Lichenodraculus matti, Braun, Holger, 2011
|
publication ID |
https://doi.org/ 10.5281/zenodo.204924 |
|
DOI |
https://doi.org/10.5281/zenodo.6184017 |
|
persistent identifier |
https://treatment.plazi.org/id/03BB87E2-FFAD-9802-FF0F-B5BA204BFAAA |
|
treatment provided by |
Plazi |
|
scientific name |
Lichenodraculus matti |
| status |
sp. nov. |
Lichenodraculus matti sp. nov.
Braun 2002, 86: Markia or Machima, Drachenschrecke
Braun 2008, 218: Dysoniini , gen. nov., “Drachenschrecke“
Etymology. Dedicated to my friend, the bat specialist Felix Matt.
Specimens and type locality. Male holotype (specimen cbt063s02) 2220 m, leg. H. Braun 6 August 1998; female allotype (cbt063s03), 2000–2500 m, leg. F. Matt 1999, both deposited in the Museo de La Plata, Argentina; male (cbt063s01), ca. 2250 m, leg. F. Matt 12 October.1997 (collected as 2nd or 3rd instar nymph and raised to adult, in collection of the author). All three specimens are from Cordillera del Consuelo, northern border of Parque Nacional Podocarpus, Provincia Zamora-Chinchipe, Ecuador.
Description. General. Most details of this beautiful insect are visible in the photographs ( Figs 1 View FIGURE 1 & 2 View FIGURE 2 ), so that the complex coloration pattern is not described in detail. The originally almost pure white general coloration turns yellowish after death, and the green background gloss of the body fades to dark brown or black.
Head. With large dorsal spine on anterior part of vertex, directed in an about 45-degree angle forward; in front of it the vertex resuming very shortly its dorsal contour and then dropping perpendicularly, forming a minute and more or less square cariniform fastigium (if not the vertex spine itself is considered as the fastigium). Below this structure, between the antennal sockets, there is a tiny and obtusely conical fastigium of the frons, in dorsal view (which is slightly obstructed by the vertex spine) not projecting beyond the sockets. Eyes in life light grey (darkening after death), with fine dark vertical stripes. White maxillary palps in life sometimes with carmine red tinge. Antennal scapes long (approximately as long as large vertex spine), antenna tips with sigmoid curl.
Thorax. Anterior margin of pronotum developed as a large spine, about as long as vertex spine but broader in lateral view (entire prozona at base); metazona elevated bulgingly from mesozona, metazona concave; disk in dorsal view not medially restricted and lateral lobes perpendicular. Sternum pubescent.
Wings. Tegmina roughly three times as long as broad, in expanded state arcuate with parallel margins (costal margin convex and anal margin concave), with terminal concave truncation, hence in lateral view upper tegmen tip conspicuously pointed. Stridulatory area of male right tegmen mostly transparent. The female’s right tegmen on dorsal side at the base, in the middle of the part that is usually covered by the left tegmen, several veinlets at the anal margin with very small stridulatory pegs (eight at the most proximal one). Hind wings longer than tegmina, hyaline, the tip that projects in folded position beyond tegmina with equal coloration as the latter (white venation on partly blackened background).
Legs. Fore coxae with spine. Fore femora on inner ventral margin with two spines; genicular lobes with long spines, the internal spine considerably longer than the external one. Tympana of fore tibiae blackened except for ventro-caudal margin, dorsal margin of tibiae at lower part of ear region with two curved spines, the internal one twice as long as the external one. Ventral margin with a few very small spines. Middle femora on ventral outer margin with two curved spines, and sometimes with a third and much smaller spine (on left middle femur of the female specimen); genicular lobes with spines, the external one twice as large as the internal one. Middle tibiae dorsally and close to the base on the outer margin with two curved spines, coinciding with two much smaller spines on the inner margin. Hind femora with ventral spines increasing in size from base to tip, the basally broadened ones in distal half being very large and strongly curved, and strongly sticking out, especially the outer ones; inner and outer genicular lobes with long spines of equal length. There is no difference in leg spination between males and females.
Abdomen. Male cerci long and strongly upcurved, at the base dorsally hollowed out, with the inner edge sporting there a flattened tooth. Male subgenital plate with minute styli. Female ovipositor broad, uniformly upcurved, only very little narrowed toward the tip with almost parallel margins, terminally broadly rounded, almost entire dorsal margin and final millimetre of ventral margin delicately serrate.
Measurements. Pure body length (without wings and terminalia) 17 to 20 mm; total length (wings included) about 30 mm; tegmina 19–21 mm; hind femora 16–19 mm (with minimum values referring to males and maximum values to the female); antennae 30–40 mm. Further measurements can be inferred from figure 2.
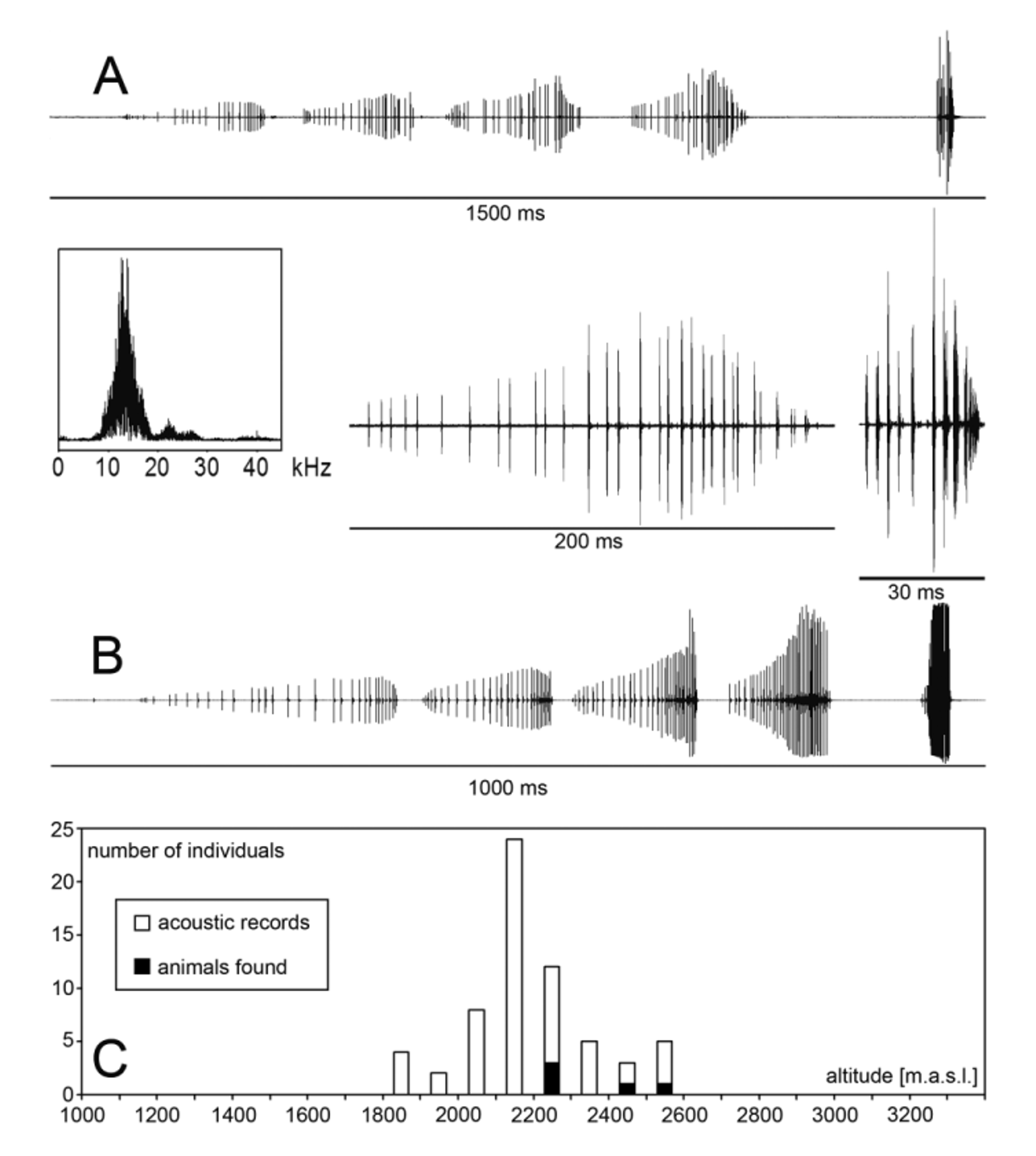
Song. In comparison to most other Tettigoniidae species encountered in the investigation area, that lead a nocturnal life, this katydid calls in the daytime, sharing this activity with only one other phaneropterine species, Syntechna cf. angulata , ocurring in the same montane rainforest habitat. Like this latter species, the little lichen dragon was found calling especially during the rare periods of sunshine. The illustrations ( Fig. 3 View FIGURE 3 A, B) show the typical call. It consists of a crescendo of four longer syllables followed after a longer interval by another short syllable. Separated by short pauses such calls are produced almost continuously. Since the carrier frequency spectrum between 10 and 20 kHz lies in the human audio range, this continuous diurnal song reminds a little bit of a calling cicada (in Fig. 3 View FIGURE 3 the call in the recording of the second male is slightly shorter due to higher temperature, but the carrier frequency remains constant).
Ecology. The conspicuous song facilitated the acoustical assessment of the otherwise inconspicuous katydid’s presence in the steep investigation area and revealed its entire altitudinal distribution ( Fig. 3 View FIGURE 3 C). Using field glasses once the calling male was encountered sitting high on a tree, another male calling from a small tree at higher elevation could be captured, and a third one escaped flying. The altitudinal distribution from 1800 to 2600 m coincides with the occurrence of an arboreal lichen ( Usnea sp.). This is not a mere coincidence but reflects two related aspects of this katydid’s interesting life history. The first individual discovered was an 8 mm tiny nymph that attracted attention when moving slowly and erratically through a small patch of terrestrial lichens ( Cladina or Cladonia sp.) at night. Numerous of the dispersed lichen aggregations covering the floor of small clearings in the more open forest on the cordillera ridge in this altitude (2200–2500) were carefully searched, but no further individual could be found. Much more probably these katydids live in the already mentioned epiphytic lichen that is much more abundant and grows on practically all the trees in the respective elevation zone. Sitting on such lichens the Lichenodraculus nymph was almost invisible with its bizarre tergal spines, pale overall body coloration, and bright pattern of fine stripes on dark glossy greenish background. It moved very slowly and somewhat hesitant and trembling. After careful antenna probing it sometimes jumped from the substrate into the open in laboratory situations. Remarkably this nymph fed exclusively on different species of lichens, although it was offered a great variety of different leaves, herbs and flowers, and also fresh cucumber slices. Living on this select lichen diet the small Lichenodraculus encountered in the night of October 12, moulted November 26, December 25, and finally on January 24 to adult. Five days later it sung for the first time and accepted also cucumber for alimentation. A second smaller nymph (4–5 mm body length, 1st or 2nd instar), possibly belonging to the same species, indicated by similar appearance and same characteristic antenna posture, actually was found on this arboreal lichen species on a fallen branch. It even had finely ramified antennae, as if they were real lichen branchlets. Unfortunately it died very soon and got lost.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.