Ectopleura crocea ( L. Agassiz, 1862 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3753.5.2 |
|
publication LSID |
lsid:zoobank.org:pub:B50B31BB-E140-4C6E-B903-1612B7B674AD |
|
DOI |
https://doi.org/10.5281/zenodo.6127355 |
|
persistent identifier |
https://treatment.plazi.org/id/03BB5A48-FFC9-3728-DC80-FE37D5CBF9CD |
|
treatment provided by |
Plazi |
|
scientific name |
Ectopleura crocea ( L. Agassiz, 1862 ) |
| status |
|
Ectopleura crocea ( L. Agassiz, 1862) View in CoL
( Figures 3–5 View FIGURE 3 View FIGURE 4 View FIGURE 5 ; Tables 1–8 View TABLE 1 View TABLE 2 )
Parypha crocea L. Agassiz, 1862: 249 View in CoL , pls 23-2a.
Tubularia mesembryanthemum Allman 1871: 418 View in CoL , figs. 83–84; Hargitt 1927: 494; Yamada 1959: 16; Schmidt 1971: 32, pl. 2B; Hirohito 1988: 18, fig. 4, pl.1, fig. B.
Tubularia crocea View in CoL ; Torrey 1902: 42, pl. 3, figs. 22–23; Rees 1963: 1223; Brinckmann-Voss 1970: 28, text-fig. 30–34; Calder 1971: 24, pl. 1C; Genzano, Cuartas & Excoffon 1991: 69, pl. 5C; Blanco 1994: 182; Genzano & Zamponi 2003: 306, 307, 309, Tables 2-3 View TABLE 2 ; Demicheli & Scarabino 2006: 530.
Tubularia ralphi Bale 1884: 42 View in CoL ; Watson 1980: 60, fig. 25–37; Watson 1982: 85, fig. 4.6.b, Plate 7.5.
Tubularia gracilis View in CoL von Lendenfeld 1885: 597, fig. 51–52.
Tubularia sagamina Stechow 1907: 194 ; Yamada 1959: 16.
Tubularia australis Stechow 1925: 196 View in CoL .
Tubularia warreni Ewer 1953: 351 View in CoL , text-fig. 1–4; Millard 1959: 299; Millard 1966: 435; Millard 1975: 35, frontispiece, fig. 15A–G.
Ectopleura warreni View in CoL ; Migotto & Silveira 1987: 101, fig. 3; Migotto 1996: 25; Grohmann et al. 1997: 230, Table 1 View TABLE 1 ; Rosso & Marques 1997: 417, 420, 421, Table 1 View TABLE 1 , Figure 4 View FIGURE 4 .
Ectopleura crocea View in CoL ; Petersen 1990: 174, fig. 27; Schuchert 1996: 107, figs. 64a–g; Schuchert 2001: 43; Bouillon et al. 2004: 104, fig. 55E–F; Genzano et al. 2009: 37, 40, Tables 2-3 View TABLE 2 , Fig. 3 View FIGURE 3 ; Schuchert 2010: 357 -362, fig. 6.
Ectopleura ralphi View in CoL ; Petersen 1990: 175; Schuchert 1996: 109; Migotto, Marques & Flynn 2001: 289, 290, 293-297, figs. 5-6, Table 1 View TABLE 1 .
Pinauay ralphi View in CoL ; Marques & Migotto 2001: 475, 478, 480, figs. 2B, 3, Table 1 View TABLE 1 ; Migotto, Marques, Morandini & Silveira 2002: 10; Marques & Migotto 1994: 173, 174, Tables 15.1, 15.2; Grohmann 2006: 103, 104, Tables 1-2 View TABLE 1 View TABLE 2 ; Oliveira, Marques & Migotto, 2006: 4, 10, fig. 5, Table 1 View TABLE 1 ; Oliveira & Marques 2007: 31; Grohmann 2007: page not numbered; Silveira & Morandini 2011: 5.
Pinauay crocea View in CoL ; Marques & Migotto 2001:480.
Examined material. Brazil: State of Rio de Janeiro, Macaé, Cavaleiros Beach, 22°24’S 41°47’W, 15.viii.2008, on rock, 95% ethanol, coll. A.C. Morandini ( MZUSP 1633); State of São Paulo: São Vicente, Vacas Beach, 23o58S 46o23W, 05.ix.1991, intertidal fringe, on rock, under Phragmatopoma stripe, at the same level of Eudendrium , 4% formalin, coll. A.C. Marques ( MZUSP 414); Itanhaém, Saudade Beach, 24o10'S 46o45'W, 26.viii.1991, intertidal fringe, on rock, under Phragmatopoma stripe, 4% formalin, coll. A.C. Marques ( MZUSP 406); Peruíbe, Centro Beach, 24o19'S 46o58'W, 12.viii.1992, intertidal fringe, on rock, sheltered place, forming an abundant stripe below Phragmatopoma , 18o C, 28‰, 4% formalin, coll. A.C. Marques ( MZUSP 433); Peruíbe, Jureia-Itatins Ecological Station, 24°34’S 47°14’W, 15.ix.2008, on rock, 95% ethanol, coll. J.M.M. Nogueira ( MZUSP 1636); Cananéia, Argolão Rocky Shore near São João Hill, 25o00'S 47o57'W, 25.viii.1992, intertidal fringe, on rock, 19o C, 28‰, 4% formalin, coll. A.C. Marques ( MZUSP 444); State of Paraná: Mel Island, Encantadas Rock, 25o34'S 48o18'W, 06.viii.1988, 4% formalin, coll. M.A. Haddad ( MZUSP 1750); Paranaguá, Yacht Club Paranaguá, 25°30’S 48°29’W, 10.x.2007, on artificial substrate, 95% ethanol, coll. M.A. Haddad, ( MZUSP 1637); Guaratuba, bottom trawling net, 4 km of shore, 25o52'S 48o33'W, 01.xii.2003, 4% formalin, coll. M.A. Haddad ( MZUSP 1751); State of Santa Catarina: Itapoá, Itapema beach, 26o05'S 48o36'W, 04.vi.2004, 4% formalin, coll. M.A. Haddad ( MZUSP 1752); Penha, on a culture of mussels, 26o45'S 48o38'W, 17.vi.2005, 4% formalin, coll. M.A. Haddad ( MZUSP 1753); Bombas, Bombas Beach, 27o07’S 48o30W, 03.xii.2006, 4% formalin, coll. M.A. Imazu ( MZUSP 1754) and 95% ethanol, coll. E. Ale, ( MZUSP 1638). Argentina: Mar del Plata, Punta Cantera, 38o04'S 57o32'W, 26.i.2002, intertidal fringe, 4% formalin, coll. G. Genzano ( MZUSP 1755), 95% ethanol, coll. G. Genzano ( MZUSP 1639).
Type specimens: Ectopleura ralphi , type specimen lost, a neotype was proposed based on specimens from Australia, Victoria, Port Phillip, Yarra River Entrance Beacon, 03.iv.1977, 1– 2m, on mussel and ascidia, formaldehyde ( NMV G3227) ( Watson 1980). Ectopleura crocea , we found no reference concerning the material described by L. Agassiz, from the port of Boston. It may be lost.
Description. Colonies dioecious, up to 55 mm high. Hydrorhiza and hydrocaulus with well-developed perisarc. Unbranched erect hydrocauli arising from stolonial hydrorhiza. Hydrocaulus’ coenosarc split into two longitudinal chambers with basal diameter 200–420 µm, apical 340–1000 µm; distal region of hydrocaulus with globular expansion supporting terminal hydranth. Hydranth with one whorl of aboral and one whorl of oral tentacles; oral tentacles adnate to hypostome up to the mouth region, circular in transversal section; aboral tentacles quadrangular in transversal section. Unbranched blastostyles of gonosomes arising immediately above aboral whorl of tentacles; main axis of each blastostyle supporting gonophores. Female gonophore cryptomedusoid, oval, with eight distal laterally compressed crests surrounding terminal aperture, terminal region of spadix projecting to outside. Male gonophore cryptomedusoid, spherical to oval, without distal crests. Early released actinulae with 8– 11 aboral capitate tentacles.
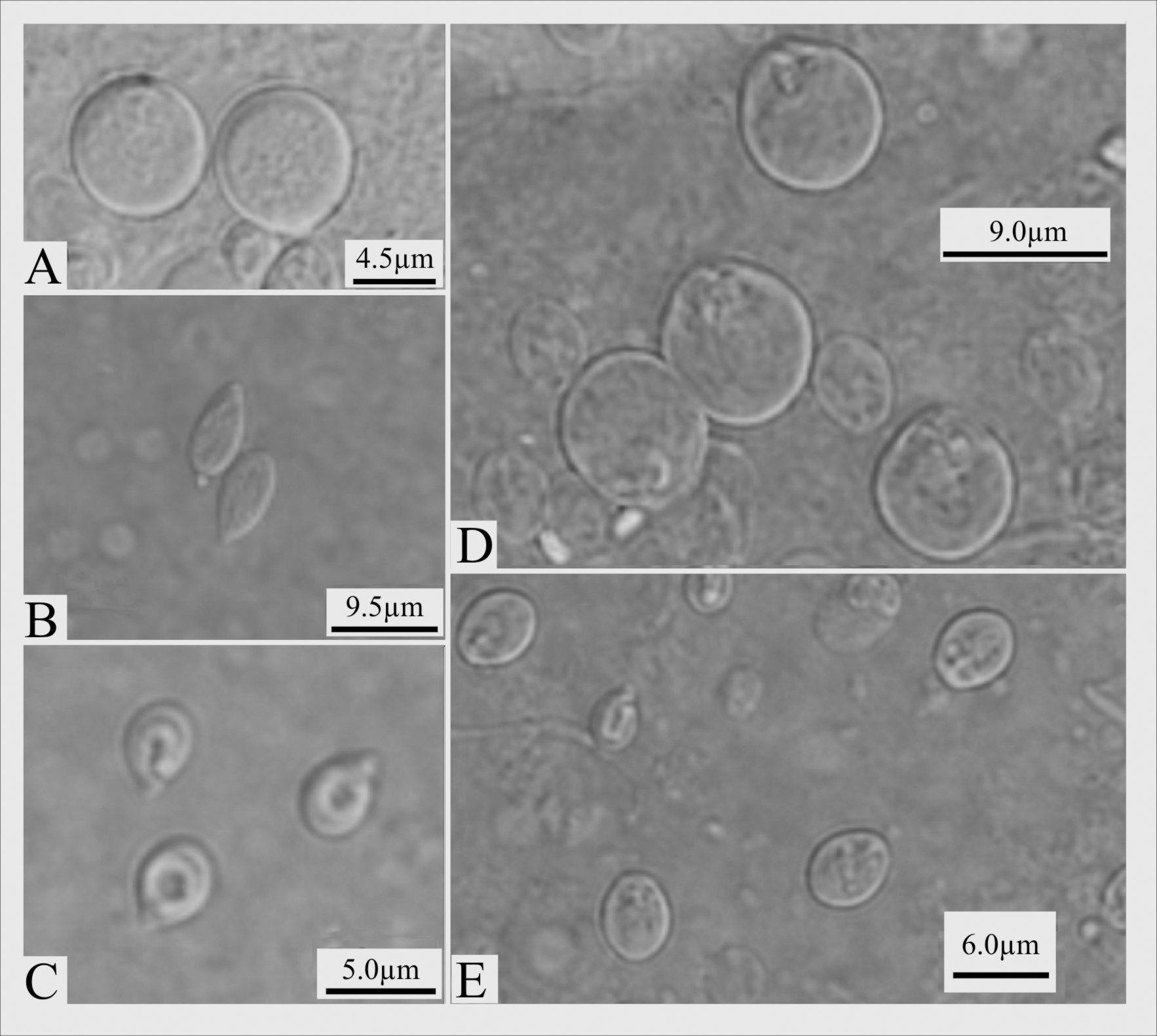
Aboral tentacles with four types of nematocysts: O-basitrichous isorhizas, rare and not measured ( Figure 3 View FIGURE 3 A); basitrichous isorhizas, common, 6.45–12.19 X 2.84–6.04 µm ( Figure 3 View FIGURE 3 B); desmonemes, abundant, spherical to oval, 3.50–6.81 X 2.41–5.13 µm ( Figure 3 View FIGURE 3 C); small stenoteles, abundant, 5.02–7.82 X 3.66–8.09 µm ( Figure 3 View FIGURE 3 E). Oral tentacles with three types of nematocysts: O-basitrichous isorhiza, rare and not measured ( Figure 3 View FIGURE 3 A); basitrichous isorhizas, rare, 7.06–14.73 X 2.87–7.1 µm; large stenoteles, abundant, 7.36–12.5 X 6.07–11.38 µm and small stenoteles 4.93–7.93 X 3. 2–7.13 µm ( Figure 3 View FIGURE 3 D).
Distribution in the South Western Atlantic Ocean. Brazil: States of Espírito Santo ( Grohmann et al. 1997, Grohmann 2006), São Paulo (Migotto & da Silveira 1987, Migotto 1996, Rosso & Marques 1997, Migotto et al. 2001, 2002, Marques & Migotto 2004, Oliveira et al. 2006, Oliveira & Marques 2007, Silveira & Morandini 2011), Paraná (Haddad, 1992), Santa Catarina ( Miranda et al. 2011) and Rio Grande do Sul ( Migotto & Silveira 1987) (see Migotto et al. 2002, Marques et al. 2003). Uruguay ( Demicheli & Scarabino 2006). Argentina: Provinces of Buenos Aires ( Blanco 1994, Genzano et al. 1991, 2009, Genzano 1994, 1998, Genzano & Rodriguez 1998), Río Negro and Chubut ( Blanco 1994, Genzano et al. 1991), Santa Cruz and Tierra del Fuego (Oliveira et al. submitted).
Remarks. General morphology. The species E. crocea and E. ralphi are morphologically similar and have been considered sister-taxa (Marques & Migotto, 2001) or synonyms ( Bouillon et al. 2006, Schuchert 2010). Historically, subtle differences have been cited to differentiate the species ( Table 1 View TABLE 1 ). For instance, Petersen (1990) differentiated the two species by describing E. crocea as having more aboral and oral tentacles than E. ralphi . However, this relationship appears to vary ( Table 1 View TABLE 1 ). Indeed, characters related to the tentacles are generally variable ( Tables 1–2 View TABLE 1 View TABLE 2 ; see also Agassiz 1862, Hargitt 1927, Ewer 1953, Calder 1971, Schmidt 1971, Migotto & Silveira 1987, Hirohito 1988).
The SWAO specimens all have hydroucauli that broaden distally ( Table 3), as described for E. crocea ( Hirohito 1988, Schuchert 1996), although specimens with the same diameter throughout the hydrocaulus were reported by Petersen (1990). Each polyp is gonochoristic, although settlement of actinulae on already developed hydrocauli ( Rungger 1969) may promote pseudo-hermaphroditism, a strategy also reported for other hydroids ( Brinkmann-Voss 1970, Sommer 1990, Marques 2001, Schuchert 2010, Nawrocki & Cartwright 2012).
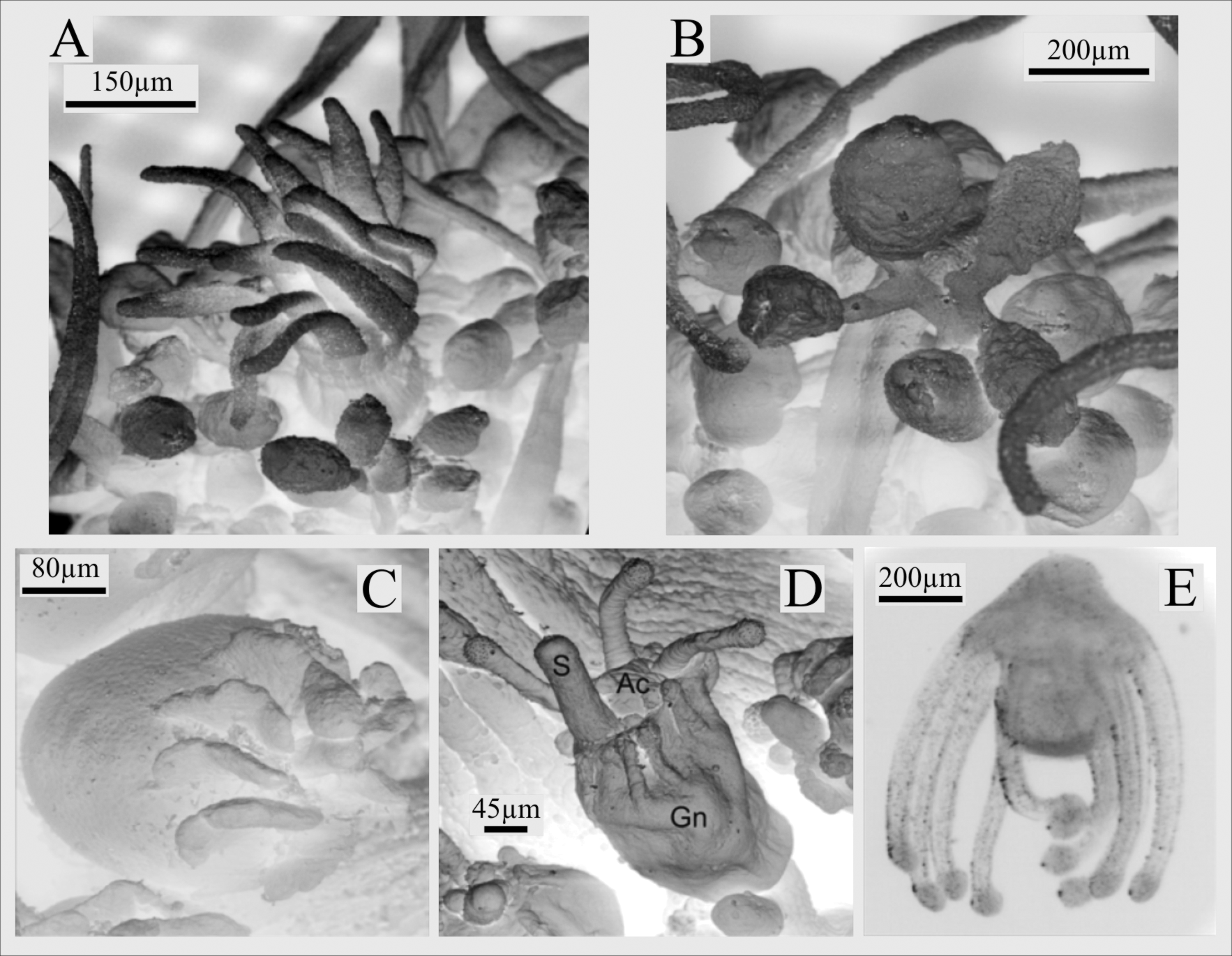
There are inconsistencies in the description of the blastostyles, either characterized as unbranched ( Ewer 1953 and Millard 1966 for E. ralphi ; Brinckmann-Voss 1970, Hirohito 1988, and Petersen 1990 for E. crocea ) or branched ( Bale 1884, Millard 1975, Watson 1980, Migotto & Silveira 1987, and Petersen 1990 for E. ralphi ; Schuchert 1996 for E. crocea ) ( Table 4). Descriptions of the neotype of E. ralphi from Melbourne ( Australia) have different ways to describe the blastostyles, characterized either as “[...] only occasionally branched” ( Schuchert 1996: 109; our underline) or as “mature blastostyles branched [...]” ( Watson 1980: 61), or (“usually unbranched, but some branching can occur”, Schuchert 2010, p. 359). Specimens from SWAO have unbranched blastostyles ( Figure 4 View FIGURE 4 A–B), similar to those described by Allman (1871) and contrasting with the long bunches of gonophores described for other localities ( Calder 1971, Millard 1975, Watson 1982, Petersen 1990). Millard (1959) commented on the difficulty of differentiating primary and secondary pedicels, which may explain the challenges of characterizing the ramification of blastostyles.
(shaded rows), expressed as minimum–maximum values. Length and diameter are given in millimeters. T = Tubularia ; E
= Ectopleura .
Hydranth Tentacles
Locality length diameter aboral oral
(n) number length number length Hydrocaulus Gonophores
Locality length diameter length diameter (n) proximal medial distal
São Vicente 14–35 240–300 300–400 340–540 260–500 180–300 (10) (20.7±6.17) (280±23.09) (350±28.67) (436±65.18) (368±68.77) (228±39.1) Author Female Male Blastostyles Actinulae (Original reference to species) gonophore gonophore
Agassiz (1862) (Paripha 6–10 crests no crests simples or branched
crocea )
Allman (1871) 8 apical processes 4 small tubercles no oral tentacles
Bale (1884) ( T. ralphi ) 4tubercles branched
Allen (1900) ( Paripha crocea ) 6–8 crests no crests branched
Torrey (1902) ( T. crocea ) 8 crests top spherical, smooth or
with processes
Stechow (1907) ( T. sagamina ) 8 tubercles 6 tentacless
Stechow (1925) ( T. australis ) 4 small tubercles 8 aboral, 5 oral tentacles
Hargitt (1927)
The morphology of the female gonophores ( Figure 4 View FIGURE 4 C) is a diagnostic feature for E. crocea ( Rees & Thursfield 1965, Schmidt 1971, Petersen 1990, Schuchert 2001, 2010). Nevertheless, some variation may be observed, mainly because of the development of the gonophores, or due to their contraction ( Torrey 1902, Schuchert 1996). It is hard to identify tubulariids without gonophores ( Watson 1982), and this may be the cause of misidentifications between E. crocea and Ectopleura larynx (Ellis & Solander, 1786) , especially for the Northwestern Atlantic (e.g. Fraser 1944).
Male gonophores do not present apical crests ( Figure 4 View FIGURE 4 B; see also Agassiz 1862, Brinckmann-Voss 1970, Calder 1971, and Schuchert 1996, 2010 for E. crocea ; Ewer 1953, Watson 1980, 1982, Migotto & Silveira 1987 for E. ralphi ), although in some cases they are described with small apical processes, varying in size and development between colonies, and even in the same colony ( Table 4) ( Allman 1871, Hirohito 1988, and Petersen 1990 for E. crocea ; Stechow 1925 and Millard 1975 for E. ralphi ).
Larval characters, such as tentacles, were already used to separate E. ralphi and E. crocea . A vague note in Schuchert (1996: 109, appears to refer to the observations of someone else) states that “the actinulae of E. ralphi , however, are reported to have rudiments of oral tentacles which are absent from E. crocea ”. Other data refer to variations in morphology and number of tentacles of the actinulae ( Ewer 1953, Brinckmann-Voss 1970, Millard 1975, Watson 1980, 1982, Migotto & Silveira 1987, Petersen 1990, Schuchert 1996, 2010; see Table 4). Presumably “the oral tentacles will develop anyway immediately after the release of the actinula and the presence or absence in liberated ones is thus only a matter of timing” ( Shuchert 2010, p. 361), an interpretation that attenuates the importance of the variation. The actinulae of the SWAO present 8–10 ( Brazil) or 8–11 ( Argentina) capitate aboral tentacles, depending on their development, and do not have rudiments of oral tentacles ( Figure 4 View FIGURE 4 D– E), but the variability of this character was never strictly assessed.
Detailed studies on anatomical and histological characters have corroborated previous observations. Among these, the histological preparations confirmed that the oral tentacles are circular in a transversal section ( Figure 5 View FIGURE 5 A–B), while the aboral tentacles are squared ( Figure 5 View FIGURE 5 C), as described by Petersen (1990) for E. crocea . Also, transversal sections of the hydrocaulus have shown that its coenosarc is split into two longitudinal chambers ( Figure 5 View FIGURE 5 D), as already noted before ( Ewer 1953 for E. ralphi ; Allman 1871 and Schuchert 1996 for E. crocea ), although this feature was considered to be inconstant in number and size ( Millard 1959, 1975 for E. ralphi ; Campbell & Campbell 1968, Hirohito 1988, and Petersen 1990 for E. crocea ).
Cnidome. The cnidome was uniform throughout all studied populations from SWAO ( Figures 3 View FIGURE 3 A, B, D, E). Literature data for E. crocea present few discrepancies ( Table 5 View TABLE 5 , contrasting with Table 6 View TABLE 6 ), for instance a cnidome restricted to stenoteles and desmonemes ( Brinckmann-Voss 1970) and a potential contamination by microbasic euryteles ( Schuchert 1996; in Schuchert 2010, p. 360, they are referred to “rare euryteles”).
Schuchert (1996) length 8.0–9.5 9.0–9.5 6.5–7.0 5.0–6.0 5.0–5.5 9.0 ( Ectopleura crocea ) width 7.0–9.5 3.0–4.0 5.0–5.5 3.5–4.5 3.0–3.5 4.0
São Vicente Itanhaém Peruíbe Cananéia Ilha do Mel Basitrichous length 8.58–10.74 6.45–10.41 7.77–12.12 8.45–10.55 *1 8.14–11.86 (9.43±0.48) (8.39±0.67) (9.39±0.67) (9.41±0.46) (9.76±0.8) Stenoteles length 5.57–7.34 5.59–7.22 5.31–7.43 5.45–7.82 5.48–7.64 (6.29±0.32) (6.29±0.31) (6.36±0.37) (6.52±0.44) (6.45±0.41) (small) width 4.2–5.96 4.27–5.5 4.14–6.67 4.33–6.05 4.49–6.04 (4.93±0.31) (4.85±0.27) (5.07±0.4) (5.06±0.36) (5.27±0.32) Basitrichous length 7.06–11.6 7.67–10.21 7.22–10.78 8.2–9.03 *2 7.68–11.97 (9.32±0.74) (8.67±0.4) (9.46±0.63) (8.62±0.59) (9.79±0.79) width 2.87–5.24 3.19–4.81 3.04–4.94 3.75–4.09 *2 2.94–5.67 (3.95±0.46) (3.91±0.31) (3.91±0.37) (3.92±0.24) (4.46±0.49) Oral Stenoteles length 7.37–11.88 7.43–11 7.36–11.37 8.27–11.47 *3 7.99–11.72 (9.72±0.89) (9.53±0.58) (9.68±0.68) (10.2±0.77) (10.61±0.59) tentacles (large) width 6.07–10.29 6.45–9.86 6.53–10.4 6.21–10.31 *3 7.07–10.42 (8.36±0.86) (8.23±0.66) (8.81±0.71) (8.79±0.86) (9.37±0.6) Stenoteles length 5.22–7.31 5.34–6.96 5.52–7.93 5.66–7.2 *4 5.64–7.47 (6.19±0.4) (6.06±0.3) (6.29±0.49) (6.4±0.37) (6.42±0.38) (small) width 4.24–6.5 4.1–6.45 4.33–7.02 4.34–5.82 *4 4.41–6.46 (5.03±0.37) (4.84±0.32) (5.14±0.53) (5.06±0.34) (5.3±0.45)
Guaratuba Itapoá Penha Bombas Mar del Plata Basitrichous length 8.04–12.19 8.91–11.91 8.65–11.56 7.37–10.61 7.81–10.43 (9.94±0.75) (10.6±0.6) (10.12±0.58) (9.24±0.56) (9.37±0.52) width 3.06–5.87 3.73–5.82 3.37–5.29 3.54–5.33 3.17–5.26
(4.41±0.5) (4.69±0.4) (4.4±0.4) (4.28±0.37) (4.35±0.43)
Aboral Desmoneme length 3.94–6.81 4.57–6.66 4.51–6.6 4.67–6.54 4.02–6.17
(5.14±0.53) (5.46±0.39) (5.67±0.41) (5.68±0.44) (5.2±0.37)
tentacles width 2.53–4.49 3.2–5.13 3.2–4.9 3.2–4.85 3.08–4.36
(3.67±0.46) (4.14±0.33) (4.12±0.38) (4.08±0.31) (3.67±0.28) Stenotele length 5.02–7.3 5.77–7.31 5.37–7.66 5.13–7.69 5.26–6.96
(6.19±0.47) (6.62±0.3) (6.47±0.42) (6.4±0.42) (6.17±0.32) (small) width 3.66–5.69 4.83–8.09 4.3–6.06 4.27–6.04 4.24–5.78
(4.72±0.42) (5.49±0.47) (5.01±0.35) (5.0±0.37) (4.88±0.3) Basitrichous length 7.9–11.16 7.72–13.05 7.94–11.38 7.8–11.34 7.65–14.73 (9.38±0.7) (10.38±0.7) (9.7±0.77) (9.39±0.65) (9.32±0.94) width 2.87–5.18 3.51–5.56 3.42–5.52 3.41–5.73 3.28–7.1
(4.21±0.45) (4.55±0.47) (4.28±0.41) (4.23±0.4) (4.24±0.55)
Oral Stenotele length 8.64–11.87 8.19–12.15 9.25–12.5 8.98–12.22 8.68–12.09 (10.31±0.58) (10.29±0.84) (10.8±0.56) (10.3±0.63) (10.42±0.71)
tentacles (large) width 7.64–9.87 7.05–11.16 8.19–11.38 7.64–10.38 7.69–10.49 (8.67±0.56) (9.19±0.8) (9.76±0.61) (9.09±0.52) (8.98±0.59) Stenotele length 5.03–7.08 5.09–7.37 5.28–7.22 4.93–7.48 5.23–7.26
(6.12±0.41) (6.4±0.46) (6.32±0.38) (6.18±0.47) (6.19±0.37) (small) width 3.2–3.2–6.00 4.21–7.13 4.43–5.78 3.9–6.02 4.05–5.84
(4.6±0.43) (5.34±0.4) (5.07±0.32) (4.95±0.43) (4.96±0.38) The basitrichous isorhizas found in SWAO specimens were characterized for “ Tubularia larynx ” Ellis & Solander, 1786 as “pseudo-microbasic b-mastigophore” ( Östman et al. 1995: 166), possibly because of the basal spines of the tubule, giving the false appearance of a shaft under light microscopy (Östman 1987). The types isorhiza/anisorhiza and basitrichous/b-mastigophore are sometimes suggested as overlapped categories ( Cutress 1955, England 1991).
The cnidome has been suggested to be a valuable taxonomic tool, even in more restricted geographic scales (Östman et al. 1987), but for the SWAO region, neither cnidome, nor dimensions of the hydroid, present any kind of geographic structure. Specimens from the states of São Paulo ( São Vicente, Itanhaém, Peruíbe e Cananéia) and Santa Catarina (Itapoá and Penha) have the smallest and largest nematocysts, respectively ( Figure 6 View FIGURE 6 A; Table 6 View TABLE 6 ). Comparing general dimensions of the hydroid ( Figure 6 View FIGURE 6 B; Tables 2-3 View TABLE 2 ), specimens from Guaratuba and Mar del Plata have the lowest and highest values, respectively.
DNA. Twenty-two specimens were analyzed for each marker. ITS1+ 5.8S (332bp) resulted in a total of six haplotypes, with the Argentine haplotype identical to that of Santa Catarina, both basically with a distance of three base pair indel from all others ( Figure 7 View FIGURE 7 A). The genetic diversity found among the six haplotypes was only 0.1% ( Table 7 View TABLE 7 ).
COI (489bp) resulted in a total of 11 haplotypes. Polymorphic sites revealed no saturation ( Table 7 View TABLE 7 ), and 20 substitutions, with one synonymous transversion and four non-synonymous transitions (two replacements of Valine per Isoleucine, both aliphatic/hydrophobic; two replacements of aromatic Phenilalanine per aliphatic Leucine, both hydrophobic). These five changes were detected in only five Brazilian haplotypes. The COI haplotype network also shows low genetic distances between haplotypes ( Figure 7 View FIGURE 7 B). It is interesting to notice that the genetic pairwise difference between the most distant sites (northernmost Macaé and southernmost Mar del Plata) is lower than those among closer intermediate localities ( Table 8 View TABLE 8 ). Corrected average of pairwise differences and pairwise fixation indexes between localities showed no significant values ( Table 8 View TABLE 8 ). A recent publication included samples of Ectopleura from both South Africa (southern hemisphere, expected to be geographically more related to E. ralphi ) and California (northern hemisphere, expected to be geographically more related to E. crocea ), and found no genetic difference between these two populations (Nawrocki & Cartwright 2012).
1Total number of mutations detected
2Singleton variable sites
3Parsimony informative sites
4Number of sites with insertion/deletion of bases 5Relation between synonymous/non-synonymous amino acid changes 6Nucleotide diversity/standard deviation
General discussion and concluding remarks. We have observed no significant difference or geographical patterns between Brazilian E. ralphi and Argentine E. crocea for both morphological and molecular data. Therefore, SWAO populations of Ectopleura likely belong to the same species.
Another important question is whether both species are valid. Schuchert (1996: 109) remarked that “until further, preferably also molecular, analysis has been made it seems advisable to keep both species separate”. For Petersen (1990:176), “despite the obvious similarities between E. ralphi and E. crocea it seems best at present to keep the two species separate since the differences listed above appear to be constant and are shared by population of E. ralphi in both Australia and South Africa ”. Traditionally, the majority of the records of E. crocea and E. ralphi are from the Northern and Southern hemisphere, respectively ( Figure 1 View FIGURE 1 ). The fragmented and disjunct distributions of the species could also be explained by bioinvasion processes, either human or naturally mediated ( Hewitt 2002, Ruiz et al. 2006, Marques, 2011, Mead et al. 2011, Rocha et al. 2013).
Considering our current knowledge of E. crocea and E. ralphi , we see no evidence to keep them apart. Considering them as synonymous, the binomen E. crocea would have nomenclatural priority. This was first proposed by Bouillon et al. (2006: 252) and reiterated by Schuchert (2010, that considered as a “new syn.”, p. 357). The proposal by Bouillon et al. (2006) offered no concrete evidence or arguments supporting the synonymy. In contrast, Schuchert (2010) proposed a long synonymic list for E. crocea , but his analysis was actually based on very few specimens (n=12) from four restricted regions ( Australia, South Africa, Mediterranean and Atlantic USA) – even in this restricted sample the phenotypic variability reported is impressive, which is also consistent with a composite of species (see Tables 1 View TABLE 1 and 4 for a summary). Schuchert (2010, p. 359) listed as diagnostic characters of E. crocea the “female sporosacs usually bearing six to eight crest-like processes around distal opening, several eggs or embryos per sporosac”.
In this study we provide substantial evidence that two different SWAO “populations”, previously assigned to E. crocea and E. ralphi , are the same. Nevertheless, whether these two different “populations” correctly represent the world nominal species E. crocea and E. ralphi is difficult to assess, and has to be considered conjectural. The proposed synonymy between E. crocea and E. ralphi ( Bouillon et al. 2006, Schuchert 2010) have not been based on strict taxonomical procedures, i.e., neither were based on the study of the type specimen of E. ralphi , nor on broad geographic analysis considering extensive phenotypic variation of abundant material of the species, nor on broad molecular analysis from a wide geographical range. Therefore, the ad hoc proposal of the synonymy, even though presently accepted by us, has to be considered tentative and subject to further assessment.
TABLE 1. Literature data of morphometric and meristic information for “ E. crocea ” (white rows) and “ E. ralphi ”
| Author (Original reference to species) | Hydrocaulus length diameter | Aboral tentacles number length | Oral tentacles number length | Locality |
|---|---|---|---|---|
| Agassiz (1862) ( Paripha crocea ) | 63.5–88.9 | 24 | 24 | Boston harbor, USA |
| Allman (1871) ( T. mesembryanthemum ) | 101 | 20–24 | 24 | Gulf of Spezia, Italy |
| Bale (1884) ( T. ralphi ) | 76–101 | Hobsons Bay, Australia | ||
| Allen (1900) ( Paripha crocea ) | 63.5–76.2 | 16–24 | Woods Holl, USA | |
| Torrey (1902) ( T. crocea ) | inconstant | 24 top | 15–18 | San Francisco and San Diego Bays, USA |
| Stechow (1907) ( T. sagamina ) | 150 | 50 9.0 | 20–25 | Misaki, Japan |
| Stechow (1925) ( T. australis ) | 50–80 | 20 | 16–18 | Fremantle, Australia |
| Hargitt (1927) ( T. mesembryanthemum ) | 35–50 | 20–25 | 20–25 | South China |
| Ewer (1953) ( T. warreni ) | 100 top 0.22–0.8 | 24–30 4 top | 22–27 0.8 top | Natal, South Africa |
| Millard (1959) ( T. warreni ) | 50 | 18–29 | 15–24 | Natal and East coast, Africa |
| Yamada (1959) ( T. mesembryanthemum ) | 30 | 20 | 10 | Sagami Bay and Seto, Japan; Amoy, South China |
| Yamada (1959) ( T. sagamina ) | 150 | 50 | 20–25 | Sagami Bay and Seto, Japan; Amoy, South China |
| Rees (1963) ( T. crocea ) | 50 | England | ||
| Millard (1966) ( T. warreni ) | 17.5 1.0 | East cost of South Africa | ||
| Brinckmann-Voss (1970) ( T. crocea ) | 28 top | 17–20 | Italy, England and France | |
| Calder (1971) ( T. crocea ) | 100 | 20–24 | 20–24 | Florida and north of Gulf of Mexico |
| Schmidt (1971) ( T. mesembryanthemum ) | 50 | 20–25 | 20–25 | Gulf Aqaba, Red Sea |
| Millard (1975) ( T. warreni ) | 50–100 | 31 top 5 or more | 27 top 1 or more | Durban Harbor, South Africa |
| Watson (1980) ( T. ralphi ) | 120 top 0.3–0.5 | 16–27 4–5 | 15–25 2–3 | Victoria, Australia |
| Watson (1982) ( T. ralphi ) | 120 | 16–27 | 15–25 | Fremantle, Australia |
| Migotto & da Silveira (1987) ( E. warreni ) | 70 top 0.18–0.6 | 12–27 0.4–6.0 | 11–30 0.4–1.9 | Southeastern and Southern coast, Brazil |
| Hirohito (1988) ( T. mesembryanthemum ) | 40–50 | 16–31 | 14–43 | Japan |
| Petersen (1990) ( E. crocea ) | 70 | 22–30 | 18–24 | East and West coast, USA |
| Petersen (1990) ( E. ralphi ) | 16–27 | 15–25 | InkermanCreek,Australia | |
| Schuchert (1996) ( E. crocea ) | 50 0.2–0.6 | 22 top | 20 | New Zealand |
| Bouillon et al. (2004) ( E. crocea ) | 70 | 22–30 | 18–24 | Mediterranean |
| Schuchert (1996) ( E. crocea ) | 30–80, 0.6–0.8, maxi 120 but up 2 | 22–28 (max. 38) | ca. 18 (max. 26) | Australia, South Africa, USA (Atlantic), Mediterranean (Italy, France) |
TABLE 5. Summary of literature data of cnidomes described for “ E. crocea ” (white rows) and “ E. ralphi " (shaded rows). The nematocysts of E. crocea by Schuchert 2010 (p. 360) have the same dimensions of those by Schuchert (1996) and Millard (1975). Measurements in micrometers, min – max. (*): only length reported.
| Author (Original reference to species) | Heterotrichous anisorhiza | Basitrichous isorhiza | Stenotele Stenotele (large) (small) | Desmoneme | Microbasic eurytele |
|---|---|---|---|---|---|
| Ewer (1959) length ( Tubularia warreni ) width | 9.5 * | 9.0 8.0 | 8.0 7.0 (stenotele) | 5.0 4.0 | |
| Brinckmann-Voss (1970) length ( Tubularia crocea ) width | present | present |
TABLE 6. Measurements of nematocysts, in micrometers, by locality. Minimum value – maximum value (average value ± Standard deviation). Number of measurements for each cell of the table is 100, except for those marked with *. (* 1 n = 26, * 2 n = 2, * 3 n = 40, * 4 n = 20.)
| width | 3.11–5.03 (4.03±0.32) | 2.84–4.9 (3.77±0.43) | 3.11–4.89 (3.94±0.3) | 3.38–5.65 *1 (4.34±0.53) | 3.16–6.04 (4.32±0.57) | |
|---|---|---|---|---|---|---|
| Aboral | Desmonemes length | 4.18–5.64 (4.85±0.3) | 3.5–5.5 (4.59±0.43) | 4.46–5.79 (5.1±0.33) | 3.9–5.98 (4.85±0.38) | 4.46–6.0 (5.19±0.29) |
| tentacles | width | 2.62–3.84 (3.35±0.26) | 2.41–4.11 (3.17±0.33) | 2.84–4.48 (3.63±0.28) | 2.69–4.62 (3.48±0.33) | 2.94–4.54 (3.62±0.28) |
TABLE 7. Summary of the genetic polymorphism observed for COI and ITS 1 + 5.8 S from the 22 samples of specimens of Ectopleura from the southwestern Atlantic Ocean in a total of five sampled localities.
| Length in bp | Haplotypes detected | Variable Sites | Mut1 | Singl2 | PIS3 | Indel4 | S/NS5 | π/sd6 | |
|---|---|---|---|---|---|---|---|---|---|
| COI | 489 | 11 | 20 | 20 | 8 | 12 | 0 | 15/5 | 0.012/0.001 |
| ITS1+5.8S | 332 | 3 | 1 | 1 | 1 | 0 | 3 | - | 0.001/0.0007 |
TABLE 8. Pairwise comparisons of localities for the CO 1 marker (10,000 permutations) of specimens of Ectopleura from the southwestern Atlantic Ocean showing no significant genetic differences between them (p> 0,05). Above the diagonal are the genetic distances expressed as corrected average of pairwise differences; below are the pairwise fixation indexes. Probability values are given in parentheses.
| Macaé (RJ) | Juréia (SP) | Paranaguá (PR) | Bombas (SC) | Mar del Plata (ARG) |
|---|---|---|---|---|
| Macaé (RJ) | 4.00 (0.10) | 3.00 (1.00) | 4.00 (0.10) | 4.78 (0.10) |
| Juréia (SP) 0.76 (0.10) | 5.00 (1.00) | 6.00 (0.33) | 7.00 (0.10) | |
| Paranaguá (PR) 0.64 (0.99) | 0.82 (0.99) | 5.00 (0.33) | 6.00 (0.25) | |
| Bombas (SC) 0.76 (0.10) | 0.86 (0.33) | 0.82 (0.33) | 7.00 (0.10) | |
| Mar del Plata (ARG) 0.70 (0.10) | 0.76 (0.10) | 0.64 (0.10) | 0.76 (0.10) |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Ectopleura crocea ( L. Agassiz, 1862 )
| Imazu, Maurício Antunes, Ale, Ezequiel, Genzano, Gabriel Nestor & Marques, Antonio Carlos 2014 |
Pinauay ralphi
| Silveira 2011: 5 |
| Oliveira 2007: 31 |
| Grohmann 2006: 103 |
| Oliveira 2006: 4 |
| Migotto 2002: 10 |
Ectopleura crocea
| Schuchert 2010: 357 |
| Genzano 2009: 37 |
| Bouillon 2004: 104 |
| Schuchert 2001: 43 |
| Schuchert 1996: 107 |
| Petersen 1990: 174 |
Ectopleura ralphi
| Migotto 2001: 289 |
| Schuchert 1996: 109 |
| Petersen 1990: 175 |
Ectopleura warreni
| Grohmann 1997: 230 |
| Rosso 1997: 417 |
| Migotto 1996: 25 |
| Migotto 1987: 101 |
Tubularia warreni
| Millard 1975: 35 |
| Millard 1966: 435 |
| Millard 1959: 299 |
| Ewer 1953: 351 |
Tubularia australis
| Stechow 1925: 196 |
Tubularia sagamina
| Yamada 1959: 16 |
| Stechow 1907: 194 |
Tubularia crocea
| Demicheli 2006: 530 |
| Genzano 2003: 306 |
| Blanco 1994: 182 |
| Genzano 1991: 69 |
| Calder 1971: 24 |
| Brinckmann-Voss 1970: 28 |
| Rees 1963: 1223 |
| Torrey 1902: 42 |
Tubularia gracilis
| Lendenfeld 1885: 597 |
Tubularia ralphi
| Watson 1982: 85 |
| Watson 1980: 60 |
| Bale 1884: 42 |
Tubularia mesembryanthemum
| Hirohito 1988: 18 |
| Schmidt 1971: 32 |
| Yamada 1959: 16 |
| Hargitt 1927: 494 |
| Allman 1871: 418 |
Parypha crocea
| Agassiz 1862: 249 |