Chiroderma salvini Dobson, 1878
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4846.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:6F6EBF63-5598-416C-8694-14C4A8687693 |
|
DOI |
https://doi.org/10.5281/zenodo.4332639 |
|
persistent identifier |
https://treatment.plazi.org/id/03BAA52E-F85D-FFCC-7090-F8BD7F015E95 |
|
treatment provided by |
Felipe |
|
scientific name |
Chiroderma salvini Dobson, 1878 |
| status |
|
Chiroderma salvini Dobson, 1878 View in CoL
Synonyms:
Chiroderma salvini Dobson, 1878: 532 View in CoL ; type locality “ Costa Rica.”
Chiroderma salvini salvini: Handley, 1966: 297 View in CoL ; name combination.
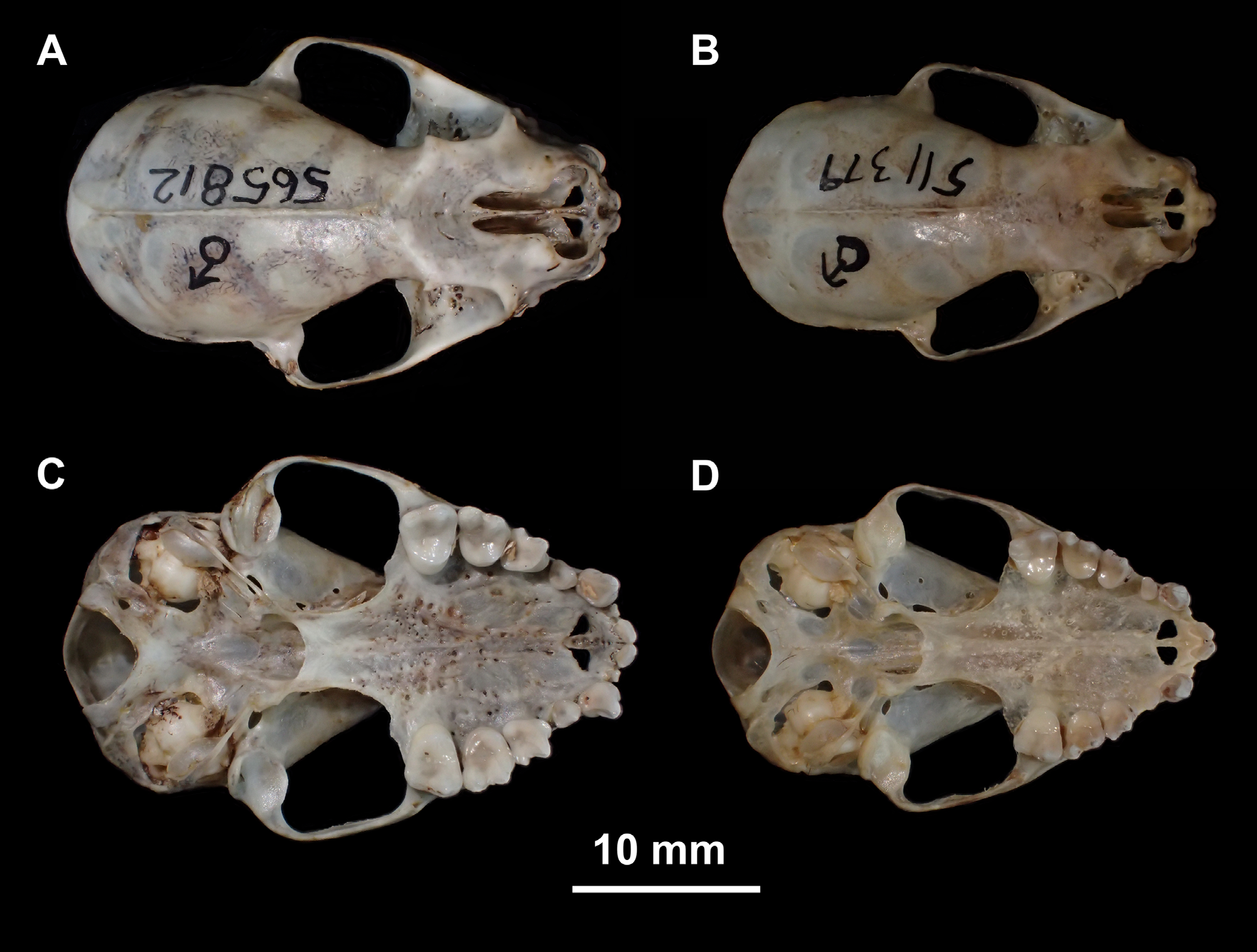
Chiroderma salvini scopaeum Reid and Langtimm, 1993: 300 View in CoL ; not Chiroderma salvini scopaeum Handley, 1966 View in CoL . Type Material. The type of C. salvini View in CoL (BMNH 68.8.16.2), fixed by original designation, is a fluid-preserved adult male with skull removed and tongue still attached to the body. The anterior half of the pelage is more faded than the posterior half. Nevertheless, it is possible to note whitish hairs above the lips and below the eyes. A thin median dorsal stripe extends from the middle dorsum to the posterior extremity of the lower back (contra Dobson 1878: 532, who considered the stripe to be absent). The cranium and mandible are in good condition with all teeth present. Some parts of the basicranium, as well as a large part of the palate have attached soft tissue. The I1s have convergent tips that do not touch each other. The angular processes of the mandible are broken.
The species was named after Osbert Salvin, a British zoologist who edited and organized, with Frederick Godman, the 40-volume “Biologia Centrali-Americana”. On the type specimen’s skull label, the locality is given as “ Costa Rica ”, and to the right it is handwritten “O. Salvin [c]”, suggesting that the collector would indeed be Salvin. However, this naturalist collected specimens exhaustively in Guatemala and bordering countries, such as Belize ( Godman 1915; Papavero 1973). Enrique Arcé, a Guatemalan field worker trained by Salvin, collected most of the bird specimens from Costa Rica described by the British zoologist ( Salvin 1864; Warren & Harrison 1971; Beolens et al. 2014). Therefore, we suggest that the type of C. salvini View in CoL probably was collected by E. Arcé during his work in Costa Rica.
In the volume on mammals of the “Biologia Centrali-Americana”, authored by E. Alston (except for a few pages in the Supplement, authored by O. Thomas) and published between 1879 and 1882 ( Lyal 2011), there is a color plate of C. salvini depicting the species as lacking the medial dorsal stripe ( Fig. 10 View FIGURE 10 ), an error that probably originated from Dobson (1878) description. Dobson (1880), however, recorded the presence of the stripe in an additional specimen of C. salvini from Popayán, Colombia.
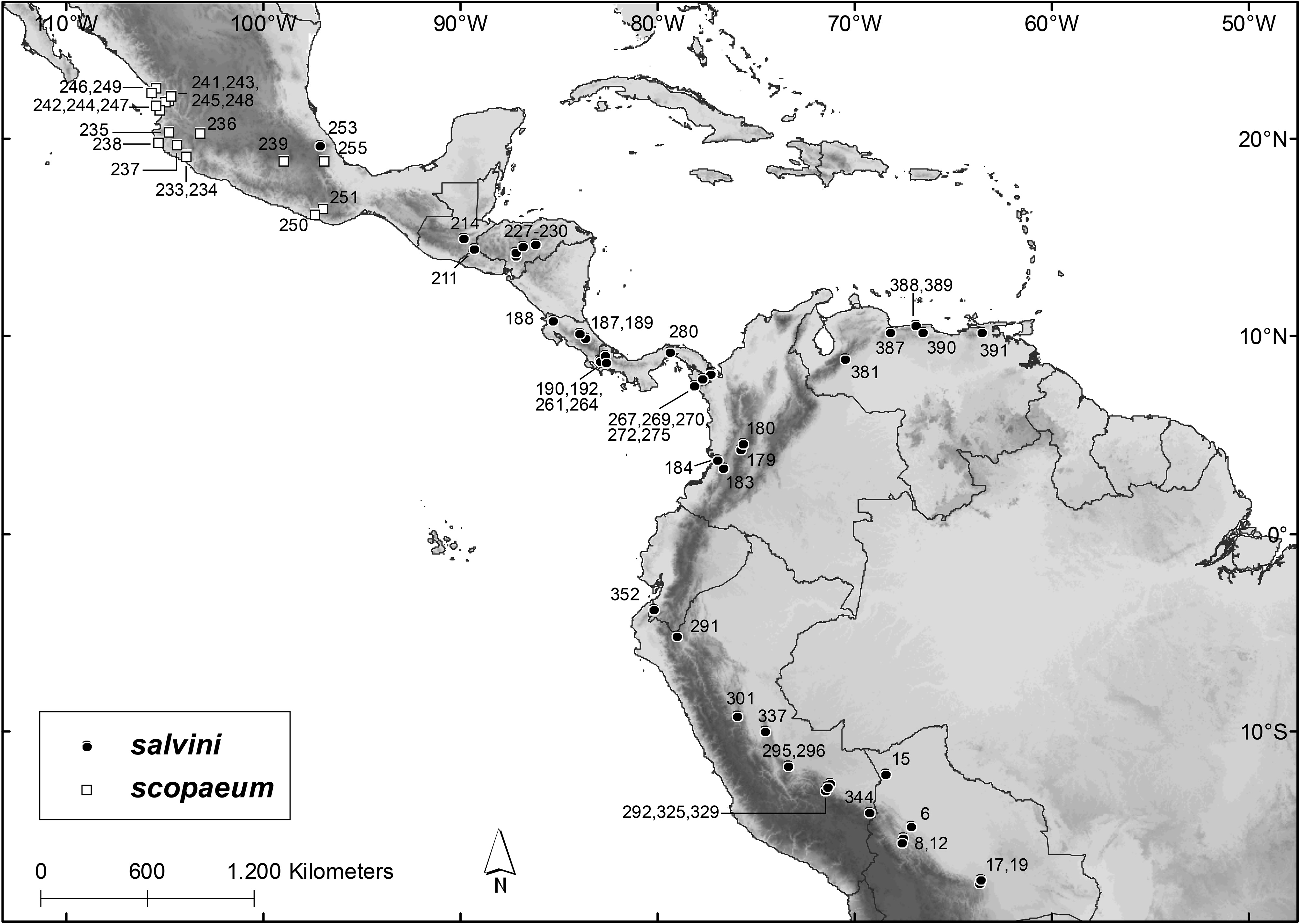
Distribution and Habitat. Chiroderma salvini is in eastern and southern México from Veracruz southeastward through Central America ( Guatemala, El Salvador, Honduras, Costa Rica, and Panamá), into South America (northern and western Venezuela, western and northern Colombia, western Ecuador, eastern Perú in the Andean foothills, and western Bolivia; Fig. 11 View FIGURE 11 ). The absence of records from Nicaragua may be a sampling artifact, possibly related to the fact that this country has lower mean elevations than the neighboring countries, and C. salvini is associated with montane forests.
Records of C. salvini are from humid tropical forests, mainly sub-montane and montane. In Guatemala, El Salvador, and Honduras, the species also occurs in seasonally dry tropical forests. The distribution of C. salvini is associated with moderate to high elevations, with records in or close to the Sierra Madre Oriental in México, the cordilleras of Central America (e.g. Sierra Madre de Chiapas in Guatemala, Cordillera de Talamanca in Panamá and Costa Rica), and on both slopes of the Andean cordillera in South America ( Fig. 11 View FIGURE 11 ). Among the 39 specimen localities with precise coordinates, the mean elevation was 1,010 m above sea level (ranging from 73 m to 2,045 m), with 32 localities (82%) above 600 m and 20 (51%) above 1,000 m. In Panamá, C. salvini was more frequently captured between 600 and 1,500 m ( Handley 1966b). In Parque Nacional Braulio Carrillo, Costa Rica, the species was recorded at 680 m ( Timm et al. 1989). In Venezuela C. salvini was captured between 611 and 2,240 m, with 93% of the captures above 1,000 m ( Handley 1976). In Parque Nacional de Manú, Peruvian Amazon, the species was documented between 450 and 1,920 m ( Solari et al. 2006). In the Department of Tolima, Colombia, records of C. salvini are between 1,380 and 2,150 m ( Bejarano-Bonilla et al. 2007; Galindo-Espinosa et al. 2010), and in the Department of Valle del Cauca, C. salvini was captured at elevations from 1,200 to 1,700 m ( Mora-Beltrán & López-Arévalo 2018).
Description and Comparisons. The dorsal pelage of C. salvini varies from pale brown to dark brown. Dorsal hairs are tricolored, with a narrow (approximately ¼ of hair length) dark brown base, a wide (approximately ½ of hair length) buff medial band, and a narrow terminal band approximating ¼ of hair length. Basal and terminal bands are usually the same color. Genal and interocular pairs of facial stripes are always present; conspicuous, wide, and brilliant-white. Interocular stripes are large, their widths varying between 1 and 4 mm, and entirely white. The median dorsal stripe is visible in most specimens, not detected in 2 of 174 specimens (1.1% of the sample): one from Venezuela (USNM 415233) had a faint suggestion of the stripe on the middle dorsum, and another from Honduras (TTU 12806) had no trace of a stripe. The spear of the noseleaf has a simple tip. The lateral margins of the horseshoe and the spear are whitish. The base and margins of the ear are yellowish.
The dimensions of the cranium of C. salvini are similar to small C. doriae , large C. scopaeum , and large C. villosum ( Tables 7 and 8). The braincase is globose, conspicuously standing out from the adjacent frontal and nasal regions. In dorsal view, the nasal notch extends posteriorly to the anterior margin of the orbits ( Fig. 12 View FIGURE 12 ). In lateral view, the anterior margin of the orbits is even with the distal margin of P4 and mesial margin of M1 ( Fig. 13 View FIGURE 13 ). A sagittal crest was present in 87.7% (186 of 212) of the specimens we examined. The sagittal crest was weakly developed in 22 (10.4%) specimens and not detected in four (1.8%). The posterior palatine process was absent in 77% of the sample (159 of 206 specimens), and was poorly developed when present. With cranium and mandible in occlusion, there is no frontal gap (as in C. improvisum and C. villosum ; Fig. 14 View FIGURE 14 ) but there is a small lateral gap, as in C. doriae , C. scopaeum , C. gorgasi and C. trinitatum ( Fig. 9 View FIGURE 9 ).
The I1s converge medially in 97% of the specimens (200 of 206) and the tips may or may not be in contact. Six specimens (3%) have parallel I1 crowns that lack any contact. The P3 is oval in occlusal outline, differing from the antero-posteriorly compressed outline of P 3 in C. doriae . The P3 contacts C. but not P4.
The lower canine is pointed and relatively tall, and the tip is approximately the same height as the coronoid process (as in C. villosum , but differing from C. doriae and C. scopaeum , in which the canines are clearly below the level of the coronoid; Fig. 13 View FIGURE 13 ). The crown of p2 is low, approximately ¼ of the crown height of p4, longer mesiodistally than tall, and does not contact p4 (similar to the morphology of C. scopaeum and C. villosum ).
Compared with the allopatric C. doriae , C. salvini can be distinguished by its smaller size, globose braincase (less rounded in doriae ), taller lower canines (lower canines in doriae are relatively shorter and below the level of the coronoid process), and smaller p2 (in doriae the p2 is approximately ⅔ of the height of p4).
Where sympatric, C. salvini can be confused with C. scopaeum and C. villosum . Externally, C. salvini can be separated from C. villosum by, on average, a longer forearm ( Tables 7 and 8); basal and apical bands of dorsal fur the same color (in villosum the base is usually darker than the tip); presence of wide and conspicuous facial stripes (narrow, faint, or absent in villosum ), and a simple tip on the noseleaf spear with pale lateral margins (notched tip and noseleaf uniformly brown in villosum ). Cranially, C. salvini differs from C. villosum by its relatively longer rostrum and shorter nasal notch (in villosum the notch is longer, ending near the level of the post-orbital constriction); smaller orbits (in villosum the anterior margin of the orbit is even with the P4); post-orbital processes less pointed than in villosum ; posterior palatine process small or absent (in villosum process usually present and conspicuous), and absence of a frontal gap when cranium and lower jaw are in occlusion ( Fig. 14 View FIGURE 14 ).
Compared with C. scopaeum , C. salvini is larger ( Fig. 15 View FIGURE 15 , Tables 8 and 10) and externally it differs in pelage color, usually being darker than scopaeum . The skull of C. salvini is more robust, and the lambdoid-suture region of C. scopaeum is rounder, as can be seen in dorsal view ( Fig. 12 View FIGURE 12 ). The nasal notch of salvini is longer, usually reach- ing the interorbital region ( Fig. 12 View FIGURE 12 ). Lower canines are relatively taller and more pointed than in scopaeum , and the medial cingulum of the lower canines is not as well developed as in scopaeum ( Fig. 13 View FIGURE 13 ).
Geographic Variation and Phylogeography. Phylogenetic analyses of 18 sequences did not recover any geographical structuring from Costa Rica to Bolivia ( Fig. 16 View FIGURE 16 ). Phenotypically, C. salvini is a relatively homogeneous species across its distribution.
Subspecies. C. salvini is monotypic.
Remarks. We found four published reports in which C. villosum from localities in Perú and Brazil were misidentified as C. salvini . The record of C. salvini for the Serra do Divisor, in the Peruvian Amazon ( Medina et al. 2015), is here reidentified as C. villosum based on the reported forearm length (45.6 mm) and on a photograph of the specimen clearly showing a notched tip on the noseleaf and ears lacking pale margins (C. Medina in litt.). We also reanalyzed the specimens from Porto Velho, Rondônia, (MZUSP 35408) and Aricá, Mato Grosso, (MZUSP 6494) reported by Rocha et al. (2016), and confirmed that they have the diagnostic characters of C. villosum . Also, the record for the Cerrado of Tocantins ( Maas et al. 2018) is recognized here as a C. villosum based on the measurements presented in the article and a photo of the skull (L.A.C. Gomes in litt.).
Natural History. Four genera and five species of plants are documented in the diet of C. salvini in Colombia: Cecropia telealba (Urticaceae) , Ficus insipida , F. cuatrecasana , Poulsenia armata (Moraceae) , and Piper phytolaccifolium (Piperaceae) ( Castaño et al. 2018). In Bolivia, one C. salvini was captured in a mist net set under a Ficus guaranitica (Aguirre 1994 apud Anderson 1997). In Veracruz, México, individuals were covered in pollen of Pachira aquatica (Malvaceae) ( Hernández-Montero & Sosa 2016). In the Peruvian Amazon, Bravo et al. (2008, 2010) recorded C. salvini visting “collpas”, which are mineral licks containing clay-rich water that is ingested by the bats. Diurnal roosts used by C. salvini are unknown, but one animal was recorded flying through a lighted tunnel in a gold mine in Panamá and, in Venezuela specimens, have been captured inside houses ( Goldman 1920; Handley 1976).
The following ectoparasites have been recorded in C. salvini in Panamá: Amblyomma sp. n. ( Ixodidae ), Chirnyssoides caparti (Sarcoptidae) , Periglischrus iheringi (Spinturnicidae) , and Paratrichobius salvini (Streblidae) (Fairchild et al. 1966; Furman 1966; Wenzel et al. 1966; Lourenço et al. 2013). In Venezuela, Periglischrus iheringi and Trichobius persimilis (Streblidae) were collected in C. salvini ( Herrin & Tipton 1975; Wenzel 1976), and in México the mites Parichoronyssus lopezi (Macronyssidae) , Periglischrus iheringi , and Eudusbabekia vampyrops (Myiobiidae) were recorded on the species ( Colín-Martínez et al. 2017).
The reproductive pattern of C. salvini in Central America is best described as seasonal polyestry, with birth peaks occurring between March and April, and in August. Based on label information, pregnant females were recorded in Panamá in January (n=1), February (n=41), March (n=1), and June (n=1), while lactating individuals were recorded in March (n=5). In Guatemala, a pregnant C. salvini was recorded in January ( Carter et al. 1966). In Honduras, pregnant or lactating C. salvini have been found in July and August ( LaVal 1969).
In South American populations, the scarcity of data does not permit generalizations. Based on the information we obtained from specimen labels, pregnant females have been recorded in Venezuela in July (n=2), August (n=1), and November (n=1), and in the Colombian Pacific there is a record for June (n=1). Based on literature records, pregnancies in Colombia are known for January, March, April, May, June, October, and December, and there are records of females that were both pregnant and lactating in March and April ( Wilson 1979). In cis-Andean South America, two pregnant females were recorded from Perú, in August and September, and a lactating C. salvini was noted in October.
Specimens Examined (N = 216): Bolivia: La Paz, Serrania Bellavista ( AMNH 246625 About AMNH ) ; Pando, Santa Rosa ( AMNH 262537 About AMNH , 262538 About AMNH ) ; Santa Cruz, 4.5 km N and 1.5 km E of Cerro Amboro ( AMNH 261666 About AMNH ) ; Santa Cruz, Estancia San Rafael de Amboro ( AMNH 261667–261670 About AMNH ) . Colombia: Quindío, Vereda El Dorado ( IAvH-M 7034 ), Vereda San Juan d’Carolina ( IAvH-M 7036 , 7039 ) , Valle del Cauca, Pance ( USNM 483743–483746 About USNM ) , Río Zabaletas ( USNM 483747–483762 About USNM ) . Costa Rica: without precise locality ( BMNH 68.8.16.2 [holotype of salvini ]) ; Cartago, Angostura ( USNM 12913/22849 ) , Guanacaste, Rincón de La Vieja ( USNM 565812 About USNM ) ; Heredia, Parque Nacional Braulio Carrillo ( USNM 562856 About USNM ) ; Puntarenas, Cañas Gordas ( AMNH 142484 About AMNH ) . El Salvador: Santa Ana, Los Planes ( TTU 62461 , 62462 View Materials ) . Guatemala: El Progreso, Rio Uyús ( ROM 99703 ) . Honduras: Francisco Morazán, 16 km by road N Tegucigalpa ( TTU 12800–12808 View Materials ) , La Flor ( AMNH 126210 About AMNH , 126211 About AMNH , 126244–126251 About AMNH , 126253 About AMNH , 126255–126264 About AMNH , 126446 About AMNH , 126448–126455 About AMNH ) , San Marcos ( AMNH 123331 About AMNH ) , Olancho, 50.4 km by road NNE Juticalpa ( TTU 12809 ) . México: Veracruz, Las Minas ( USNM 329445 About USNM ) . Panamá: Bocas del Toro, Río Changena Camp ( USNM 319415–319425 About USNM , 319499 About USNM , 319500 About USNM ) , Rancho Mojica , Río Changena ( USNM 319286 About USNM ) , Chiriquí, Cuesta de Piedra ( USNM 331684–331686 About USNM ) , Darién , Cana ( USNM 179718 About USNM ) , Cerro Malí ( USNM 338042 About USNM , 338043 About USNM ) , Cerro Pirre ( LSUMZ 25468–25474 View Materials ) , Cerro Tacarcuna ( USNM 338044 About USNM ) , Jaqué ( USNM 362919 About USNM ) , Tacarcuna Village Camp ( USNM 209969 About USNM , 305387 About USNM , 309443–309445 About USNM , 309906 About USNM , 309908–309910 About USNM , 309912–309942 About USNM , 309946–309968 About USNM , 309972– 309977 About USNM ) , Panamá, Cerro Azul ( USNM 305388 About USNM , 323445–323447 About USNM ) . Perú: Cajamarca, San Ignacio ( MUSM 12637 ) , Cusco, Consuelo ( MUSM 19663–19665 , 19667 ) , Comunidad Nativa Tangoshiari ( MUSM 13377 ) , Ridge Camp ( USNM 588032 About USNM ) , Madre de Dios, Hacienda Amazonia ( MUSM 9742 , 9751 ) , Quebrada Aguas Calientes ( MUSM 16650 ) , Pasco, Palmira ( MUSM 10878–10880 ) , Puno, Yanacocha ( MUSM 34980 ) , Tumbes, Quebrada Naranjos ( MUSM 19177 ) . Venezuela: Carabobo, La Copa ( USNM 440740–440744 About USNM ) , La Vega del Río Santo Domingo ( USNM 440746 About USNM ) , Distrito Federal, Los Venados ( USNM 370526 About USNM , 370527 About USNM ) , Hotel Humboldt ( USNM 370528 About USNM , 370530–370532 About USNM ) , Miranda , Guatopo Natural Park ( USNM 387191 About USNM ) , Monagas, Hacienda San Fernando ( USNM 415233–415235 About USNM ) , San Agustín ( USNM 415236 About USNM , 415237 About USNM ) .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Chiroderma salvini Dobson, 1878
| Garbino, Guilherme S. T., Lim, Burton K. & Tavares, Valéria Da C. 2020 |
Chiroderma salvini scopaeum
| Reid, F. & Langtimm, C. 1993: 300 |
| Dobson, G. E. 1878: 532 |
Chiroderma salvini
| Dobson, G. E. 1878: 532 |