Scyliorhinus capensis ( Müller & Henle, 1838 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4601.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:8A695352-8382-458F-A86A-17A198F780CA |
|
persistent identifier |
https://treatment.plazi.org/id/03B94378-D07C-062E-FF7D-FC5CFD65A90F |
|
treatment provided by |
Plazi |
|
scientific name |
Scyliorhinus capensis ( Müller & Henle, 1838 ) |
| status |
|
Scyliorhinus capensis ( Müller & Henle, 1838) View in CoL
( Figs. 19B View FIGURE 19 , 20–27 View FIGURE 20 View FIGURE 21 View FIGURE 22 View FIGURE 23 View FIGURE 24 View FIGURE 25 View FIGURE 26 View FIGURE 27 ; Tabs. 3 View TABLE 3 , 6 View TABLE 6 , 7 View TABLE 7 )
Common names: yellowspotted catshark ( South Africa and United Kingdom), roussette à taches jaunes ( France), alitán de manchas amarillas ( Spain).
Scyllium capense Müller & Henle, 1838 View in CoL –41: 11–12 (original description, type locality: Cape of Good Hope); Smith,1837: 85 (name only); Duméril, 1865: 320 –321 (compilation); Günther, 1870: 404 (listed, British Museum); Gilchrist, 1902: 165 (listed, “ Cape Seas”); Leigh-Sharpe, 1922: 559 –560, fig. 6 (clasper description).
Catulus capensis: Garman, 1913: 74 –75 (brief account, classification).
Scyllium (Betascyllium) capense Leigh-Sharpe, 1924: 326 View in CoL (clasper description).
Scylliorhinus capensis: Gilchrist, 1921: 71 (listed, Cape of Good Hope); Barnard, 1925: 40 –41 (catalogued, South Africa); Barnard, 1947: 16, pl. 3, fig. 1 (catalogue, South Africa).
Scyliorhinus capensis: Regan, 1908: 458 View in CoL (brief account); Fowler, 1941: 35 (catalogue, South Africa); Smith, 1949: 54, fig. 38 (catalogue); Bass et al., 1975: 32, fig. 19 (catalogue, south coast of Africa); Springer, 1979: 132 –133, fig. 84 (taxonomic review); Cadenat & Blache, 1981: 181, fig. 123a (catalogue, western coast of Africa); Compagno, 1984: 359 –360 (FAO catalogue); Bass, 1986: 95, figs. 11, 16, pl. 3 (catalogue, off southwestern Cape until Natal); Compagno, 1988b: 606, figs 2, 6b, 7b, 8c–d (compared to S. comoroensis View in CoL , from off Namibia until Natal); Compagno et al., 1991: 83 –84 (distribution, off south Namibia until Natal); Bianchi et al., 1999:79 (catalogue South Africa); Compagno, 1999: 480 (listed); Compagno et al., 2005: 248, pl. 41 (compilation); Ebert, Compagno & Cowley, 2006: 1053 –1065 (reproductive biology); Ebert et al., 2013a: 372, 379, pl. 51 (compilation); Ebert & Mostarda, 2013: 48 (FAO catalogue, southeastern Atlantic); Weigmann, 2016: 43 (listed).
Haploblepharus capensis: White, 1937: 121 (systematics, listed).
Lectotype. NHMUK 1845.7 About NHMUK .3.141, male, 620 mm TL (Cape Seas , South Africa) [designated herein].
Paralectotypes. NHMUK 1845.7 About NHMUK .3.144, male, 950 mm TL (Cape Seas , South Africa) ; NHMUK 1953.5 About NHMUK .10.2, male, 910 mm TL (Cape Seas , South Africa) .
Additional material examined. 81 specimens (see Appendix).
Diagnosis. Scyliorhinus capensis differs from all congeners by presenting a dark brown body with light yellow to golden spots (vs. light spots absent in S. cervigoni , S. garmani , S. meadi , and S. retifer ; spots beige or cream in S. boa , S. cabofriensis , S. canicula , S. comoroensis , S. duhamelii , S. haeckelii , S. hesperius , S. stellaris , S. torazame , S. torrei , and S. ugoi ), predominantly larger than spiracles throughout the body (vs. predominantly smaller in S. boa , S. cabofriensis , S. canicula , S. duhamelii , S. stellaris , and S. ugoi ); males with pelvic apron extending almost entire length of the pelvic fin inner margins (vs. extending to only 2/ 3 in congeners, except in S. canicula , S. duhamelii , S. torazame , and S. torrei ); clasper with rough terminal dermal cover (vs. smooth in congeners, except in S. canicula ); counts of monospondylous vertebrae 44–46 (vs. counts lower than 43 in other species, except in S. garmani , S. meadi and S. stellaris ); large sized adult males between 600–760 mm TL and adult females between 720–760 mm TL (vs. other species with smaller sizes at sexual maturity, except S. cervigoni , S. meadi and S. stellaris ). The following combination of characters, although less conspicuous, also helps distinguish this species: anterior nasal flaps not reaching the upper lip (vs. flaps reaching the lip, sometimes covering it in S. canicula , S. cervigoni , S. comoroensis , S. duhamelii , S. garmani , and S. stellaris ); mandibular canal of lateral line system presenting 5 or 6 pores (vs. 3–4 in S. hesperius ); oral canal of lateral line system with 8–10 pores (vs. 5–6 in S. hesperius ; 10–12 in S. duhamelii ; 9–13 in S. torrei ); commissural teeth with two or three cusplets (vs. one in S. cervigoni , S. torazame and S. torrei ); interdorsal space 0.6–1.0 times the anal base (vs. greater than anal base in S. boa , S. cabofriensis , S. haeckelii , S. hesperius , S. meadi , S. retifer , S. torrei , and S. ugoi ); males with distal tip of pelvic fins triangular and sharp (vs. square-tipped and nearly straight in S. torazame ); clasper with cover rhipidion covered by dermal denticles (vs. no dermal denticles in S. boa , S. cervigoni and S. retifer ); terminal 3 cartilage present (vs. absent in S. cabofriensis , S. cervigoni , S. comoroensis , S. duhamelii , S. haeckelii , S. stellaris , S. torrei , and S. ugoi ); dorsal terminal 2 cartilage reduced and subtriangular (vs. elongated in S. boa , S. canicula , S. comoroensis , S. duhamelii , S. retifer , S. stellaris , S. torazame , and S. torrei ); width across the nasal capsules 73.3–90.1% NL (vs. 90–92.6% in S. stellaris ; 92.2–98.5% in S. torazame ); distance between nasal apertures 20.1–26.7% NL (vs. 12.8–15.6% in S. boa ; 27.8–37.6% in S. capensis ; 17.9–21.6 in S. hesperius ; 27.6–29.8% in S. stellaris ; 15–18.3% in S. torrei ); basal plate width 65.8–75.3% NL (vs. 58.2–65.1% in S. canicula ); width across postorbital processes 72.1–82% NL (vs. 63.3–70.6% in S. hesperius ).
Description. Morphometric and meristic data are given in Table 6 View TABLE 6 , and neurocranial measurements in Table 7 View TABLE 7 .
Body robust, tapering considerably posterior to cloaca ( Fig. 20 View FIGURE 20 ). Prepectoral length 0.3–0.4 times the prepelvic length. Trunk shorter than tail; snout-vent length 0.8 times vent-caudal length. Pectoral-pelvic space 1.6–1.7 times the pelvic-anal space. Interdorsal space 1.8–2.9 times the dorsal-caudal space ( Tab. 6 View TABLE 6 ). No interdorsal, postdorsal or postanal ridges; lateral crest on caudal peduncle absent.
Head moderately broad and depressed; head length 1.7 times head width ( Figs. 20 View FIGURE 20 , 21 View FIGURE 21 ). Snout relatively short, preoral length 0.4–0.6 times mouth width and 0.6–0.7 times smaller than preorbital length. Prenasal length 0.7 times internarial space; preorbital length 0.3–0.6 times interorbital space.
Eye large and slitlike, eye length 2.4–4 times its height and 0.2–0.3 times smaller than head length ( Figs. 20 View FIGURE 20 , 21 View FIGURE 21 ). Eye dorsolateral on head, with lower edge medial to horizontal head rim in dorsal view; subocular ridge strong. Nictitating lower eyelid of rudimentary type, with shallow subocular pouch and secondary lower eyelid free from upper eyelid. Spiracle close behind but well separated from eyes, dorsolaterally on head and somewhat lower than level of eye notch. Spiracle diameter goes 3.8–6 times in eye length and 6.7–11.5 times in interorbital width.
First two gill openings about equally wide; first one twice as long as fifth. All gill openings slightly concave and not elevated on dorsolateral surface of head; gill filaments not visible externally.
Nostril with broad incurrent aperture, without nasoral groove or nasal barbel, and small and oval excurrent aperture. Anterior nasal flap large, triangular, and covering posterior nasal flap and excurrent aperture, extending just anterior to mouth, close to the upper lip but not touching it ( Figs. 22 View FIGURE 22 A–B). Mesonarial ridge distinct but not exceeding the posterior border of the anterior nasal flap. Posterior nasal flap rectangular, situated on the posterior border of the excurrent aperture and corresponding to 1/3 of the anterior flap. Mesonarial superior and inferior flaps triangular and corresponding to 1/3 of anterior nasal flap. Internarial distance 0.7–0.9 times smaller than interorbital distance.
Mouth arched, moderately large and short, its length goes 1.7–2.1 times in mouth width ( Figs. 22 View FIGURE 22 A–B). Lower labial furrow short and narrow, 3.2–4.1 times smaller than mouth width. Dorsal labial cartilage 1.3 times the ventral cartilage; anterior tip of dorsal labial cartilage reaching the orbital process of the palatoquadrate. Tongue flat and rounded, light-colored, with oral papillae hardly detectable.
Monognathic heterodonty gradual well developed; anterior teeth abruptly larger than the parasymphysial ones and lateral teeth smaller distally, with smaller and thicker principal cusps. Sexual heterodonty present with females presenting anterior teeth with more developed cusplets and shorter principal cusps in relation to males ( Figs. 23 View FIGURE 23 , 24 View FIGURE 24 ). Tooth counts 24–38 26–38/22–41 23–40. Parasymphysial teeth with a principal cusp flanked by one cusplet on each side; proximal cusplets 2/3 the height of the principal cusp and marginal ones poorly developed. Protuberances on the crown base or striae inconspicuous. Anterior teeth larger than the parasymphysial and principal cusp less stout. Anterior teeth with two cusplets; principal cusp greater and more slender than parasymphysial teeth. Anterior upper teeth with protuberances in crown base and striae extending through 1/3 the height of the principal cusp; striae absent in lower teeth. Females with anterior teeth with cusplets distally sharp and more developed than in males, corresponding to half the height of the principal cusp. Lateral teeth with four cusplets; marginal cusplets as high as proximal ones. Principal cusp slightly oblique in both jaws, proximal cusplets 2/3 the height and half the width of principal cusp. Protuberances present in crown base and striae extending throughout the crown. Commissural upper teeth with two or three cusplets; principal cusp stronger and slightly oblique and cusplets corresponding to more than half the height of principal cusp. Marginal cusplets, when present, poorly developed. Protuberances present and striae extending slightly more than half the height of the principal cusp. Ectodermal pits present in lateral and commissural teeth, restricted to the crown base.
Lateral trunk denticles with flat, elongated teardrop-shaped crowns, 1.3–1.8 times longer than wide ( Tab. 3 View TABLE 3 ); anterior part covered with ectodermal pits. Crown with a strong medial ridge extending its entire length onto long principal cusp. Dermal denticles above the pectoral fin with five ridges, medial ridge less prominent than in denticles of other regions and lateral ridges not extending to the angle between principal cusp and cusplets. Dermal denticles below dorsal fins longer and presenting prominent ridges, extending to the distal edge of cusplets ( Fig. 25 View FIGURE 25 ).
Pectoral base 0.7–0.8 times mouth width ( Fig. 22C View FIGURE 22 ). Pectoral anterior margin 2–2.3 times its base and 1.4–1.6 times the posterior margin. Pectoral fin skeleton aplesodic with radials mostly divided into three segments. Propterygium and mesopterygium trapezoidal; the former smaller than the latter. Propterygium with one proximal segment; mesopterygium with 3–4 proximal segments fused proximally. Metapterygium with 9 proximal segments. Metapterygial axis rectangular and corresponding to 1/5 of metapterygium.
Pelvic fin subtriangular in males and trapezoidal in females ( Fig. 22F View FIGURE 22 ); pelvic anterior margin 0.8–0.9 times the posterior margin and 0.8–1.0 times the pelvic base. Pelvic inner margins of males fused by through almost all of their extension, with sharp edges; claspers of juveniles covered by the pelvic apron and evident only with lifted apron.
Clasper short and cylindrical, sometimes extending beyond free rear tips of pelvic fins; clasper inner length 0.8–1.0 times the pelvic anterior margin, 1.4–2.4 times the clasper outer length and 5.4–5.6 times the clasper base. Most of clasper surface except dorsomedial surface of glans, medial border of cover rhipidion, rhipidion, and terminal dermal cover, covered by dermal denticles with anteriorly directed crowns ( Fig. 26A View FIGURE 26 ). Clasper hooks absent. Rhipidion well-developed, partly covered medially by a prominent exorhipidion and anteriorly by the cover rhipidion; insertion of rhipidion at posterior portion of dorsal terminal 2 cartilage and extending to the end of glans. Cover rhipidion expanded medially reaching, and sometimes covering, a nearly straight and poorly developed exorhipidion; both cover rhipidion and exorhipidion covering the clasper groove. Envelope absent; pseudosiphon distinct and robust. Terminal dermal cover rough and restricted to the glans tip, close to cover rhipidion and exorhipidion.
Clasper skeleton relatively simple ( Fig. 26B View FIGURE 26 ). Ventral marginal cartilage shorter than dorsal one; ventral terminal beginning anteriorly, but ending together with the dorsal terminal. Terminal 3 cartilage present and anteromedial to the ventral terminal cartilage. Dorsal terminal 2 cartilage rhomboidal, dorsally projected and posterior to terminal 3 cartilage. Ventral terminal 2 cartilage rodlike, situated on and corresponding to half-length of ventral terminal cartilage; ventral terminal 2 beginning at the same level as the dorsal terminal 2 cartilage.
First dorsal fin subrectangular or triangular, with nearly straight anterior margin, rounded apex and angular free rear tip ( Fig. 20 View FIGURE 20 ). First dorsal fin origin opposite or slightly posterior to the insertion of pelvic fin and its insertion above the anterior third of pelvic-anal distance. Anterior margin 1.4–1.5 times first dorsal fin base; first dorsal fin height 0.6–0.7 times its base.
Second dorsal fin smaller than the first and triangular ( Fig. 20 View FIGURE 20 ). Second dorsal fin origin opposite to posterior 2/5 of anal fin base and its insertion posterior to posterior end of the anal fin. Anterior margin 1.3–1.4 times base of second dorsal fin; second dorsal base 1.6–1.8 times its height and 1.3–1.7 times the dorsal-caudal distance. First dorsal fin 1.3 times larger than the second dorsal fin.
Anal fin triangular, apically narrow and not falcate ( Fig. 20 View FIGURE 20 ); anal fin base 1.6–1.7 times the second dorsal fin base. Anal fin anterior margin nearly straight, apex narrowly rounded, free rear tip acutely pointed, and inner margin straight. Anal fin base 0.9–1.1 times the interdorsal distance and 2–2.7 times the dorsal-caudal distance. Anal anterior margin 1.5–1.6 times the posterior margin; anal fin height 0.3–04 times its base.
Caudal fin narrow-lobed and asymmetrical ( Fig. 20 View FIGURE 20 ). Dorsal caudal lobe 1.7–1.8 times larger than preventral lobe; subterminal caudal margin as long as the terminal margin. Caudal crest of enlarged denticles absent on caudal fin margins.
Neurocranium broad and somewhat flattened, corresponding to 7.8–8.7% TL. Rostrum length corresponding to 1.3–1.7 times the distance between lateral rostral cartilages. Distance between lateral rostral cartilages greater in males. Width across nasal capsules proportionately smaller in males (73.3–77.3% NL) than in females (77.6–90.1% NL). Anterior fontanelle and basal plate proportionately larger in females (22.5–29.4% and 42.8–52.9% NL, respectively) than in males (20.1–21.8% and 38.3–41.9% NL). Orbital region goes 2.1–2.2 times in the nasobasal length. Optic capsule proportionately narrower in males (51.5–54.4%) than in females (57–62.5%). Optic capsules width 1.9–2.4 times its length. Width across postorbital processes 1.2–1.3 times the preorbital processes width ( Tab. 7 View TABLE 7 ).
Coloration in alcohol. Lectotype and paralectotypes taxidermized and dark colored on the dorsal surface; light spots inconspicuous on lateral surfaces ( Fig. 20 View FIGURE 20 ). In the other specimens, body light brown, marked by seven or eight saddles darker than background; caudal saddles less evident than other saddles ( Fig. 21 View FIGURE 21 ). Spots yellow to golden predominantly greater than spiracles throughout the body, sometimes forming longitudinal rows and becoming greater on lateral surfaces; present on proximal tip of pectoral and dorsal fins, less distinct on pelvic and anal fins. Spots can be fused, forming rows in intersaddles and separating saddles in two portions. Circles formed by large spots between saddles anterior to first dorsal fin, greater and less evident in females and juveniles. Lunate spots with dark center and double spots present in some specimens. Belly and ventral surface of paired fins with no spots, cream in color.
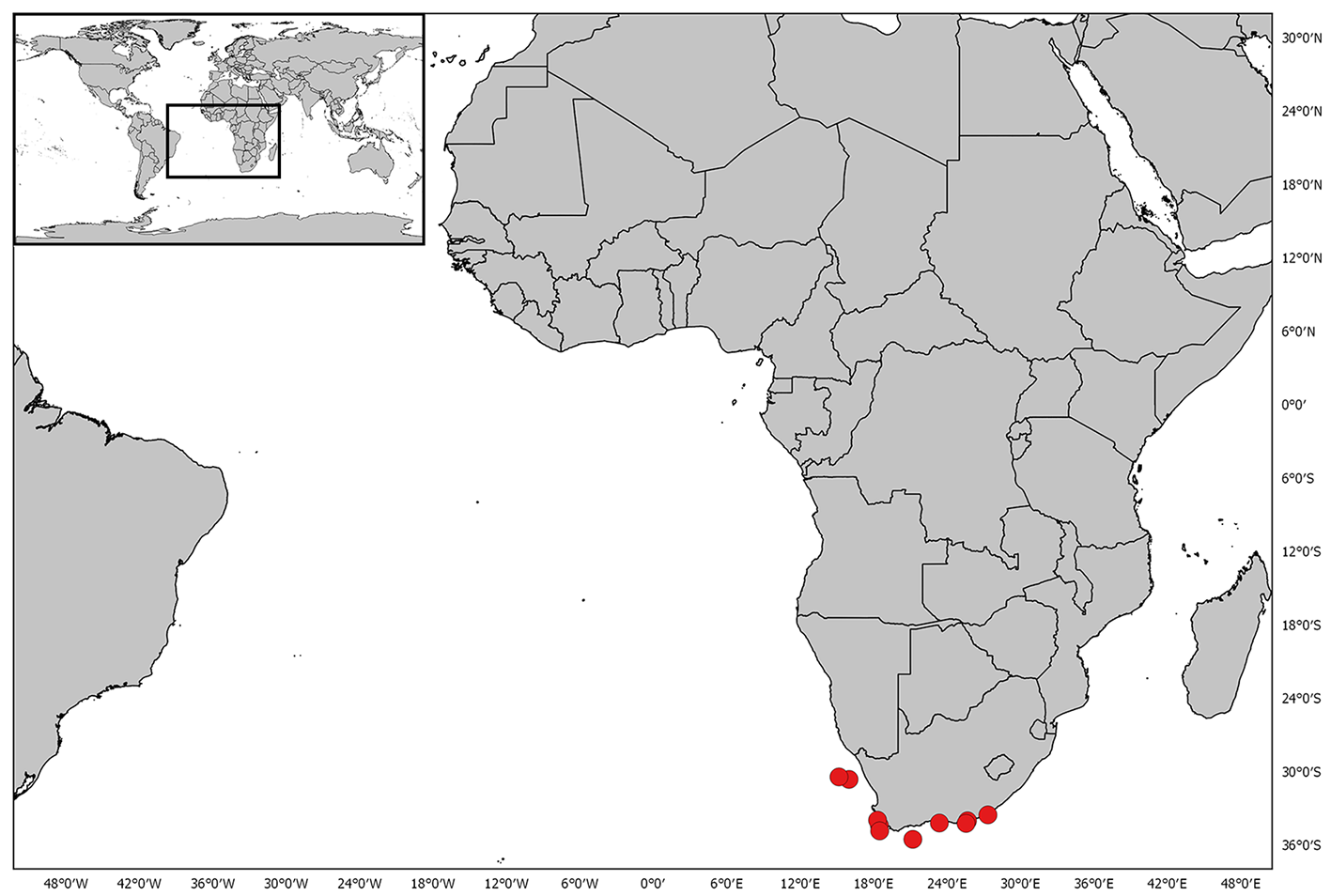
Distribution. This species is endemic to Southern Africa, distributed from the Karas region of southern Namibia, occurring off the Cape of Good Hope, and to Waterloo Bay, King William’s Town, in the Eastern Cape ( Fig. 27 View FIGURE 27 ).
Biological data. Adult males between 600–760 mm TL; largest male examined was 1062 mm TL. Adult females between 720–760 mm TL; largest female examined was 886 mm TL. Newly born 250—270 mm TL. Largest specimens reported to reach 1220 mm TL ( Compagno et al. 2005).
Egg capsules extracted from a 700 mm TL female. Egg capsules elongated, lateral edges lighter than the rest and continuous with lateral tendrils. Striae poorly developed or absent. Anterior edge slightly concave and posterior border concave and narrower, with tendrils and filaments coiled together. Measurements of egg capsules 82.3 mm in length, 31.8 mm in width, 14.5 mm in height; anterior edge 22.1 mm, posterior edge 9.3 mm ( Fig. 19B View FIGURE 19 ). This species is a benthic dweller recorded in depths of 26–795 m (Ebert et al. 2013a). Conservation status ‘Near Threatened’ ( Compagno et al. 2004).
Etymology. The specific name ‘capensis’ refers to the type locality of the species, Cape Town, South Africa.
Remarks. The taxidermized syntypes of S. capensis (NHMUK 1845.7.3.141, NHMUK 1845.7.3.144, NHMUK 1953.5.10.2; here designated as lectotype and paralectotypes, respectively) were examined and it was observed that the nasal flaps, labial furrows, position of fins and clasper external morphology correspond to the pattern observed in the other specimens of S. capensis ( Fig. 22 View FIGURE 22 ). The specimen NHMUK 1953.5.10.3 was misidentified and belongs, in fact, to the genus Haploblepharus based on the extension and distance between anterior nasal flaps, presence of upper labial furrows and clasper external morphology, all of which agree with the patterns observed in species of Haploblepharus .
The specimen cited by Günther (1870) and Day (1878), measuring approximately 102 cm and collected in India and supposedly housed in the Natural History Museum, London, was not found. Bass et al. (1975) noted that the specimen illustrated by Day (1878, fig. 190, n. 1) differs from S. capensis in color pattern and position of the first dorsal fin, suggesting that the specimen would correspond to another species, possibly not described. Day (1878, page 725) reported that the second dorsal fin was totally posterior to the anal fin; however, examined specimens present the origin of the second dorsal fin opposite to the hindquarter of the anal fin. In relation to the color pattern, Compagno (1988a) described 24 saddles along the dorsolateral surface in the Indian specimens, very different from the eight saddles observed in S. capensis . Compagno (1984, 1988a), visiting the Indian collections, did not find specimens of Scyliorhinus , nor were specimens obtained at the time in surveys and fisheries around the country, but did not discard the existence of a species of this genus in this region. Fischer & Bianchi (1984) and Akhilesh et al. (2014) also questioned the record of S. capensis in India and, therefore, this specimen is not included in the geographic range of this species.
TABLE 6. Morphometric and meristic data of Scyliorhinus capensis. SD, standard deviation; n, number of examined specimens. Total length (TL) in mm, other measurements as percentages of TL.
| Characters | n | Range | Mean | SD |
|---|---|---|---|---|
| Total length (TL) | 75 | 176.3–1062.0 | 508.2 | 167.0 |
| Precaudal length | 75 | 72.0–84.5 | 75.1 | 1.8 |
| Eye-spiracle length | 75 | 0.7–1.5 | 1.1 | 0.2 |
| Prenasal length | 75 | 1.5–3.7 | 2.6 | 0.4 |
| Preoral length | 75 | 2.8–5.8 | 4.3 | 0.5 |
| Preorbital length | 75 | 4.4–7.8 | 6.2 | 0.8 |
| Prespiracular length | 74 | 7.3–11.7 | 9.9 | 0.8 |
| Prebranchial length | 74 | 10.6–16.6 | 14.1 | 1.0 |
| Head length | 74 | 15.4–22.0 | 19.0 | 1.2 |
| Prepectoral length | 75 | 13.4–20.0 | 17.1 | 1.3 |
| Prepelvic length | 74 | 36.9–46.6 | 42.1 | 1.7 |
| Snout-vent length | 74 | 40.2–51.1 | 44.4 | 1.9 |
| Vent-caudal length | 73 | 51.2–62.1 | 55.6 | 2.2 |
| Pre-first dorsal length | 74 | 45.0–56.1 | 48.9 | 1.9 |
| Interdorsal distance | 75 | 8.2–12.9 | 10.4 | 1.0 |
| Dorsal-caudal distance | 75 | 2.8–7.0 | 4.6 | 0.8 |
| Pectoral-pelvic distance | 75 | 14.0–23.1 | 19.0 | 2.5 |
| Pelvic-anal distance | 75 | 8.5–13.4 | 10.6 | 1.2 |
| Anal-caudal distance | 75 | 4.4–8.2 | 6.5 | 0.7 |
| Interorbital distance | 75 | 4.6–8.7 | 6.4 | 0.7 |
| Internarial distance | 75 | 4.0–6.1 | 5.2 | 0.4 |
| Mouth length | 75 | 3.2–6.0 | 4.8 | 0.5 |
| Mouth width | 75 | 6.6–10.1 | 8.6 | 0.7 |
| Lower labial furrow length | 74 | 1.6–3.1 | 2.2 | 0.3 |
| Eye length | 75 | 2.4–5.0 | 3.7 | 0.5 |
| Eye height | 75 | 0.6–2.1 | 1.3 | 0.3 |
| Spiracle length | 72 | 0.4–1.3 | 0.8 | 0.2 |
| First gill slit height | 75 | 2.1–3.6 | 2.9 | 0.3 |
| Fifth gill slit height | 75 | 1.2–2.4 | 1.6 | 0.2 |
| Pectoral length | 75 | 9.4–14.9 | 13.1 | 0.8 |
| Pectoral anterior margin | 75 | 10.7–16.2 | 14.5 | 0.9 |
| Pectoral base | 75 | 4.7–8.0 | 6.5 | 0.5 |
| Pectoral posterior margin | 75 | 6.5–11.8 | 9.0 | 0.9 |
| Pectoral inner margin | 75 | 4.3–7.1 | 5.9 | 0.5 |
| Pelvic length | 75 | 9.5–14.9 | 11.9 | 1.1 |
| Pelvic anterior margin | 75 | 5.1–9.2 | 7.2 | 0.6 |
| Pelvic posterior margin | 74 | 5.5–10.7 | 7.4 | 1.0 |
| Pelvic base | 75 | 5.2–11.1 | 7.6 | 0.8 |
| Pelvic inner length | 75 | 2.1–7.9 | 4.7 | 1.1 |
| Clasper outer length | 34 | 1.6–6.3 | 2.6 | 1.4 |
| Clasper inner length | 34 | 3.8–9.0 | 5.0 | 1.6 |
......continued on the next page
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
ParvPhylum |
Chondrichthyes |
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Scyliorhinus capensis ( Müller & Henle, 1838 )
| Soares, Karla D. A. & De, Marcelo R. 2019 |
Haploblepharus capensis:
| White, E. G. 1937: 121 |
Scyllium (Betascyllium) capense
| Leigh-Sharpe, W. H. 1924: 326 |
Scylliorhinus capensis:
| Barnard, K. H. 1947: 16 |
| Barnard, K. H. 1925: 40 |
| Gilchrist, J. D. F. 1921: 71 |
Catulus capensis:
| Garman, S. 1913: 74 |
Scyliorhinus capensis: Regan, 1908 : 458
| Weigmann, S. 2016: 43 |
| Ebert, D. A. & Mostarda, E. 2013: 48 |
| Ebert, D. A. & Compagno, L. J. V. & Cowley, P. D. 2006: 1053 |
| Compagno, L. J. V. & Dando, M. & Fowler, S. 2005: 248 |
| Bianchi, G. & Carpenter, K. E. & Roux, J. P. & Molloy, F. J. & Boyer, D. & Boyer, H. J. 1999: 79 |
| Compagno, L. J. V. 1999: 480 |
| Compagno, L. J. V. & Ebert, D. A. & Cowley, D. 1991: 83 |
| Bass, A. J. 1986: 95 |
| Compagno, L. J. V. 1984: 359 |
| Cadenat, J. & Blache, J. 1981: 181 |
| Springer, S. 1979: 132 |
| Bass, A. & D'Aubrey, J. D. & Kistnasamy, N. 1975: 32 |
| Smith, J. L. B. 1949: 54 |
| Fowler, H. W. 1941: 35 |
| Regan, C. T. 1908: 458 |
Scyllium capense Müller & Henle, 1838
| Leigh-Sharpe, W. H. 1922: 559 |
| Gilchrist, J. D. F. 1902: 165 |
| Gunther, A. 1870: 404 |
| Dumeril, A. H. A. 1865: 320 |
| Smith, A. 1837: 85 |