Nyctimene robinsoni Thomas, 1904
|
publication ID |
https://doi.org/ 10.1093/mspecies/sex007 |
|
publication LSID |
lsid:zoobank.org:pub:019E5BA2-06B8-4FF7-A15F-6D73ED4C2244 |
|
persistent identifier |
https://treatment.plazi.org/id/03B987DA-FFB9-4D62-FF0C-41FD6E3F7ABB |
|
treatment provided by |
Felipe |
|
scientific name |
Nyctimene robinsoni Thomas, 1904 |
| status |
|
Nyctimene robinsoni Thomas, 1904 View in CoL
Eastern Tube-nosed Bat
Nyctimene Robinsoni Thomas, 1904:196 View in CoL . Type locality “Cooktown, Queensland,” Australia. Nyctimene tryoni Longman, 1921:179 . Type locality “Canungra, Queensland,” Australia.
CONTENT AND CONTEXT. Context as for genus. Nyctimene robinsoni View in CoL is monotypic. Synonymies follow Jackson and Groves (2015).
NOMENCLATURAL NOTES. The taxonomy of Nyctimene in the Australo-Papuan region remains unresolved at this time. Taxonomic designations including species distributions have been difficult to determine for species in the genus Nyctimene due to the uncertainty of the identity of collected individuals in Australia, New Guinea, and Moa Island ( Thomas 1904; Hall and Richards 2000; Hall et al. 2008b). Species and subspecies assignments within Nyctimene have changed multiple times (e.g., Gray 1863; Thomas 1904; Andersen 1912; Simmons 2005). N. robinsoni is thought to have been misidentified on New Guinea and Moa Island as N. cephalotes and early on as N. albiventer in Australia. Lack of molecular data has also made taxonomic descriptions complex; some genetic studies have been unable to distinguish N. robinsoni from N. albiventer ( Donnellan et al. 1995) . Possibility of another Nyctimene species, collected in Cape York and confirmed by mtDNA to be of a different species than N. robinsoni , is yet to be described (Hall and Richards 2000).
The generic name, Nyctimene , is Greek, meaning “night moon.” The specific epithet, robinsoni , was chosen by Thomas (1904) to honor the naturalist Herbert C. Robinson, collector of the first 2 specimens from Cooktown, Australia (Hall and Richards 2000).
DIAGNOSIS
Nyctimene robinsoni is currently the only recognized species of the genus Nyctimene in Australia. It can be distinguished from all other Australian bats by its tubular nostrils that protrude 5–6 mm from the end of the rostrum and the yellow to lime-green colored spots that are distributed on its wings, face, and ears ( Fig. 1 View Fig ; Thomas 1904; Andersen 1912). Its general coloration is gray to brown with a dark vertebral stripe extending down its dorsal side; this is distinguishable from the Pallas’s tube-nosed bat ( Nyctimene cephalotes ) with which it may be sympatric on Moa Island, Torres Strait (Hall and Richards 2000). Additionally, N. robinsoni , is unique from other members of Nyctimene by lacking dark coloration at the base of hairs ( Andersen 1912). The ears of N. robinsoni are also recognizable, being larger than most others in this genus and acutely pointed. The ear measures 13 mm at its greatest width ( Andersen 1912) and 18 mm in height ( Thomas 1904), generally equal in length to the hind foot with claws ( Andersen 1912). N. robinsoni also has a tail that is typically 20–25 mm in length and free from the body, this is unlike other species of Pteropodidae ( Thomas 1904; Andersen 1912).
GENERAL CHARACTERS
The following measurements come primarily from original studies by Thomas (1904) of 1 adult male holotype specimen, and expanded by Andersen (1912) of 2 adult males, the holotype and a paratype; these quantitative descriptions are corroborated by other more recent descriptions (e.g., Hall and Richards 1979; Hall 1983; Hall and Richards 2000).
Nyctimene robinsoni weighs 40–60 g ( Richards 1986; Hall and Pettigrew 1995) with a head and body length of 100–110 mm ( Thomas 1904; Andersen 1912). The wings are described as short and broad (see “Form and Function” for digit measurements). Forearm length is 60–70 mm ( Thomas 1904; Hall and Richards 1979). The tibia of N. robinsoni ranges from 24 to 24.5 mm. The ears are approximately equal in length to the hind foot and claws (17 mm — Andersen 1912).
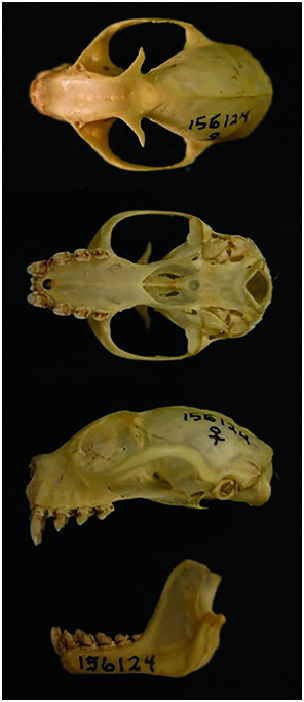
Structurally, N. robinsoni is perhaps most recognizable by its skull ( Fig. 2 View Fig ). The greatest length of skull is about 32.5 mm ( Thomas 1904). The cranium is between 21.7 and 22.3 mm in width across the zygomatic arches, and the braincase is considered narrow for a species of Nyctimene at 13.8–14.8 mm at the zygomatica. The distance from the lambda to gnathion, representing an angular anterior–posterior skull width, is 32.8–33 mm ( Andersen 1912). The cranium exhibits basicranial flexion with a frontal convexity above the palate and behind the cheek teeth of 11.7 mm ( Thomas 1904). N. robinsoni also has an alveolar ridge that projects posteriorly beyond the condyle. The condylobasal length measures 30.8–31.0 mm ( Andersen 1912).
Characteristic of members of Nyctimene are tubular nostrils that protrude 5–6 mm from the end of the rostrum ( Andersen 1912; Hall and Richards 1979). The depth of the rostrum from the top of the nasal tubes to the inferior surface of the mandible is equal in length from the end of the rostrum to the anterior canthus of the eye. The length from the palation to the incisive foramen is 12.2–12.7 mm, and from the palation to the basion, 13–13.2 mm. The premaxillae are fused with a depth at the symphysis of 2.5–2.8 mm. As a consequence of a shortened rostrum, the nasal branches of the premaxillae are so reduced as to completely lose contact with the nasal bones, and the posterior narial passage and mesopterygoid fossa are widened (5.0– 5.7 mm in width). The length from the orbit to the nares is 6.0– 6.2 mm, less than the lacrimal width of 8.8–9.2 mm. The height from the alveolar of the canine is between 6.7 and 7.3 mm. The mandible length from the condyle is 24.2–24.6 mm and the coronoid height is 14.5–14.7 mm ( Thomas 1904; Andersen 1912). The angular process is greatly reduced. The orbital diameter is 9.3–9.5 mm ( Andersen 1912). The interorbital width is 6.0– 6.2 mm and postorbital width is 5.7–5.8 mm ( Thomas 1904; Andersen 1912). Females exhibit an open, V-shaped pelvic girdle, and males have a closed, O-shaped pelvic girdle ( Chapman et al. 1994).
The coloration of N. robinsoni can range from gray to a reddish-brown with a narrow darker brown vertebral stripe extending from the head posteriorly along the middorsal line. The venter generally has a paler coloration. All hairs are uniform in color from base to tip and the characteristic yellow to green spots exhibited on wings, face, and ears are unique between individuals ( Thomas 1904; Andersen 1912).
DISTRIBUTION
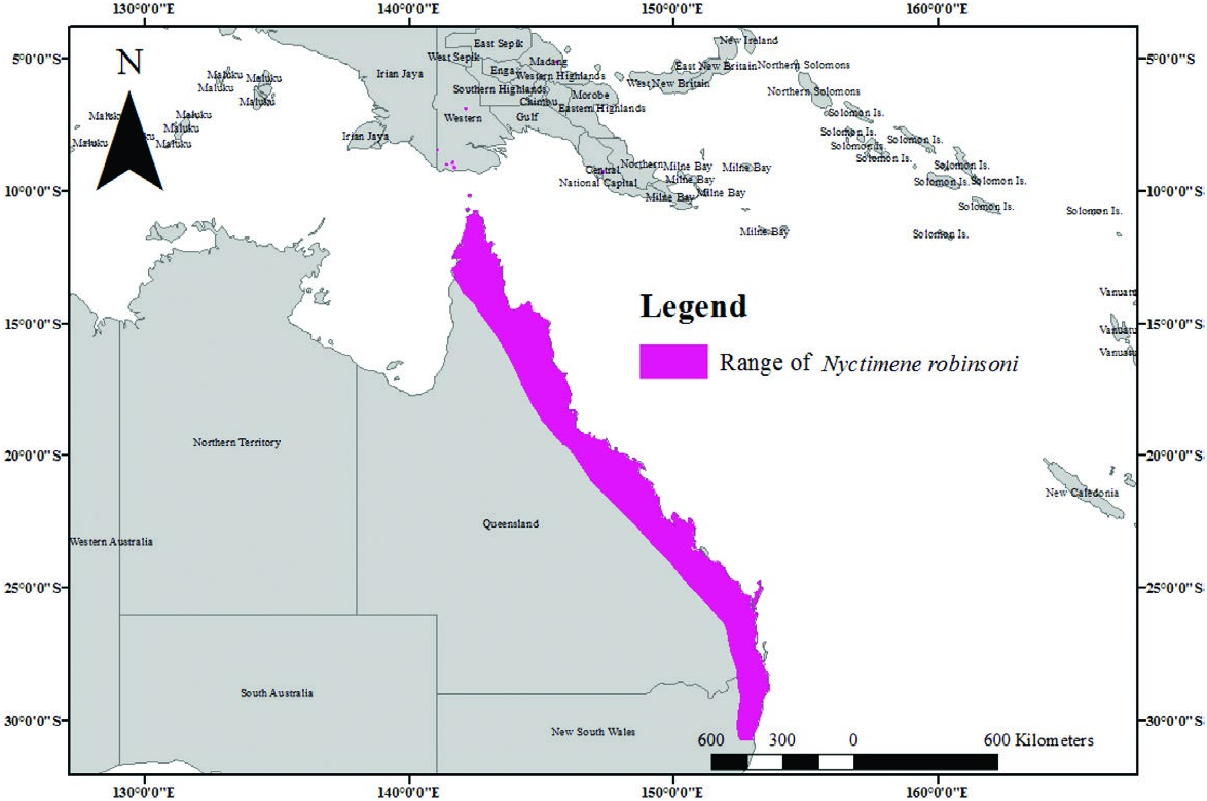
Nyctimene robinsoni is distributed from eastern coastal Queensland, generally from Cape York Peninsula, to northern New South Wales ( Fig. 3 View Fig ). It is considered to be rare in New South Wales. The most southern occurrences of N. robinsoni are those most inland, about 84 km east of the coastline at Culmaran Creek Valley in the Richmond Range State Forest ( Schultz 1997).
Previous records of N. cephalotes , identified from Moa Island, are now considered to be misidentifications of N. robinsoni ( Hall et al. 2008b) . Similarly, a subspecies of the common tube-nosed bat N. albiventer was identified from Australia; however, these specimens are now considered to be N. robinsoni . Specimens from mainland New Guinea, previously identified as N. cephalotes , possibly are N. robinsoni ( Hall et al. 2008b) . N. robinsoni possibly also occupies areas westward of New Guinea including the islands of Halmahera, Obi, Batjan, Waigeo, and Salawati; there are unresolved misidentifications with N. albiventor and N. cephalotes among others. Hall et al. (2008b) suggest that further taxonomic research is needed to determine the precise distribution of N. robinsoni . No fossils are known.
FORM AND FUNCTION
Form. —The presence of the nasal tubes has required some modification to the rostral structure in members of Nyctimene . The tubes are supported by a slight anterior–inferior projection of the nasal bones to meet the mesethmoid cartilage, along with a dorsal expansion of the alveolar branches of the premaxillae ( Andersen 1912).
The total length of the pollex, including the claw, is 27.0– 28.5 mm (metacarpal: 10 mm; 1st phalanx: 13 mm). Nyctimene robinsoni has a well-developed 2nd digit claw with the entire digit including claw measuring 47–48 mm (metacarpal: 31–32.5 mm; 1st phalanx: 5.5–6 mm; 2nd and 3rd phalanx: 10 mm). The 3rd digit measures a total of 120–122.5 mm (metacarpal: 45–47 mm; 1st phalanx: 32.5–33.5 mm; 2nd phalanx: 42–42.5 mm); the 4th digit is about 95 mm long (metacarpal: 40.5–42.5 mm; 1st phalanx: 25–25.5 mm; 2nd phalanx: 29 mm); the 5th digit is a total of 91– 90 mm in length (metacarpal: 45–46.5 mm; 1st phalanx: 20.5–21 mm; 2nd phalanx: 23–25 mm). The calcar or calcaneum is also well developed and measures about 10 mm ( Andersen 1912).
Members of Nyctimene lack lower incisors. The upper incisors are bilobed and large with a well-differentiated crown and a large posterior ledge; the outer lobe is shorter and narrower than the inner lobe. The lower canines are modified and curved so that they are broadest in the middle and nearly in contact with one another; they occlude with the upper incisors when the mouth is closed and contain no secondary cusp. The surfaces of the upper and lower canines are perfectly smooth and exhibit no vertical groove. Although some species of the genus Nyctimene contain a secondary cusp on the upper canines and distinct notch on p3, p4, and m1, these are, respectively, absent and simple in N. robinsoni ( Andersen 1912) . Dental formula is i 1/0, c 1/1, p 3/3, m 1/2, total 24. Length of the upper toothrow from canine to M1 is 11.7–12.0 mm and length of the lower toothrow from the canine to the m2 measures 13.0– 13.5 mm ( Andersen 1912). The P2 and p2 are equal in height to the canines, whereas other cheek teeth are molariform with well-developed anterior cusps. Cheek teeth measure (length followed by width, mm) as follows: P3 2.7, 1.9–2.0; P4 2.5, 1.9–2.0; M1 2.3, 1.7–1.9; p3 2.7, 1.8–1.9; p4 2.7–2.8, 1.8–1.9; m1 2.5–2.7, 1.7–1.8; m2 1.6–1.8, 1.5–1.6 ( Andersen 1912).
The palate of N. robinsoni has slight to no narrowing behind the maxillary toothrow. All species in the genus Nyctimene have a rough palate with coarse ridges that extend from side to side across the midline. The ridges immediately posterior to canines are relatively straight and become progressively more convex posteriorly. Interspaces between ridges are reduced in a posterior pattern and become somewhat asymmetric beyond the maxillary toothrow. Lateral measurements of the palate are as follows: width externally across crowns for canines, 6.7 mm; width between bases of canines, 2.2–2.3 mm; width externally across the upper 1st molars, 10.2–10.7 mm; width between upper 4th premolars, 6.0– 6.5 mm ( Andersen 1912).
The tongue of N. robinsoni contains 4 circumvallate papillae, unlike any other Chiropteran. The general shape of the tongue uniformly tapers to a blunt end with no apparent extensibility. Birt et al. (1997) documented types and locations of various forms of papillae using scanning electron microscopy. Tridentate papillae are concentrated in the upper anterior region of the tongue and contain relatively long prongs with a mean length of 730 µm. Behind the tridentate papillae are fleshy papillae forming a whorl at the center of the tongue. The tongue also contains mechanical papillae in the form of simple fringe arranged in circular or “leaf-shaped” patterns on the surface of the tongue. These papillae become more “basket-like” in shape and dominate the posterior one-half of the tongue, elongating as they progress posteriorly. The basket-like papillae are separated to lateral sides on the posterior surface of the tongue by the 4 circumvallate papillae, 2 large and 2 small. The larger of the circumvallate papillae are located anterior and lateral to the smaller circumvallate papillae. The anterior tip of the tongue is dominated by tridentate, simple-fringed, and few fungiform mechanical papillae scattered irregularly. Tongue structure corroborates evidence that N. robinsoni is an obligate frugivore (Spencer and Fleming 1989; Birt et al. 1997).
A comparative study on brains of Australian Chiroptera found that means (± SE) of 4 measurements of the brains of 8 individuals of N. robinsoni were: width, 119 ± 1.1 mm, total height, 99 ± 2.3 mm, length, 192 ± 2.4 mm, and length of cerebral hemispheres, 121 ± 2.9 mm (Stephan and Nelson 1981). The lobulus petrosus is large, but a hemispheral part of the paraflocculus is not obvious. The mean encephalization index (brain mass relative to body mass, considered a measurement of intelligence) for N. robinsoni is 196, with the average index for the family Pteropodidae being 197. The corresponding mean (and SE) body and brain masses used to calculate the encephalization index based on a regression line were 47.1 ± 5.5 g and 12.32 ± 6.6 g, respectively (Stephan and Nelson 1981).
Bhatnagar et al. (1986) described the pineal gland in Chiropterans. This gland is located in the epithalamus, between the 2 hemispheres of the brain and produces melatonin, a hormone that affects sleep patterns. In N. robinsoni , the pineal gland has an anteroposterior extent of about 570 µm, a greatest width of 790 µm, and a volume of 0.2303 mm 3 ( Bhatnagar et al. 1986).
Examinations of nervous structures of the wing, in particular somatosensory nuclear complex or ventrobasal complex of the dorsal thalamus, indicate they closely resemble those of other megachiropterans and are not atypical of other mammalian species ( Manger et al. 2001).
Function. —Despite being perhaps the most intriguing characteristic of Nyctimene robinsoni , the exact purpose of tubular nostrils is currently unknown. Hall and Pettigrew (1995) suggested that the tubes, which are able to move independently of one another, might be used for locating fruit via olfaction by concentrating aromas and following odor plumes through the rainforest. This function is often called stereo olfaction (Hall and Pettigrew 1995; Schwab and Pettigrew 2005).
Hall and Pettigrew (1995) observed incredible maneuverability by N. robinsoni that is rare, particularly in larger bat species. N. robinsoni demonstrated the ability to hover for several seconds and change facing direction while hovering, even when holding fruit. It has been suggested that such maneuverability is due to the short and broad shape of their wings (Hall and Pettigrew 1995).
Richards (1986) reported on the action of the lower canines and upper incisors during feeding. The skin of the fruit is 1st pierced by the lower canines, which are used as a pivot point as the upper incisors are pulled downward tearing the skin from the fruit to reveal the inner flesh ( Richards 1986). The tongue of N. robinsoni is specially adapted for eating fruit. The fungiform papillae found near the tip of the tongue and the circumvallate papillae along the posterior central region, all contain secretory pits associated with taste buds. The tridentate papillae that are located near the blunt tip of the tongue are highly keratinized and rough and are thought to be useful for piercing the skins of fruit. The dominant presence of tridentate papillae along with the central whorl may provide a flat surface and pressure point with which the fruit can be crushed against the ridged upper hard palate. Additionally, the presence of the fungiform papillae in proximity with the tridentate papillae at the tip of the tongue would allow N. robinsoni to taste the fruit immediately upon piercing it ( Birt et al. 1997). Type and number of taste buds are reduced in N. robinsoni compared with other megachiropterna nectivores and frugivores. The index of olfactory bulbs is relatively high (143— Baron et al. 1996) and it has been hypothesized that the increased ability for olfaction compensates for the reduced number of taste buds ( Birt et al. 1997).
The pineal organ, correlated with sleep patterns, is similar to that of other megachiropterans ( Bhatnagar et al. 1986). This suggests an association with factors such as habitat, neocortical and cerebellar development, and activity patterns (e.g., daytime roosting and no hibernation). Stephan and Nelson (1981) also detected a correlation between the encephalization index and trophic roles. Fruit eaters, such as N. robinsoni , tend to be highly encephalized. Their study suggests that members of Pteropodidae have the most highly developed brain of all Australian Chiroptera .
The basal metabolic rate of N. robinsoni was measured at 54.7 ml O
2
per hour, similar for other species of this family. Moderate decreases in the basal metabolic rate during the tropical winter indicate that thermoregulatory costs for this species in the cold season are low. The resting metabolic rate also has been shown to be highly affected by ambient temperatures ( Riek et al. 2010).
ONTOGENY AND REPRODUCTION
Little is known about the reproductive cycles, biology, or ontogeny of Nyctimene robinsoni . It is a seasonal breeder with a gestation period of 3–3.5 months (Atlas of Living Australia, http:// bie.ala.org.au/species/ NYCTIMENE +ROBINSONI. Accessed 9 November 2015). Females give birth to 1 young per year between October and December, followed by a long lactation period that is often obvious by a pinkish coloration on the ventral side of the female ( Hall 1983; O’Brien 1993; Hall et al. 2008a).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Nyctimene robinsoni Thomas, 1904
| Loveless, Allison Marcella & McBee, Karen 2017 |
Nyctimene Robinsoni
| LONGMAN, H. 1921: 179 |
| THOMAS, M. R. O. 1904: 196 |