Reticulipeurus Kéler, 1958
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4742.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:CA0AD801-C329-4D41-B081-1647491DF842 |
|
DOI |
https://doi.org/10.5281/zenodo.3684873 |
|
persistent identifier |
https://treatment.plazi.org/id/03BA7024-9B17-EC54-55EF-FBE4FE7DFE3A |
|
treatment provided by |
Plazi |
|
scientific name |
Reticulipeurus Kéler, 1958 |
| status |
|
Reticulipeurus Kéler, 1958: 332 .
Oxylipeurus ; Price et al. 2003: 202, 231 (in partim).
Type species: Lipeurus tetraonis Grube, 1851 , by original designation.
Diagnosis. Reticulipeurus is morphologically similar to Cataphractomimus and Sinolipeurus , all sharing the following characters: (1) the reticulation of several abdominal plates (the exact extent of clearly visible reticulation is variable within Reticulipeurus ), (2) morphology of the mesosome (antero-lateral corners with hooked extensions, distal margin rugose, gonopore originating at proximal end), (3) a rounded frons, and (4) an enlarged male scape and pedicel, with the scape bearing a tooth-like projection.
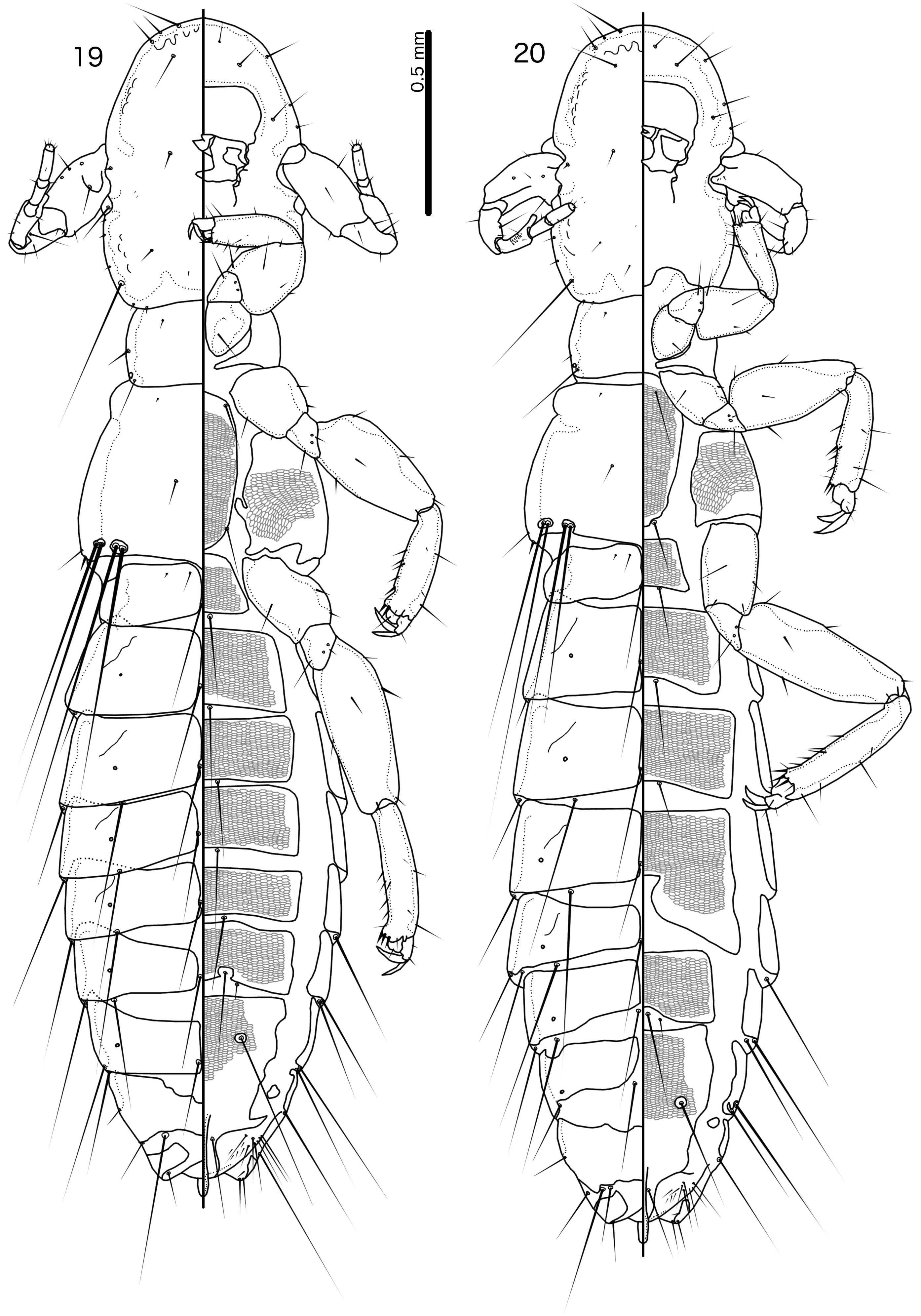
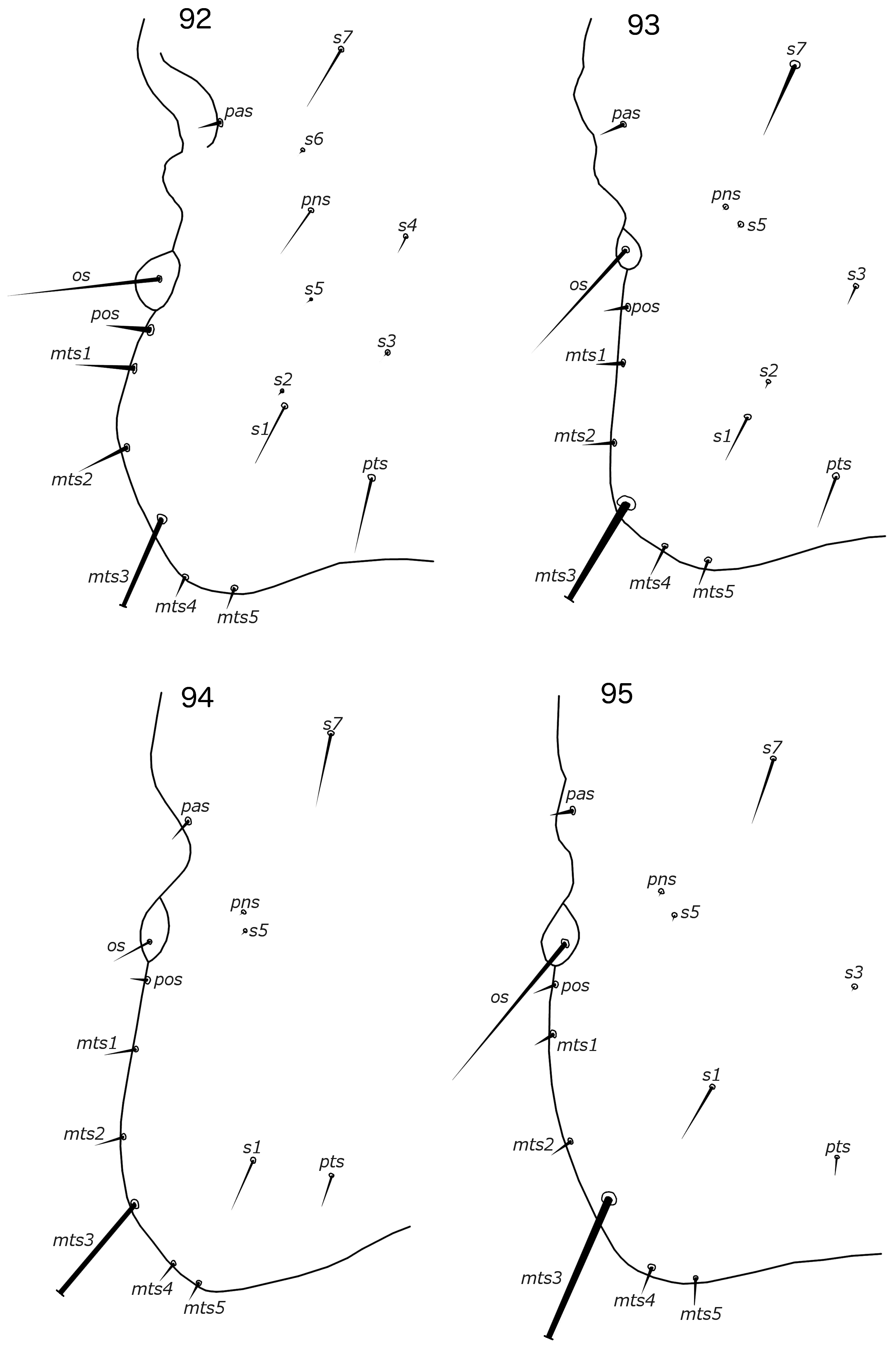
Reticulipeurus can be separate from Cataphractomimus by the characters given above, under the diagnosis of Cataphractomimus . Reticulipeurus can be separated from Sinolipeurus by the following characters: (1) dorsal preantennal suture present in Reticulipeurus ( Fig. 7 View FIGURES 7–8 ), but absent in Sinolipeurus ( Fig. 19 View FIGURES 19–20 ); (2) male tergopleurites IX+X and XI fused and both medianly continuous in Reticulipeurus ( Fig. 7 View FIGURES 7–8 ), but separate and tergopleurite XI medianly separate in Sinolipeurus ( Fig. 19 View FIGURES 19–20 ); (3) differences in postantennal head chaetotaxy ( Figs 93, 95 View FIGURES 92–95 ); (4) male genitalia proportionately smaller in Reticulipeurus ( Figs 42–59 View FIGURES 33–44 View FIGURES 45–56 View FIGURES 57–68 ) than in Sinolipeurus ( Figs 63–68 View FIGURES 57–68 ); (5) mesosome with large, lateral rugose nodi in distal end in Sinolipeurus ( Fig. 65 View FIGURES 57–68 ), but without such nodi in Reticulipeurus ( Fig. 47 View FIGURES 45–56 ); (6) parameres gently curved or roughly straight in Reticulipeurus ( Fig. 46 View FIGURES 45–56 ), but S-shaped in Sinolipeurus ( Fig. 64 View FIGURES 57–68 ); (7) gonopore markedly different in structure ( Figs 47 View FIGURES 45–56 , 65 View FIGURES 57–68 ); (8) stylus long and slender, arising from center of subgenital plate, with underlying posterior extension of subgenital plate in Sinolipeurus ( Fig. 86 View FIGURES 85–87 ), but short and blunt, arising subterminally, without underlying extension of subgenital plate in Reticulipeurus ( Fig. 80 View FIGURES 79–81 ); (9) head setae as2–3 marginal in Reticulipeurus ( Fig. 5 View FIGURES 5–6 ), but clearly dorsal in Sinolipeurus ( Fig. 19 View FIGURES 19–20 ); (10) marginal pterothoracic setae grouped in two distinct clusters on each side in Sinolipeurus ( Fig. 19 View FIGURES 19–20 ), but all in one cluster or in a short row in Reticulipeurus ( Fig. 5 View FIGURES 5–6 ); (11) mandibular seta present in Reticulipeurus ( Fig. 6 View FIGURES 5–6 ) (this seta is a microseta and not always clearly visible in all specimens), but absent in Sinolipeurus ( Fig. 19 View FIGURES 19–20 ).
Description.
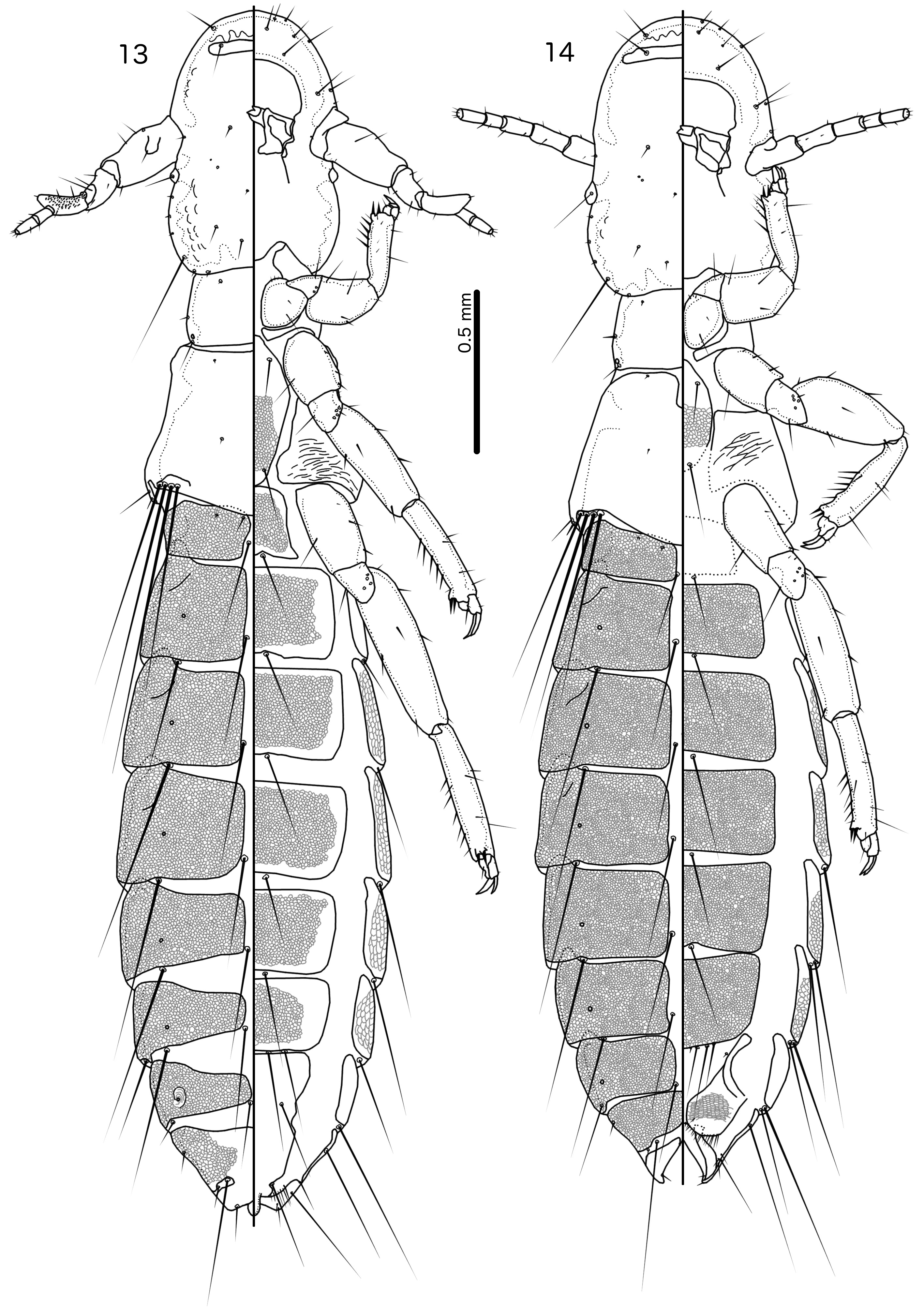
Both sexes. Head slender, frons rounded, either narrowly ( Fig. 5 View FIGURES 5–6 ) or broadly ( Fig. 15 View FIGURES 15–16 ). Marginal carina uninter- rupted. Dorsal preantennal suture present, arising at ads and medianly continuous. Extensive internal thickenings present posterior to marginal carina across most of head, in some species in double lines (e.g. Fig. 6 View FIGURES 5–6 ). Head chaetotaxy as in Figs 5 View FIGURES 5–6 and 93 View FIGURES 92–95 (those of R. polytrapezius are different, see below); as2–3 marginal; avs3 situated far anterior, near vsms1–2; mds present as microsetae which may be hard to see; mts3 macroseta; s1–3, s5, s7 present, sizes as in Fig. 93 View FIGURES 92–95 . Coni short, at most about half-length of female scape. Antennae sexually dimorphic ( Figs 5–6 View FIGURES 5–6 ): male scape, pedicel and flagellomere I modified. Eyes not prominent. Gular plate indistinct.
Prothorax with 1 dorsal anterior seta (pdas), 1 pronotal marginal-lateral seta (pmls) and 1 pronotal postspiracular seta (ppss) on each side. Pterothorax with 1 anterior and 1 posterior submarginal meso-metanotal seta (asmns and psmns, respectively), 1 pterothoracic trichoid seta (ptrs) and 1 pterothoracic thorn-like seta (pths) on each side. Marginal pterothoracic setae in single cluster or short row on each side ( Fig. 5 View FIGURES 5–6 ). Leg chaetotaxy as in Fig. 89 View FIGURE 89 ; cII-v3 and cIII-v3 dorsal. Postero-lateral corners of pterothorax not extended into horns. Shape of abdomen and degree of visible reticulation differing among species ( Figs 5 View FIGURES 5–6 , 13 View FIGURES 13–14 ).
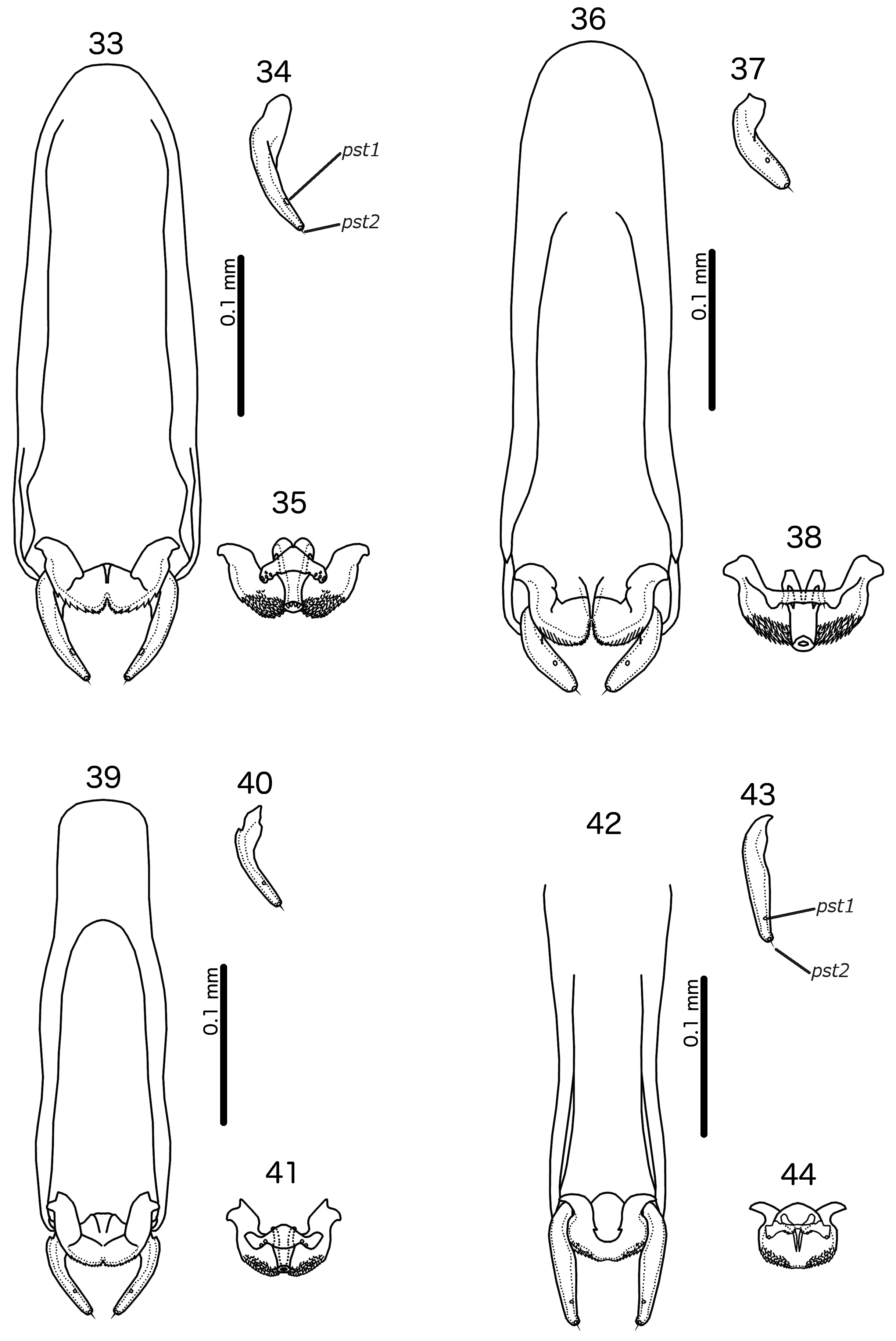
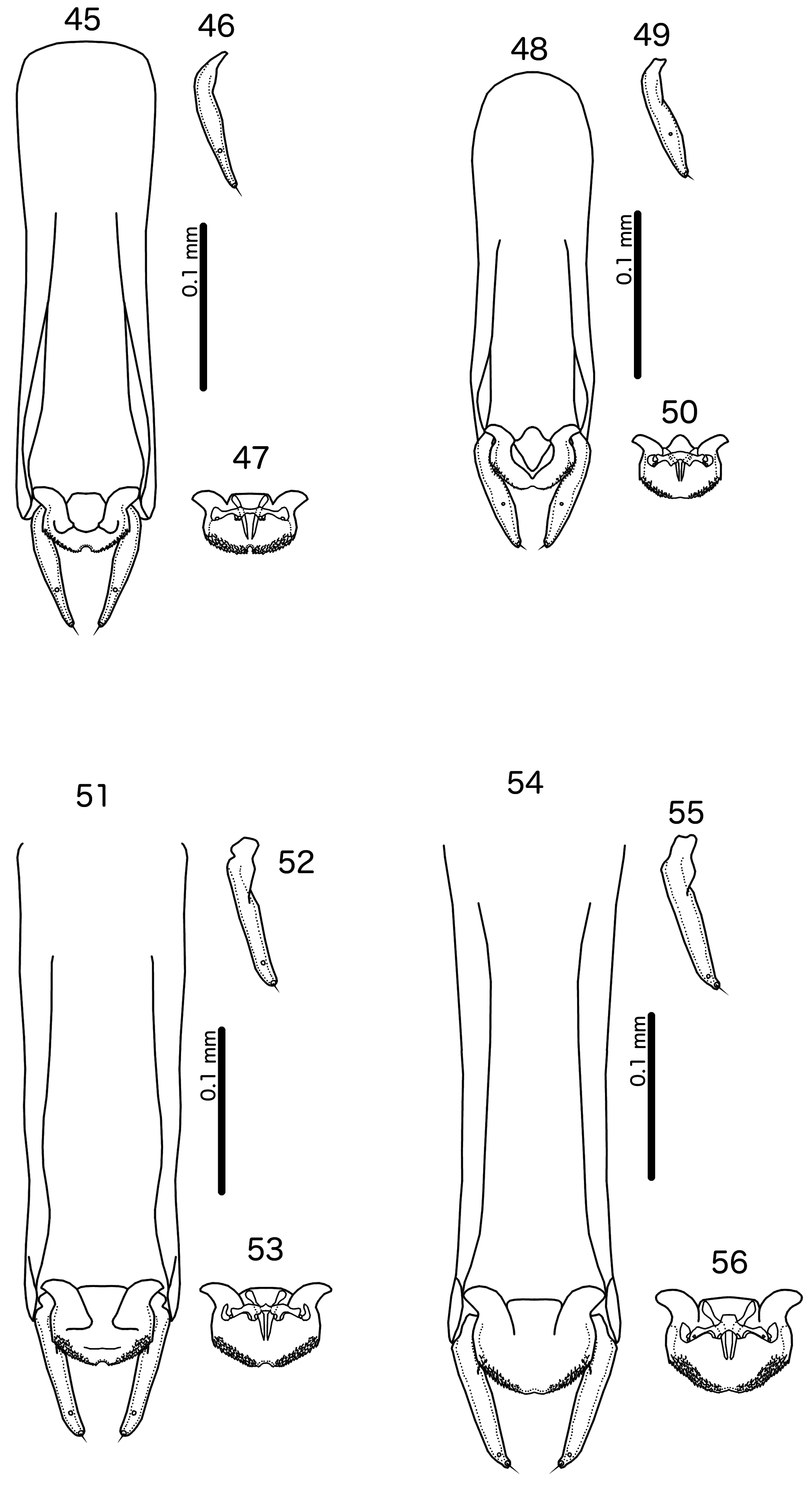
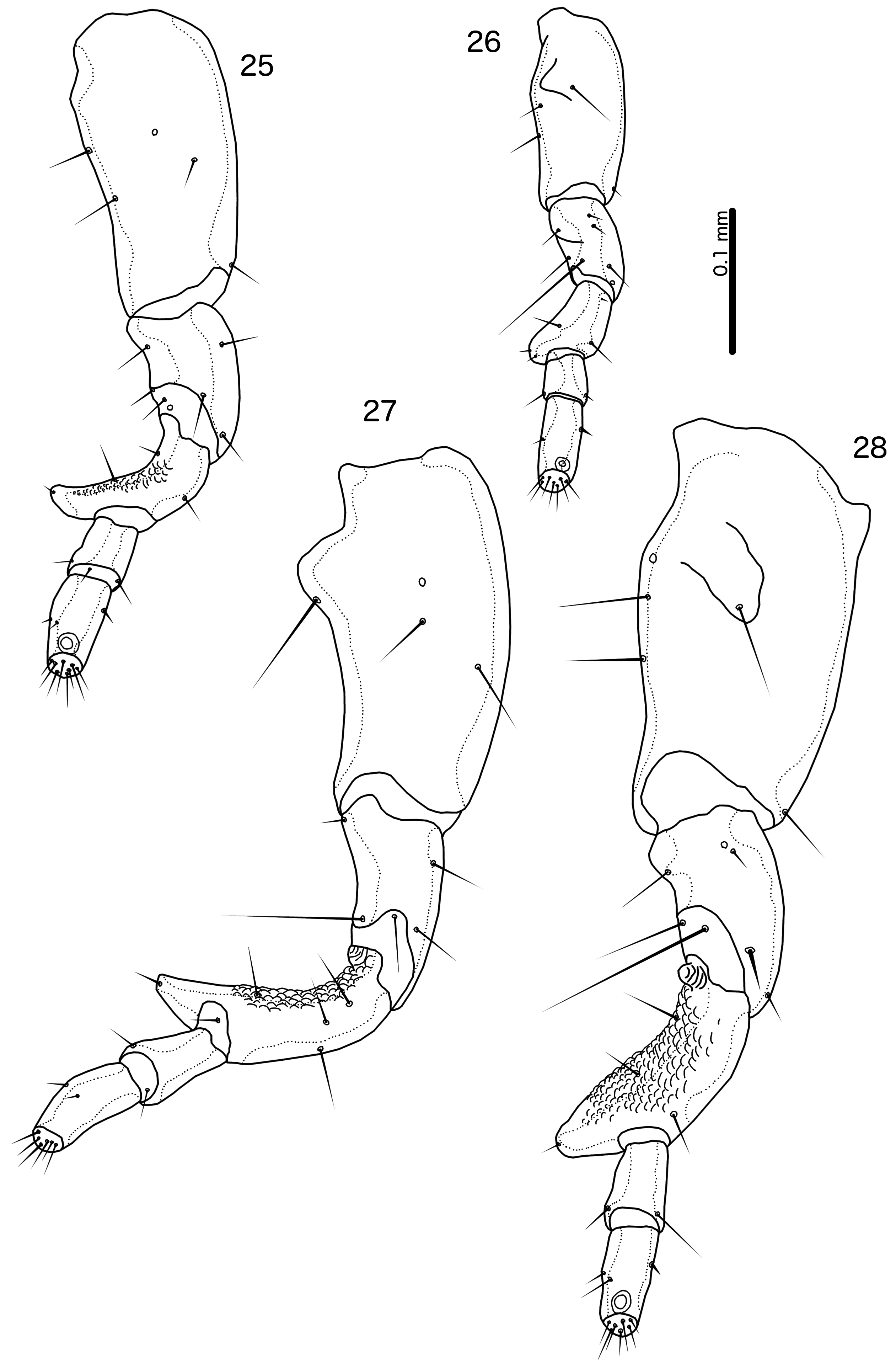
Male. In most species, the scape has a prominent tooth-like projection ( Fig. 27 View FIGURES 25–28 ), but in species without such projection, the scape is much larger than in the female; flagellomere I with hook-shaped extension distally, and may have extensive rugose area on dorsal margin ( Fig. 28 View FIGURES 25–28 ). Abdominal plates as in Fig. 5 View FIGURES 5–6 ; tergopleurites II–VIII separated medianly; tergopleurites IX+X and XI fused, each medianly continuous. Inter-tergal plates absent. Sternal plates present on segments II–VII. Subgenital plate formed by fusion of sternal plates VII–IX+X, not incised laterally between segments; accessory sternal plate may be present on anterior end of segment IX+X ( Fig. 5 View FIGURES 5–6 ); stylus short, blunt, arising subterminally, typically not extending far beyond distal margin of abdomen ( Fig. 80 View FIGURES 79–81 ) (the stylus is typically longer, spatulate and arising terminally in species parasitising species of Arborophila Hodgson, 1837 ). Male genitalia as in Figs 42–59 View FIGURES 33–44 View FIGURES 45–56 View FIGURES 57–68 : basal apodeme slender, often with similar width throughout; mesosome seemingly fused to basal apodeme proximally, antero-laterally with prominent hooks on each side and distal section with extensive rugose area (in R. polytrapezius with second rugose area more anteriorly); gonopore short, not approaching distal margin of mesosome, not fused distally; ventral sclerite wider than long, typically with at least one pore near lateral ends on each side; parameres generally slender, tapering gently, either roughly straight or curing gently; pst1–2 typically separated ( Fig. 49 View FIGURES 45–56 ), but may be close together ( Fig. 55 View FIGURES 45–56 ).
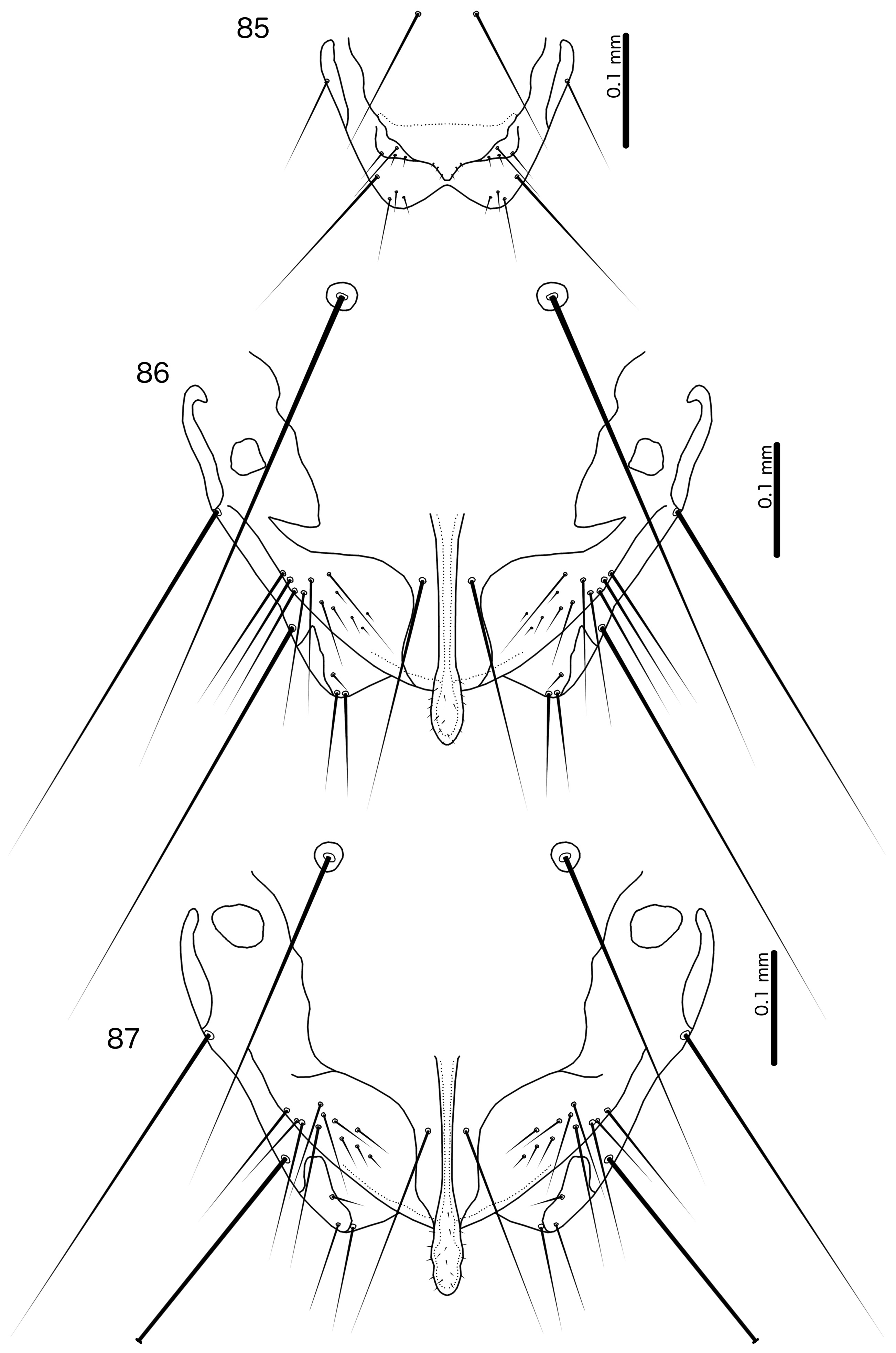
Female. Abdominal plates as in Fig. 6 View FIGURES 5–6 . Tergopleurites II–VIII separated medianly; tergopleurite IX+X medianly continuous, either separate from tergopleurite XI ( Fig. 6 View FIGURES 5–6 ), or fused with it ( Fig. 8 View FIGURES 7–8 ). Sternal plates present on segments II–VII. Subgenital plate varying between species, often diffuse and much reduced; either medianly continuous ( Fig. 70 View FIGURES 69–70 ) or separated medianly ( Fig. 71 View FIGURES 71–72 ); lateral accessory vulval plates absent; subvulval plates present; vulval margin concave, but shape differing between species and with numerous marginal setae and one submarginal seta on each side ( Figs 70–75 View FIGURES 69–70 View FIGURES 71–72 View FIGURES 73–74 View FIGURE 75 ). In lice parasitising species of Arborophila the vulval margin is folded into large, semicircular lobes. Distal ends of abdomen may be extended into claspers in lice parasitising species of Arborophila .
Host distribution. Species of Reticulipeurus are parasitic on members of the families Cracidae , Numididae and Phasianidae in the order Galliformes .
Geographical range. Almost cosmopolitan, except Antarctica and unknown from wild, native birds in Australasia, Oceania, and other islands lacking native galliform hosts.
Remarks. Besides the species treated here, we have not examined any specimen of most of the species of Reticulipeurus listed below. Therefore, our inclusion of those species in Reticulipeurus is based on Clay’s (1938) Oxylipeurus Group VI, Złotorzycka (1966) and Kéler (1958). A thorough revision of this genus is necessary to establish whether all the species included below belong to this genus, but that task is beyond the scope of this paper. Louse species from hosts in the Cracidae (including Clay’s “Group V”) are tentatively included here based on Carriker (1944), Kéler (1958) and Mey (2009), but we have not examined any specimen of any of the species from cracid hosts. Synonyms follow Price et al. (2003).
Although Clay (1938) included Oxylipeurus tetraophasis in her Group VI, we consider this species to belong to a separate genus (see below) because it shows significant differences from Reticulipeurus , in particular the structure and chaetotaxy of the head and the morphology of the male genitalia. Furthermore, Clay (1938) included Oxylipeurus agriocharis Clay, 1938 , Oxylipeurus corpulentus Clay, 1938 , Oxylipeurus ocellatus Clay, 1938 and Lipeurus polytrapezius Burmeister, 1838 in her Group VI. These species are morphologically very distinct and should be grouped in a separate genus. Also, Lipeurus dentatus Sugimoto, 1934 was included in Group VI by Clay (1938), but it is morphologically distinct from all other species in the Oxylipeurus- complex and should be placed in a monotypic genus. However, considering that all these species have not been recorded from China, they are not treated further here. Oxylipeurus forcipatus ( Piaget, 1885) may also belong in Reticulipeurus , but we have not examined Piaget’s specimens to confirm its inclusion.
In addition to the specimens redescribed below, we have examined four unidentified Chinese specimens of Reticulipeurus deposited at the NHML from three other host species, as follows: one male from Lerwa lerwa (Hodgson, 1833) , which is too poorly preserved to be described, two females from Crossoptilon harmani Elwes, 1881 and one female from Arborophila gingica . Considering that many of the useful characters to identify lice of the Oxylipeurus -complex are in the male genitalia, female-only samples cannot be reliably identified at this time. However, when the species of Reticulipeurus parasitising members of the host genus Arborophila have been revised, it might be possible to identify females.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Reticulipeurus Kéler, 1958
| Gustafsson, Daniel R., Lei, Lujia, Chu, Xingzhi & Zou, Fasheng 2020 |
Oxylipeurus
| Price, R. D. & Hellenthal, R. A. & Palma, R. L. & Johnson, K. P. & Clayton, D. H. 2003: 202 |
Reticulipeurus Kéler, 1958: 332
| Keler, S. von 1958: 332 |