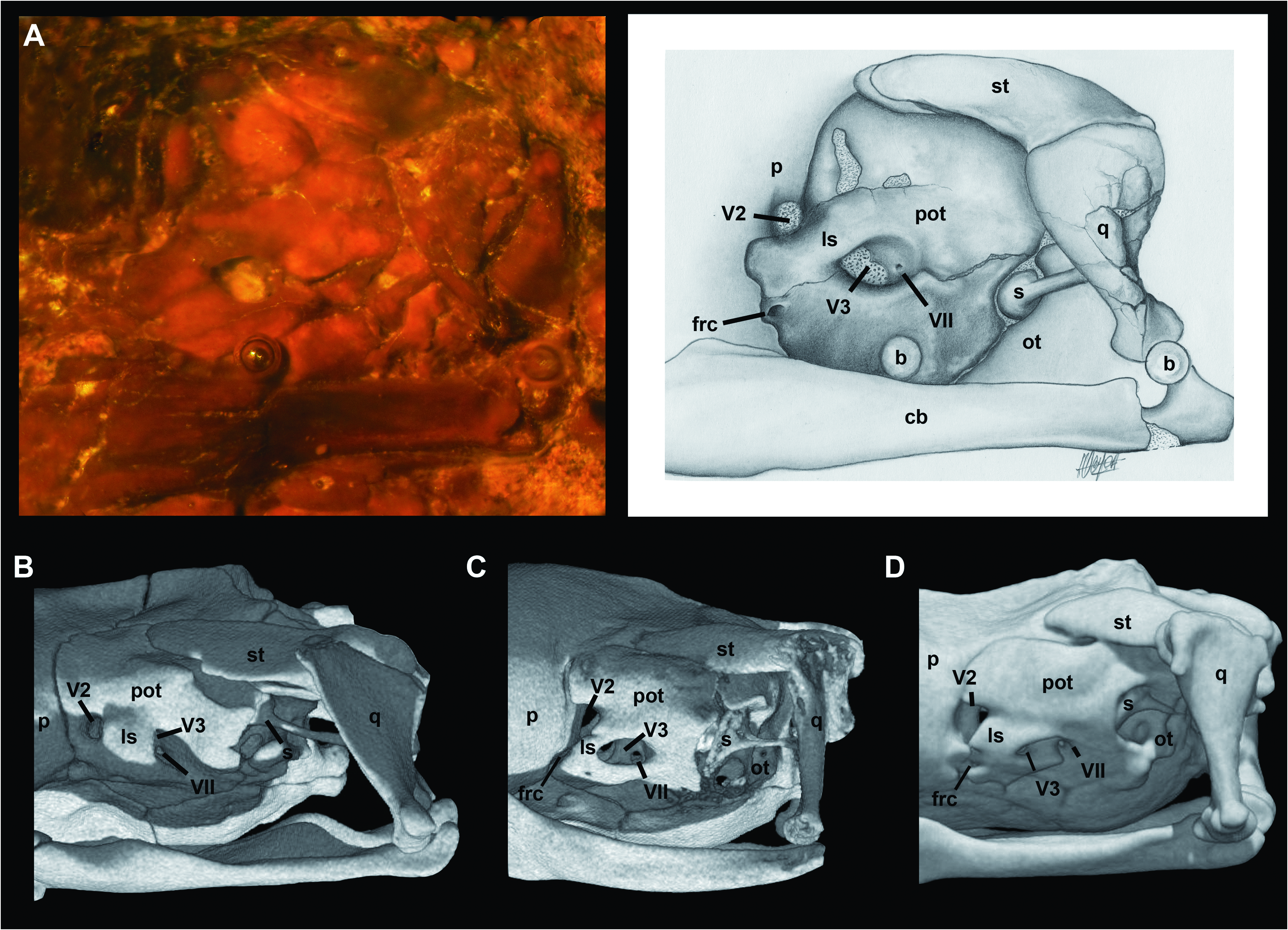
A thorough examination of the embedded side of SMF ME 1828 a+b gives a satisfactory view of the (left) lateral side of the braincase of M. variatus ( Fig. 6A View Figure 6 ). macrostomatans, the crista interfenestralis, which is interposed between the lateral aperture of the recessus scalae tympani and the fenestra ovalis, forms an individualized component in the ventral region of the juxtastapedial recess.
The prootic is similar to that of small booid snakes such as Lichanura ( Fig. 6C View Figure 6 ) and Ungaliophis ( Fig. 6D View Figure 6 ). The anterior (V2) trigeminal foramen is delimited anteriorly by the parietal and the prootic because of the weak development of the dorsal and ventral anterior processes of the prootic. In tropidophiids ( Fig. 6B View Figure 6 ) and most booids, in contrast, these processes in adult specimens are in contact anterior to the anterior (V2) trigeminal foramen, enclosing it ( Scanferla & Bhullar, 2014). The laterosphenoid is present as a broad strip of bone that separates the anterior and posterior parts of the trigeminal chamber. Ventral to the anterior end of this bone is a small foramen that topologically corresponds to the foramen for the re-entry of the cid nerve described by Rieppel (1979) in several macrostomatans. At the ventral region of the posteri- or trigeminal chamber is a small foramen that corresponds with the hyomandibular branch of the facial nerve (VII). In contrast to small booids, the fenestra ovalis is moderate in size. Additionally, the crista prootica is weakly developed and exposes part of the stapedial footplate.
Otooccipital
Parts of the left otoocipital can be seen in the embedded side of SMF ME 1828 a+b ( Fig. 6A View Figure 6 ); however, only the crista interfenestralis and the apertura lateralis recessus scalae tympani are recognizable. As in most Stapes
The left stapes is preserved and visible in articulation in SMF ME 1828 a+b ( Fig. 6A View Figure 6 ). The stapedial shaft is present but partially covered by the quadrate shaft. The observable portion of this structure indicates that its length is much greater than the diameter of the footplate, as in most macrostomatans. The shaft extends posterodorsally to attach to the cephalic condyle or the quadrate shaft. Messelophis variatus has a small footplate, similar to that of large booids, but in contrast to the typical condition in small-sized macrostomatans, which exhibit a large footplate with respect to the size of the neurocranium (e.g. Lichanura ; see Fig. 6C View Figure 6 ). Furthermore, the footplate in M. variatus is not covered by any component of the crista circumfenestralis; however, we cannot rule out the possibility that this condition is a product of the compression suffered by the holotype specimen.
Supratemporal
The embedded side of SMF ME 1828 a+b also offers a lateral view of the left supratemporal in articulation ( Fig. 6A View Figure 6 ). The former is flat bone and is applied to the dorsal surface of the prootic without the development of a distinct facet on the latter bone, and its posterior part articulates with the cephalic condyle of the quadrate. Although the articulation with the skull is surely slightly distorted, it is clear that the posterior end of the supratemporal does not surpass the posterior part of the neurocranium (i.e. a free-ending process was minute or absent). Although the posterior skull roof of specimen AMNH FARB 30650 is badly damaged, it can be inferred that supratemporals have the same short length as in the holotype specimen ( Fig. 1C View Figure 1 ). Moreover, the articular relationship of the supratemporal with the quadrate and the skull roof in both specimens supports the (near) absence of a free-ending process, as is found in most small-sized booids like Lichanura ( Fig. 6C View Figure 6 ). This interpretation contrasts with Baszio (2004), who described a slender supratemporal in the paratype specimen SMF ME 2379 and compared it with that in Boa , which has a long, free-ending process in adult specimens.
Lower jaw
Several relevant features of the lower jaw can be recognized in the embedded side of the holotype specimen SMF ME 1828 View Materials a+b and SMF ME 958 View Materials b, which offer lateral and medial views of the right lower jaw and a lateral view of the right compound bone, respectively ( Fig. 7 View Figure 7 ). The lateral aspect of the dentary reveals a large mental foramen located at the fourth tooth position ( Fig. 7A View Figure 7 ). Several teeth are visible in lateral view; they are recurved and somewhat slender. The medial view of SMF ME 1828 View Materials a+b shows a wide Meckelian groove that opens medially along its length ( Fig. 7B View Figure 7 ). The splenial is long and somewhat narrow anteriorly; there is a long anterior process that tapers anteriorly to a pointed tip, thus closing the Meckelian groove ventromedially. Posteriorly the dorsal margin of the splenial is notched, forming an anterodorsal spine. The splenial and angular meet in an abutting contact, thus forming a well-developed intramandibular joint. The coronoid bone is present, but its dorsal portion is incomplete. This postdentary element is a sliver of bone applied to the medial side of the coronoid prominence of the compound bone. It shows a long anteroventral process that narrowly contacts the posterodorsal margin of the splenial, the medial side of the posterior region of the dentary, and the anterodorsal margin of the angular. The compound bone ( Fig. 7C View Figure 7 ) exhibits a well-developed, asymmetrical surangular crest, the apex of which lies near the anterior end of the bone. Its dorsal margin forms a straight line from the coronoid prominence to the glenoid fossa. The anterior surangular foramen is situated at the base of the coronoid prominence. Posteriorly, the compound bone possesses a short and blunt retroarticular process .
ANATOMICAL REDESCRIPTION OF THE SKULL OF MESSELOPHIS ERMANNORUM
Premaxilla
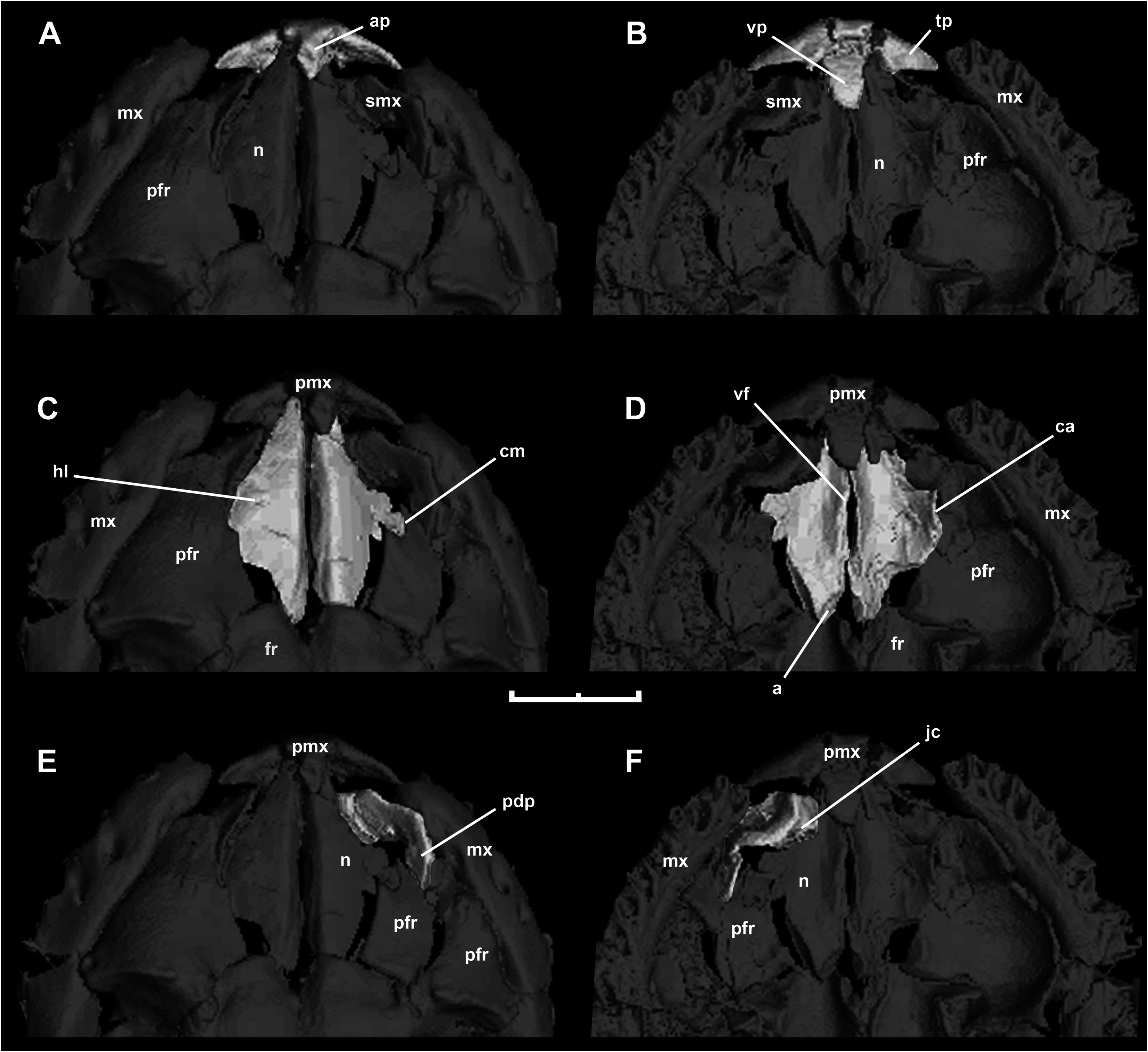
This bone is well preserved in HLMD-Me 7915 ( Fig. 8 View Figure 8 , Fig. 9A, B View Figure 9 ). Schaal & Baszio (2004) stated that forming a slightly concave lateral margin. Laterally, the lamina curves downwards, and part of the lateral margin inserts into a small notch in the dorsal edge of the dorsal lamina of prefrontal ( Fig. 9C View Figure 9 ). Moreover, the ventral view of the left nasal ( Fig. 9D View Figure 9 ) shows that the distal terminus of the dorsal lamina established contact with the dorsal lamina of the prefrontal, as occurs in some boines such as Eunectes and Boa (see Fig. 4D View Figure 4 ). The vertical (medial) nasal flange is well developed, and reaches its maximum extent in the area of contact with the premaxilla. Posteriorly, the vertical (medial) flange displays a distinct articular surface, which would have been in contact with the medial frontal pillar, as is typical of the booid nasofrontal joint ( Rieppel, 2007).
M. ermanorum has an edentulous premaxilla, a condition that strongly contrasts with respect to the toothed premaxilla of M. variatus . New mCT data of the specimen confirm that observation, revealing that the premaxilla of M. ermannorum lacks teeth and tooth positions. Another important difference between these forms is the shape of the transverse process, which is larger and more gracile in M. ermannorum than in M. variatus . As in M. variatus and boines, there is a well-developed ascending process, but its length cannot be ascertained because the distal end is broken. In ventral view, the premaxilla of M. ermannorum exhibits a conspicuous vomerine process. Although its morphology is difficult to determine because of the damage suffered by this specimen to its ventral side, it does not appear to be paired as in erycines.
Nasal
Both nasals are well preserved in HLMD-Me 7915, only slightly displaced from their anatomical position ( Fig. 9C, D View Figure 9 ). Anteriorly, the dorsal (horizontal) lamina tapers to a pointed tip, as in M. variatus ( Baszio, 2004) , thus Septomaxilla
HLMD-Me 7915 preserves a portion of the right septomaxilla ( Fig. 9E, F View Figure 9 ). As in most non-caenophidian alethinophidian snakes, the lateral vertical flange exhibits a broad base and a well-developed posterior dorsal process. Usually, this posterior process is relatively large and slender, but M. ermannorum exhibits a remarkably expanded posterior dorsal process. Among extant snakes, only Exiliboa approaches this condition. In ventral view ( Fig. 9F View Figure 9 ), it is possible to distinguish the contribution of the septomaxilla with the anterior wall of the cavity that houses Jacobson’s organ.
Prefrontal
Both prefrontals are preserved in HLMD-Me 7915 ( Fig. 10 View Figure 10 ). This bone resembles the typical booid condition previously observed in M. variatus . The lateral aspect of both prefrontals exhibits an expanded lateral lamina, as in most booids, suggesting that this structure covered the nasal gland laterally. The dorsal margin of this lamina exhibits a notch that contacts part of the lateral edge of the dorsal (horizontal) lamina of nasal, as previously mentioned. Despite its resemblance to boines and pythonines, the prefrontal lacks the dorsal lappet typically present in these groups. The lateral aspect of the prefrontal shows a conspicuous outer orbital lobe, as in M. variatus and many booids. There is a well-developed lateral foot process, which constitutes the only region of the ventrolateral margin of the prefrontal that establishes contact with the dorsal surface of the maxilla. The medial foot process is strong, although it is relatively shorter than the same structure in M. variatus . As in M. variatus , boines, erycines, and ungaliophiines, the lacrimal notch opens ventrally. We interpret the right prefrontal as being close to its natural position with respect to the maxilla ( Fig. 10A View Figure 10 ). If this interpretation is correct, the medial foot process is in contact with the dorsal surface of the medial portion of the palatine process of the maxilla. This articular condition – characterized by the aforementioned relation plus a well-developed lateral foot process in contact with the dorsal surface of the maxilla – strongly resembles the boine prefrontal–maxilla articulation (compare with Fig. 4D View Figure 4 ).
Postorbital
Both postorbitals are present and undistorted in specimen HLMD-Me 7915 ( Fig. 10 View Figure 10 ). The morphology of this bone strongly resembles that of M. variatus described above. The unforked dorsal region is applied to the anterolateral corner of the parietal and the most posterior part of the supraorbital margin of the frontal. The slight displacement of the right postorbital demonstrates that this region articulates along a shelf modelled in the parietal bone. As in M. variatus , the dorsal portion of the bone is strongly anteriorly expanded and confers a laminar shape to this part of the postorbital. The ventral portion, located posterior to the orbit, is more slender than the dorsal portion; it curves posteroventrally and tapers distally. The ventral tip, which would have contacted the maxilla or ectopterygoid, lacks the foot-like shape present in most pythonines and boines.
Maxilla
Both maxillae are present and undistorted in HLMD- Me 7915 ( Fig. 11 View Figure 11 ), but only the right maxilla is well exposed. This bone is slender, and its anterior portion shows the distinct medial curvature seen in most booids. The posterior region is straight, and the region of contact with the ectopterygoid is lower and more delicately built. In lateral view, there are two labial foramina, the anterior larger than the posterior, located at the sixth and eighth tooth positions, respectively. This condition contrasts with the single labial foramen present in M. variatus , which is located more anteriorly (at the fourth tooth position). Schaal & Baszio (2004) identified on the basis of superficial examination the presence of 15 maxillary teeth; however, a ventral view obtained through CT reveals 22 ± 1 tooth positions. The teeth are mostly crushed or absent, with the exception of two teeth at the middle of the maxilla. The preserved bases of some anterior teeth and the size of tooth positions indicates that there is a strong anteroposterior decrease in tooth size, similar to the condition in most booids. The bases of the teeth are robust and rounded, without signs of folded enamel or dentine. Preserved entire teeth are conical, recurved, and pointed. The palatine process is located at the middle of the bone and posteromedially oriented. At the posterolateral corner of this process there is a small foramen and a shallow groove corresponding to the superior alveolar foramen and its groove. Messelophis ermannorum lacks the ectopterygoid process at the posterior end of the maxilla, in contrast with derived macrostomatans. Posteriorly, there is a shelf onto which the ectopterygoid articulated. The foramen for the superior alveolar nerve pierces the posterodorsal margin of the palatine process close to its junction with the main body of the maxilla.
Ectopterygoid
The mCT reconstruction provides satisfactory dorsal and ventral views of both ectopterygoids of HLMD- Me 7915 ( Fig. 11C, D View Figure 11 ). The ectopterygoid shaft is robust and straight, in contrast to the weakly curved shaft previously described for M. variatus . Anteriorly is a forked maxillary process that overlapped the posteri- or end of the maxilla. The anterolateral prong of the maxillary process is somewhat bulbous and longer than the anteromedial prong, which would have extended to the level of the ventral tip of the postorbital. The pterygoid process of the ectopterygoid is barely differentiated from the shaft. In dorsal view ( Fig. 11C View Figure 11 ) the medial region of this process exhibits a gently convex surface that is in close proximity to the concave articular surface on the lateral margin of the ptery- goid. Furthermore, the right ectopterygoid shows the same morphology and relationship with the right pterygoid ( Fig. 11D, E View Figure 11 ). This kind of articular relationship between ectopterygoid and pterygoid bones resembles the condition present in erycines, boines, and Ungaliophis , where a laterally positioned welldelimited concave surface receives the posteriormost medial (convex) surface of the pterygoid process of the ectopterygoid.
Pterygoid
A small portion each of the left and right pterygoids is present in HLMD-Me 7915 ( Fig. 11 View Figure 11 ). The right pterygoid fragment shows a laterally positioned concave area that received the ectopterygoid ( Fig. 11E View Figure 11 ). The same articular area is seen in the dorsal view of the preserved portion of the left pterygoid ( Fig. 11C View Figure 11 ). Additionally, the left pterygoid fragment preserves part of the quadrate ramus ( Fig. 11F View Figure 11 ), which exhibits a laminar conformation, as in most macrostomatans.
Palatine
The right palatine bone is preserved in SMF ME 11426 only, yet is slightly compressed and still articulated with a small fragment of the palatine ramus of the pterygoid ( Fig. 12 View Figure 12 ). Despite the poor state of preservation, it is possible to observe some relevant traits. Most of the palatine is composed of a prominent and toothed anterior (dentigerous) process that reveals at least six tooth positions, most of these occupied by the base of teeth only. The anteriormost tooth is the best preserved of the palatine dentition, showing that palatine teeth were similar in size to the maxillary teeth, as in macrostomatans. As in most boids, both choanal and maxillary processes are located at the posterior end of the palatine, located on either side of the palatine–pterygoid joint. The choanal process comprises a narrow-based strip of bone, but the exact length of this process is not possible to ascertain. In the same way, the boundaries of the maxillary process are not defined in this specimen. Unfortunately, the poor preservation of this specimen precludes any interpretation about the nature of the palatine–pterygoid joint. Frontal
Both frontals are present, but their lateral downgrowths are broken. In dorsal view, each frontal exhibits a rectangular shape and a slightly convex posterior border with the parietal. This bone lacks the supraorbital shelf that is consistently present in boines and that confers the square shape in dorsal view. In ventral view, it is possible to distinguish a well-developed medial frontal pillar, which is a typical alethinophidian trait ( Rieppel, 2007).
Prootic
Little of the prootic bones is preserved: only the dorsal region that contacts the skull roof. Therefore, the extensive crushing of these preserved portions precludes any description of the most relevant features of this braincase bone. A depression that is a continuation of the respective structure on the parietal is present in the dorsal region of the right prootic. The combined depression received the anterior portion of the supratemporal bone.
Parietal
As was described by Schaal & Baszio (2004), the parietal of this species is broad anteriorly ( Fig. 13C View Figure 13 ), a common condition in small-sized booids. As in M. variatus and other small booids, such as Ungaliophis , there are small supraorbital processes that embrace the posterior part of the frontals and give the parietal a concave anterior margin. The anterolateral corner bears a narrow shelf that extends from the supraorbital process to the postorbital process, and supported the dorsal end of the postorbital. A cup-shaped parietal table is well developed. Consequently, the sagittal crest is restricted to the posterior third of the bone. This crest continues posteriorly as a pointed posterior sagittal process, as in M. variatus , erycines, and boines, concealing the mid-sagittal region of the supraoccipital bone. The supratemporal process is present as a posteriorly directed lobe that covers part of the anterolateral corner of the supraoccipital. Laterally, this process exhibits a conspicuous longitudinal depression that received the anterior region of the supratemporal bone ( Fig. 13C View Figure 13 ); this depression is absent in M. variatus . The mCT reconstruction reveals the endocranial view of the parietal of HLMD-Me 7915. As can also be inferred from the external view, the endocranial form of the parietal clearly shows a bulbous cavity for the telencephalon. Also, a strong medial parietal pillar, a typical feature present in all alethinophidian snakes, is well developed and delimits the space occupied by the telencephalic and mesencephalic regions of the brain.
Supraoccipital
Superficial examination and the CT reconstruction of HLMD-Me 7915 reveals the external and internal structure of the supraoccipital. It is extensively exposed in dorsal view, in contrast to boines and Eryx , where it is poorly exposed or not at all; however, as in these extant snakes the posterior process of the parietal covers the sagittal crest dorsally. Curving anteromedially and then posterolaterally from the distal end of the posterior process of this crest are the paired nuchal crests, which barely continue onto the otooccipitals. As in most macrostomatans (except tropidophiids and caenophidians) a pointed projection of the supraoccipital is interposed between the paired otooccipital atlantal processes. The internal view of the supraoccipital shows that the supraoccipital contributes to the dorsomedial part of the vestibular cavity, as in other snakes.
Otooccipital
Only the dorsal region of this bone is preserved in HLMD-Me 7915 ( Fig. 14C View Figure 14 ). As a result of the displacement of the right supratemporal, the small paroccipital process can be seen. Amongst booids, relicts of the paroccipital process are present in pythonines, which display a relatively long spike-like structure, and in most boines and Ungaliophis ( Fig. 14G View Figure 14 ). In this latter genus, as in M. ermannorum , the paroccipital process is represented by a small, acuminate projection of the posterolateral corner of the otooccipital. Both otooccipitals meet dorsally in a broad dorsomedial contact behind the supraoccipital ( Fig. 14C View Figure 14 ), their atlantal crests forming the dorsal margin of the foramen magnum.
Quadrate
Both quadrates are preserved in HLMD-Me 7915 and SMF ME 11426. The quadrate of M. ermannorum has a broad cephalic condyle that lacks a suprastapedial process, and the shaft is relatively short ( Fig. 15A View Figure 15 ). The stapedial contact, that is the stylohyal, lies at the posterior end of the cephalic condyle ( Fig. 15B View Figure 15 ), as in small booids such as Ungaliophis ( Fig. 15D, E View Figure 15 ) and Exiliboa . The mandibular condyle is broken medially; however, the preserved lateral portion suggests the condyle exhibited the typical saddle-shaped articular surface of most snakes. The quadrate is suspended from the pos- terior portion of the supratemporal. Because the posterior end of the latter bone does not project as a freeending process beyond the otooccipital ( Fig. 15C View Figure 15 ), the mandibular condyle of the quadrate lies at the level of the occipital condyle.
Supratemporal
Left and right supratemporals are preserved in HLMD- Me 7915, although they are displaced from their anatomical position. The supratemporal of M. ermannorum is relatively short, with two different regions ( Fig. 15C View Figure 15 ). The anterior portion is a tongue-like lappet that fits into the previously described depression on the supratemporal process of the parietal, the dorsal margin of the prootic, and the distal portion of the supraoccipital ( Fig. 14A–C View Figure 14 ). The contour of this complex area of contact allows us to infer the position of the supratemporal in life, which was mostly applied above the braincase, and in particular the location of its anterior tip ( Fig. 15C View Figure 15 ). This location shows that the supratemporal projected slightly beyond the rear margin of the otooccipital, a widespread condition among small- sized booids like Ungaliophis ( Fig. 15F View Figure 15 ). The posteri- or free-ending portion exhibits a marked lip, obliquely oriented with respect to the supratemporal body, which received the quadrate cephalic condyle.
Lower jaw
The left lower jaw of HLMD-Me 7915 is almost completely preserved ( Fig. 16 View Figure 16 ). Schaal & Baszio (2004) mentioned only 13 teeth in the dentary of M. ermannorum , but the mCT reconstruction demonstrates that 23 tooth positions are present. Preserved teeth occupy several of these sockets, which are implanted as in alethinophidians (see Zaher & Rieppel, 1999). As in a number of macrostomatans, there is a distinct anteroposterior decrease in tooth size, although the difference between anterior and posterior teeth is not as marked as in some boids, like Eryx ( Fig. 16F View Figure 16 ). The teeth exhibit a conical shape, and the tooth tips are recurved. The alveolar ramus of the trigeminal nerve emerges from a large mental foramen located at the posterior region of a shallow groove approximately positioned between the sixth and seventh teeth, whereas in M. variatus the same foramen is at the fourth tooth position. The posterior dentigerous process is well developed, surpassing the limits of the posterior ventral process. Meckel’s canal is deep and reaches the anterior tip of the bone. The left compound bone is well preserved. As in other alethinophidians, it bears an elongated mandibular fossa delimited by surangular and prearticular crests. As in Eryx ( Fig. 16F View Figure 16 ), the long and straight surangular crest runs from the coronoid prominence anteriorly to the glenoid fossa; it is relatively weak in comparison with M. variatus . In medial view, the prearticular crest displays a convex dorsal edge. The surangular process of the compound bone has a particularly rounded anterior tip, in contrast with the anterior pointed process present in M. variatus and most snakes. The retroarticular process is short and robust. A relatively large coronoid bone is applied to the medial surface of the coronoid prominence and anterior part of the compound bone. Its anteroventral process is well developed and extends ventrally to meet the angular and splenial, although postmortem displacement separated it slightly from these postdentary bones. The splenial and angular bones are pierced by the anterior and posterior mylohyoid foramina, respectively. These bones meet in a straight vertical contact, thus forming the intramandibular joint, with articular facets on the splenial and dentary to accommodate processes of the coronoid and angular. At the joint, the splenial has a prominent anterodorsal spine that covers Meckel’s groove and bounded the anterior inferior alveolar nerve.
PHYLOGENETIC ANALYSIS
In order to test previous conclusions about the phylogenetic relationships of M. variatus and M. ermannorum , we performed morphology-based and combined (morphology + DNA) phylogenetic analyses. The 156-character morphological matrix of Scanferla et al. (2013) was modified slightly for this purpose. We changed several terminals to species level in order to diminish the number of polymorphic codings, and added several terminals that represent all recognized groups of macrostomatans (Boinae, Pythoninae, Erycinae , Ungaliophiinae , Tropidophiidae , and Bolyeriidae ). Nine additional characters were obtained from the literature ( Kluge, 1991, 1993a, b; Gauthier et al., 2012), and four new unpublished characters were defined from personal observations. The resulting matrix with 169 osteological characters was analysed alone and in com- bination with DNA sequences for four mitochondrial (12S, 16S, cytochrome b, and NADH4) and four nuclear (BDNF, Cmos, PNN, and NGFB) genes, all taken from GenBank. The choice of these particular genes corresponds with their availability in GenBank for select- ed terminals in order to reduce the quantity of missing data. If multiple sequences were available in GenBank for a given taxon, we selected only one sequence and chose the most complete sequence for inclusion. We employed static homology via multiple alignment using default settings in Clustal X ( Thompson et al., 1997). After alignment, each sequence was trimmed of its leading and lagging gaps using BIOEDIT ( Hall, 1999). Thus, the resulting lengths of the DNA sequences in base pairs and gaps are: (317) 12S, (354) 16S, (519) cytochrome b, (522) NADH4, (658) BDNF, (295) Cmos, (507) NGFB, and (899) PNN. See Appendix S1 for further information.
Our morphological and combined matrices contain 37 taxa coded for 169 (morphological) and 4240 (169 morphological + 4071 molecular) characters (available at MorphoBank, http://www.morphobank.org, as project P1179). The trees were rooted using the anguimorph lizard Varanus salvator (Laurenti, 1768) as an outgroup, based on recently proposed anguimorph– snake relationships ( Forstner, Davis & Arévalo, 1995; Lee, 2009; Wiens et al., 2010). The matrices were analysed in TNT ( Goloboff, Farris & Nixon, 2008), with all characters treated as unordered and gaps coded as missing data. The general analytical method employed was maximum parsimony ( Farris, 1983). The search strategy employed in TNT was ‘Traditional search’ (using tree bisection and reconnection, TBR) with 1000 replications, with the objective of encountering all possible tree islands. Two alternative support measures (Bremer support and bootstrap resampling) were obtained with TNT to evaluate the robustness of the nodes of the most parsimonious trees. Bootstrap values were calculated with 1000 pseudoreplicates.
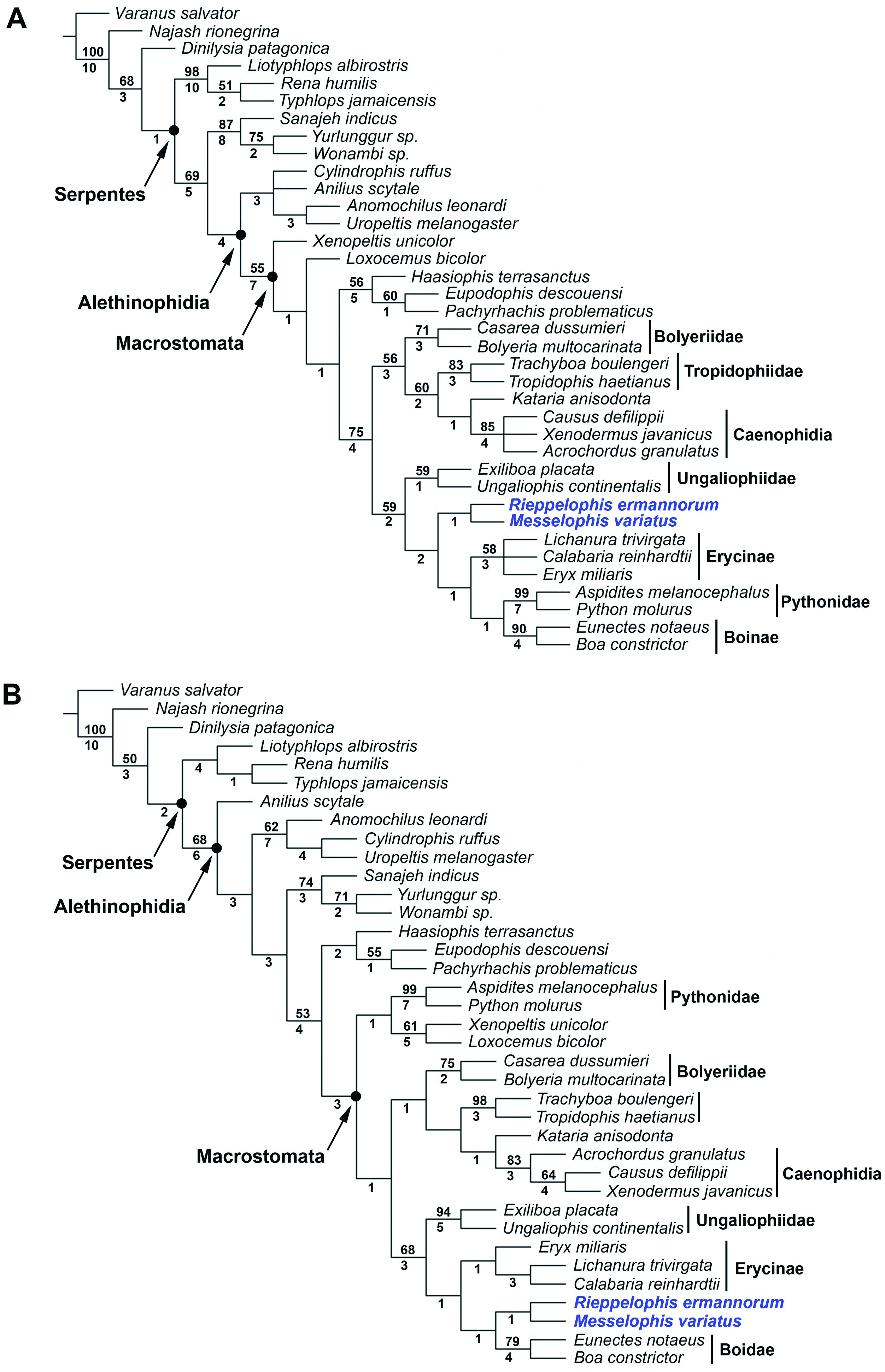
Despite the incompleteness of Messelophis species and of several other fossils added to the analysis, the obtained trees exhibit rather good resolution and strong support values for several nodes ( Fig. 17 View Figure 17 ). The analysis including all taxa and morphological characters yielded eight most-parsimonious trees, each with 456 steps (consistency index, CI 0.46; retention index, RI 0.75), whereas the analysis of the combined data set (morphological plus DNA) yielded one mostparsimonious tree with 6332 steps (CI 0.48; RI 0.41). Despite some topological differences with respect to fossil Indoaustralian ‘madtsoiids’ and marine simoliophiids, both consensus trees show a similar internal arrangement for Macrostomata. The only remarkable difference between them is the placement of pythonine snakes, which are nested with the rest of booid clades in the morphological analysis, whereas in the combined analysis they form, together with Xenopeltis and Loxocemus , an exclusive clade located at the base of Macrostomata. Despite the differences mentioned above, we recover M. variatus and M. ermannorum nested in a clade formed by ungaliophiines, erycines, and boines, i.e. the family Boidae sensu Vidal, Delmas & Hedges (2007) . This clade is supported by 30 molecular and four morphological synapomorphies, three of them unambiguous and unreversed (see Appendix S1). Nevertheless, only two of these synapomorphies could be scored in Messelophis specimens: the presence of a long finger-like medial foot process in the prefrontal (character 30) and the posterior position at the level of the palatine–pterygoid joint of the maxillary process of the palatine (character 76).
| SMF |
Forschungsinstitut und Natur-Museum Senckenberg |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.