Dorippoidea, MacLeay, 1838
|
publication ID |
https://doi.org/10.11646/zootaxa.3665.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:8358B363-BEE3-416D-96CA-8614E38B61D5 |
|
persistent identifier |
https://treatment.plazi.org/id/03BB9C75-FF8B-FFF9-FF78-FD7EFA97F852 |
|
treatment provided by |
Felipe |
|
scientific name |
Dorippoidea |
| status |
|
Superfamily Dorippoidea View in CoL
In both Dorippidae and Ethusidae the genital area is visible in dorsal view as a result of the dorsal position of the P4 and P5 and of the first abdominal somites.
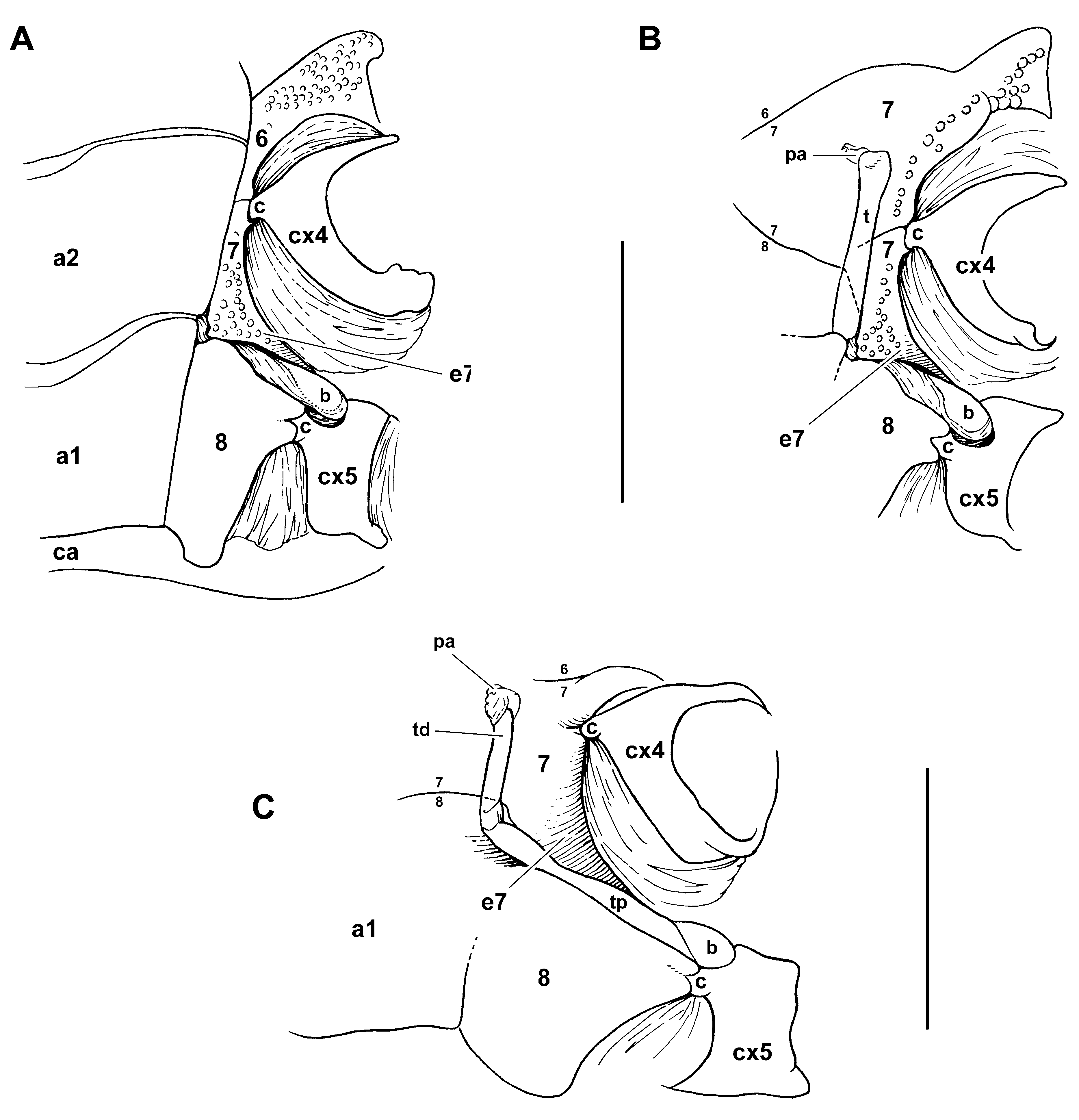
Family Dorippidae View in CoL . The male gonopore is coxal with several coxo-sternal modalities of penis protection (see Modalities of penis protection: Coxo-sternal penial tube). The penis typically consists of four portions: (1) proximal sclerotised moveable penial bulb (the “penial tubercle” of Guinot 1978a: 248; 1979a: 195, figs. 28, 46F; 1979b: fig. 2A); (2) membranous portion of variable length, which may be partially covered by the bulb; (3) long penial tube, typically at an angle, and (4) soft terminal papilla, which may be rather long when fully everted. The bulb constitutes a protection at the emergence of the penis. The penial tube runs along a deep groove on the lateral side of the G1 basipodite, its distal end prolonging into a soft papilla inserted in a lateral foramen located at the base of the G1 endopodite. The G2 is inserted in a foramen located along the mesial side of the G1. The G1 has a long protopodite that consists of an elongated coxopodite, with a visible articular condyle, and a well-developed basipodite, which encircles most of the twisted endopodite. The G2 also has a long protopodite (long coxopodite + basipodite). The long protopodites of both G1 and G2, which show a similar organisation among all dorippoids, are related to the dorsal position of the first three abdominal somites.
That the male gonopore is coxal in Dorippidae View in CoL was not readily recognised by carcinologists. De Haan (1833: xvii; 1841:120) indicated in his diagnosis a male orifice opening “ in sterno ”. According to Miers (1886: 326) the vasa deferentia “are exserted from the sternum” and according to Alcock (1896: 273) they “perforate the 5th sternite on either side”. Conversely, Ortmann (1890 –1901: 1157), Bouvier (1940: 195), and Barnard (1950: 387) stated that the dorippid male genital openings were coxal. Calman (1900: 29) observed a “penial tube” lying “between two processes of the sternum, which may meet above and form a complete ring”. Ihle (1916: 106, 112) also noted the presence of a “tube” in the dorippids. The penis passing “below the sternite 8, like under a bridge” was observed in Paradorippe granulata View in CoL by Serène & Rohmimohtarto (1969: 17, fig. 29). Yet Balss (1957: 1608) indicated a sternal location for the gonopore, “at the vicinity of the coxae of P5”, as well as Glaessner (1969: R492). Curiously, the male gonopore location and the modalities of penis protection were not mentioned by H. Milne Edwards (1837a: 151) when establishing the tribe Dorippiens , or by Bouvier (1897a, b; 1898), nor used in revisions ( Manning & Holthuis 1981; Holthuis & Manning 1990; Chen 1986 a, b, 1988, 1993, 1997, 2000; Chen & Sun 2002). Monod (1933: 36, fig. 5A–D) briefly discussed and figured the penial condition in three dorippid species.
The diagnosis of Dorippidae by Guinot (1978a: 247) was incomplete since it was based only on Medorippe lanata , where the conditions of the P5 coxa and penis differ from those of other dorippids. The configuration in dorippids was briefly redefined by Guinot (1979b: 45, fig. 2A). Holthuis & Manning's (1990: 2) statement that “It was rather surprising to us that so little is known about a group that has attracted the attention of carcinologists for so long” certainly applies to the organisation of the genital region of Dorippidae . Monod (1933: fig. 5B–D, as Dorippe ) had accurately identified the differences in the penis disposition (observed in dorsal view of the crab) between three dorippids assigned at that time to the same genus Dorippe but now distributed in three distinct genera (Guinot & Lai manuscript).
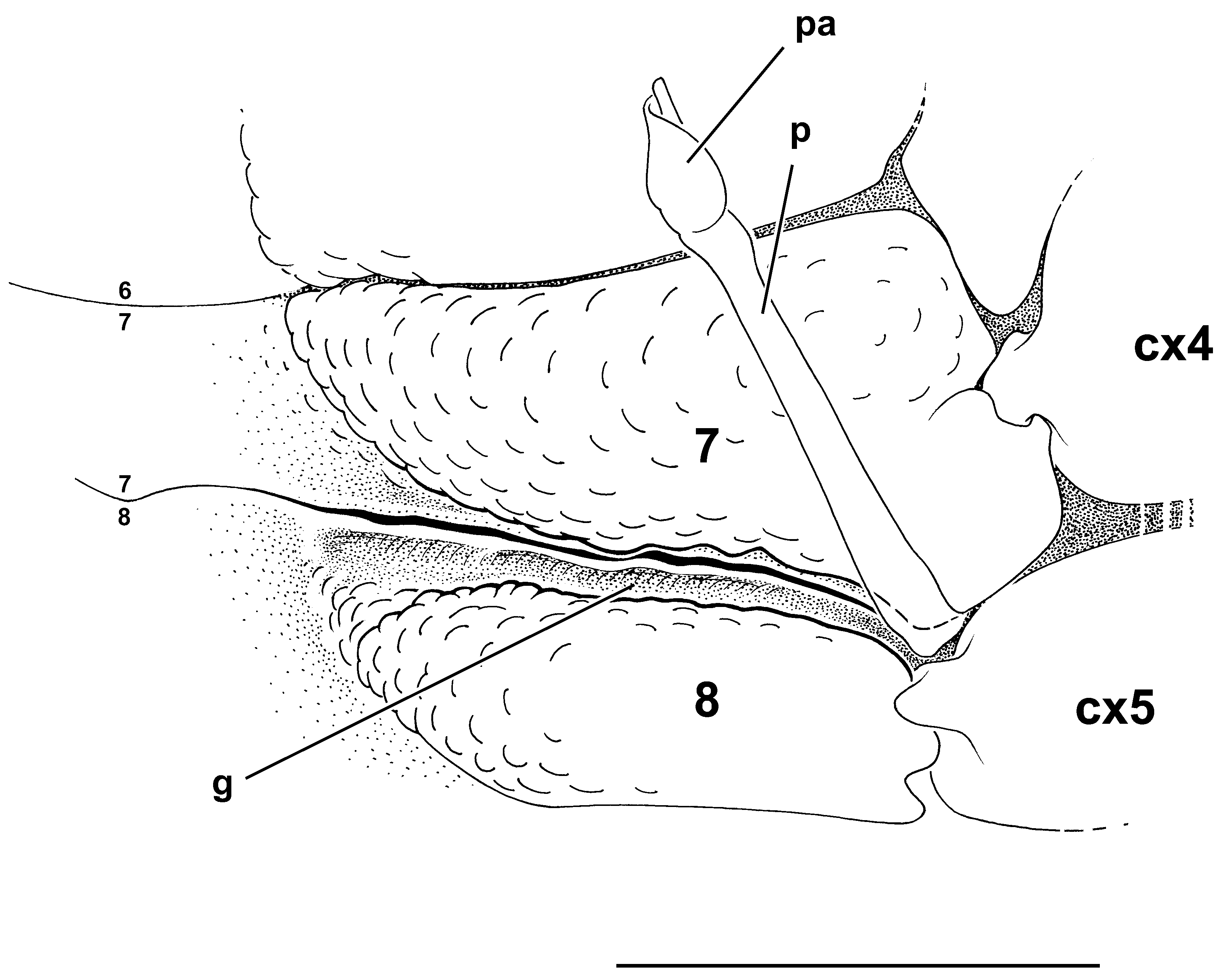
The penial tube in Dorippidae ( Figs. 15–19 View FIGURE 15 View FIGURE 16 View FIGURE 17 View FIGURE 18 View FIGURE 19 ) varies from vertical and unprotected by the thoracic sternum (only covered by the abdomen) to inclined and variously covered by sternal portions. That in most species the penial tube emerges from the sternum led to misinterpret the gonopore as sternal. Only Medorippe Manning & Holthuis, 1981 , and Phyllodorippe Manning & Holthuis, 1981 , have penises that are oriented nearly vertically ( Fig. 15 View FIGURE 15 ). In Medorippe lanata ( Figs. 15A, B View FIGURE 15 , 46B View FIGURE 46 ) sternites 7 and 8 are laterally expanded at the level of the bulb, leaving, however, a marked gap for the passage of the penis; the bulb and a small portion of the penial tube (hidden by setae) are the only exposed portions of the penis; the remaining portion is concealed only by the abdomen. In P. armata ( Fig. 15E View FIGURE 15 ) the penis, thicker than in M. lanata ( Fig. 15B View FIGURE 15 ), is proximally inclined. In both Medorippe and Phyllodorippe , the penis is lying on the steep slope formed by sternite 7.
In Dorippe Weber, 1795 , Philippidorippe Chen, 1985 , Paradorippe Serène & Romimohtarto, 1969 , Dorippoides Serène & Romimohtarto, 1969 , and Neodorippe Serène & Romimohtarto, 1969 , sternites 7 and 8 are expanded over the penis for a rather long distance, close to one another, sometimes joining completely. Sternite 8 is expanded as a bifid process over the P5 coxo-sternal condyle and partially covers the penial bulb. The penis is markedly angled. The thick, sclerotised penial bulb and the sclerotised proximal portion of the tube are exposed, whereas the following portion is covered by the abdomen; a membrane separates the inclined portion of the tube from the vertical portion. Sternites 7 and 8, being very close to each other for a short distance, do not completely join in Dorippe quadriden s ( Fig. 16A, B View FIGURE 16 ). In D. tenuipes Chen, 1980 ( Fig. 17A, B View FIGURE 17 ) sternites 7 and 8 are partially joined; a strong process in sternite 8 partially covers the short penial bulb, overhanging the inclined portion of the penis. In Philippidorippe philippinensis ( Fig. 17D View FIGURE 17 ) sternites 7 and 8 are not completely joined, and the condition is similar to that of D. quadriden s, but the penial bulb is shorter, not well demarcated from the thick, long, sclerotised subsequent portion, which is exposed between sternites 7 and 8. In Paradorippe granulata ( Fig. 17C View FIGURE 17 ) sternites 7 and 8 are close but not in contact, the inclined penial portion is weakly sclerotised, the vertical portion is short, with a long soft papilla.
In Dorippoides facchino ( Fig. 18A, B View FIGURE 18 ) sternites 7 and 8 are in contact for a very short distance, the bulb is more exposed than in Dorippe quadriden s and nearly completely fills the space between the two sternites, and there is no membrane between the inclined and the vertical portions of the penial tube. The G1 has a very elongated coxa, with a visible articular condyle, and a well-developed basis, which encircles most of the twisted endopodite. Dorippoides nudipes ( Fig. 19A, B View FIGURE 19 ) is similar, but the bulb is not as distinct as in Dorippoides facchino and is followed by a more exposed soft portion.
In Heikeopsis japonica the elongated bulb prolongs into the penis without a clear demarcation, the inclined portion is exposed for a long distance, sternites 7 and the expanded 8 are joined only briefly, the vertical portion is covered by the well-developed G1 protopodite, and the small papilla enters the G1 endopodite.
In Neodorippe callida View in CoL ( Fig. 19C View FIGURE 19 ) sternite 8 is very wide, with a large exposed area, and sternites 7 and 8 are close to one another for a long distance (much longer than in any other dorippid), so that the penis is very long. The vertical portion is covered by the long G1 protopodite. The inclined portion is not completely covered by the sternites, but in a large specimen from Singapore (cw 11 mm; MNHN-B29083), sternites 7 and 8 are partially joined and the penis is completely covered at this level. In Neodorippe simplex View in CoL (see Rahayu & Ng 2002), from Australia (MNHN), the vertical portion is longer. In a smaller (cw 9 mm) male from the same location, the penis, located inside a well-developed but exposed gutter between sternites 7 and 8, is very short, tapering distally and reduced to its inclined portion; there is no vertical portion or a well sclerotised bulb, features indicating a non-adult condition. The lengthening of the penis in Neodoripp e, as well as other features (small size; smooth, flattened, and elongated carapace), somewhat suggest an ethusid, with the difference that in ethusids the penis is for the most part located along sternal suture 7/8 and is never angled. With its two distinct portions (proximal inclined, distal vertical to the body axis), Neodoripp e is a true dorippid. The other characters of Neodorippe View in CoL , the “leaf-porter crab” (see Ng & Tan 1986; Ng & Rahayu 2002), are typically those of dorippids, even with modified P4 and P5 for carrying leaves and with a subchelate structure that is dorippid in nature, instead of an ethusid, with only a hook-like dactylus (see Castro 2005). It is noteworthy that for mating the male N. callida View in CoL holds the female with its P4 and P5, which usually are used to carry a leaf for camouflage ( Ng 1987b: 15; Lim et al. 1994: 143). The pattern of Neodorippe View in CoL is clearly linked to the posterior widening of the sternum, especially the strong expansion of sternite 8 (as in Ethusidae View in CoL ), and the condition is typically coxo-sternal.
There is a clear transformation series in Dorippidae View in CoL . The multistate characters do not necessarily indicate close relationships between the genera grouped with respect to a similar condition. In the simplest pattern the penis emerges as a nearly vertical tube from the coxal bulb, which is followed by a short, flexible, membranous area at the proximal part of the penis ( Medorippe lanata View in CoL ; Figs. 15A, B View FIGURE 15 , 46B View FIGURE 46 ), or a small portion of the penis is inclined and the membranous area is more extensive ( Phyllodorippe armata View in CoL ; Fig. 15E View FIGURE 15 ). Sternites 7 and 8 are not joined together in either genus.
In a more elaborate pattern, the penis, after its emergence from the coxal bulb, is divided into two portions by the membranous area: one variously inclined and long, and the second variously vertical and angled as it makes contact with the G1. The inclined portion may be exposed between sternites 7 and 8, or variously concealed by joined sternal parts showing various degrees of junction, including cases where the juxtaposition is complete and the penial tube is completely covered (e.g., Dorippe , Dorippoides , Paradorippe , Philippidorippe ; Figs. 15–18 View FIGURE 15 View FIGURE 16 View FIGURE 17 View FIGURE 18 , 19A, B View FIGURE 19 ). In the most elaborate pattern, the penis, after its emergence from the bulb, has a very long and inclined portion followed by a short vertical portion. Sternites 7 and 8 are joined along most of their length, but leaving a narrow gap ( Neodorippe callida ; Fig. 19C View FIGURE 19 ).
Family Ethusidae View in CoL . The male gonopore is coxal, the condition coxo-sternal. In the numerous species of Ethusa View in CoL and Ethusina Smith, 1884 , as well as in the two species of Parethusa Chen, 1997 View in CoL , and in the monotypic Serpenthusa Naruse, Castro & Ng, 2009 View in CoL . There is a single coxo-sternal modality of penis protection, with only a few variations. Sternite 8 is much more expanded posterolaterally than sternite 7 in all ethusids, and a juxtaposition of sternites 7 and 8 occurs over the penis, which varies in length among species. Sternites 7 and 8 thus form a channel and then progressively separate from each other. The penis, which emerges from the gonopore located above the P5 coxo-sternal condyle, lies in a long, deep gutter formed by a partial invagination of sternite 8 along its anterior margin. The gutter sheltering the penis is visible only when the contiguous sternites 7 and 8 are separated. Most of the penis is concealed, except for the proximal portion and the papilla that protrudes distally. A convergent arrangement, including the presence of a supplementary suture 7/8, is found in other taxa with broad posterior sterna, as in the panopeid Prionoplax View in CoL ( Fig. 14 View FIGURE 14 ).
The papilla emerges at the sternal suture 7/8 level, at its base a small sclerite covering its soft portion
( Ethusa mascarone View in CoL , Fig. 20A, B View FIGURE 20 ). The papilla is small, soft and translucent in many species, as in E. crosnieri Chen, 1993 View in CoL , E. hawaiiensis Rathbun, 1906 View in CoL , E. hirsuta McArdle, 1900 View in CoL , E. indica Alcock, 1894 View in CoL , and most of the examined Ethusina species. This small papilla is sometimes partially sclerotised. In other cases, it is large and partially sclerotised, with a short mesial scale as in Ethusa furca Chen, 1993 View in CoL , and E. longidentata Chen, 1997 View in CoL , or very large and with a sclerotised base as in Ethusa obliquidens Chen, 1993 , E. rosacea View in CoL ( Fig. 21B View FIGURE 21 ), Ethusina abyssicola View in CoL ( Fig. 22A–C View FIGURE 22 ), and E. talismani View in CoL ( Fig. 21A View FIGURE 21 ). A particular shape is found in Ethusa dilatidens View in CoL ( Fig. 21C View FIGURE 21 ), where the soft tip protrudes from a funnel-like, sclerotised structure.
abdomen indicated by dotted line. Scale bars: 2 mm (A, B); 3 mm (C); 1 mm (D).
The conspicuous penial bulb so characteristic of Dorippidae is absent in Ethusidae . The proximal portion of the penis may be non-sclerotised, as in Ethusa dilatidens ( Fig. 21C View FIGURE 21 ), E. hawaiiensis , E. hirsuta , Ethusina microspina Chen, 2000 , and E. robusta ( Miers, 1886) . Or it is partially sclerotised, as in Ethusa granulosa , E. indica , E. longidentata , E. obliquidens , E. rosacea ( Fig. 21B View FIGURE 21 ), Ethusina challengeri ( Miers, 1886) , E. talismani ( Fig. 21A View FIGURE 21 ), E. abyssicola ( Fig. 22A–C View FIGURE 22 ), E. macrospina Ng & Ho, 2003 , E. paralongipes Chen, 1993 , and E. taiwanensis Ng & Ho, 2003 . The proximal portion of the penis may even have a visible, small sclerite on its surface, as in Ethusa mascarone ( Fig. 20A, B View FIGURE 20 ) and E. izuensis Sakai, 1937 .
The organisation of the G1 and G 2 in Ethusidae ( Figs. 20–22 View FIGURE 20 View FIGURE 21 View FIGURE 22 ) is similar to that of Dorippidae ( Figs. 15–19 View FIGURE 15 View FIGURE 16 View FIGURE 17 View FIGURE 18 View FIGURE 19 ). The G1 has a long protopodite, a coxopodite with a visible articular condyle, and an endopodite with a shallow, long mesial groove for the insertion of the G2. The ethusid G2 also has a rather long protopodite. The dorippid G1 shows an extreme diversity, as highlighted by Holthuis & Manning (1990: 6) and Sin et al. (2009: fig. 4). The dorippid G2 is shorter than the G1 but only about half of the G1, straight, without a demarcated flagellum, and with only a small cleft at the apex ( Stephensen 1946: fig. 4B, D; Serène & Rohmimohtarto 1969: fig. 29; Chen 1986: fig. 3f). The ethusid G1 is slender and weakly setose ( Castro 2005: 505, figs. 2C, 3B, 6D, 7, 8, 14B, 15, 21C, 28C, 30C, 31B) or stout and more ornamented ( Castro 2005: 505, figs. 10B, 16C, 18B, 19B, 20A, 23C, 25B, 32D); see also Hendrickx (1989: figs. 1–3). The ethusid G2 is longer than the G1 and has an elongated flagellum ( Castro 2005: figs. 2D, 6C, 8, 14C, 21D, 30D, 31B) or as long as or even shorter than G1 ( Castro 2005: figs. 10C, 16D, 20B, 25C, 32E; see also Hendrickx 1989; Chen 1986 a, b, 1988, 1993, 1997, 1998, 2000; Chen & Sun 2002). The gonopod morphology of Dorippoidea is linked to the long-term evolutionary history of the group (see Position of the Dorippoidea in the Brachyura ).
Ethusa View in CoL , generally considered close to Dorippe View in CoL , had been always included in Dorippinae or Dorippidae View in CoL . The confusion regarding the location of the male gonopores of Dorippe View in CoL and allies also applied to Ethusa View in CoL , being regarded as either coxal (Alcock 1896: 273; Ortmann 1890 –1901: 1157; Bouvier 1940: 195; Barnard 1950: 388), sternal (De Haan 1841: 117, 119; Miers 1886: 326, 328; Alcock 1896: 273; Balss 1957: 1608, 1610), or not described at all ( Rathbun 1937: 75, 89). The same is true for Ethusina View in CoL . Ihle (1916: 113, fig. 64) correctly figured a “ Penis-Tubus ” in Ethusa View in CoL . In erecting the subfamily Ethusinae, Guinot (1977b: 1052) diagnosed the penis as “emerging from the sternum but passing first by the P5 coxa” and later ( Guinot 1979b: 45, footnote, fig. 2B1–B3, 3) the condition was denoted “coxo-sternal” (see also Guinot 1978a: 249; 1979a: 103; Manning & Holthuis 1981: 38; Guinot & Bouchard 1998: 651; Števčić 2005: 105; Castro 2005: 505; Ng, Guinot & Davie 2008: 59).
A main difference between Dorippidae View in CoL and Ethusidae View in CoL involves their mouthparts, notably the shape of their afferent branchial openings ( Fig. 42A, C View FIGURE 42 ). Another difference is the penis, which is long, continuous, oblique (never angled), mostly enclosed in Ethusidae View in CoL , instead of the bipartite, angled dorippid tube. Furthermore, the ethusid penis lies in a gutter excavated on sternite 8 (compare with Fig. 14 View FIGURE 14 ). This arrangement, which is uniform among ethusids ( Figs. 20–22 View FIGURE 20 View FIGURE 21 View FIGURE 22 ), results from the widening of the posterior sternum, especially sternite 8, and the almost complete dorsal junction of sternites 7 and 8 over the penis along most of its length, except for the proximal sclerite and papilla. The papilla enters directly into the base of the G1 ( Figs. 20B View FIGURE 20 , 22C View FIGURE 22 ) in all Ethusidae View in CoL , whereas in Dorippidae View in CoL the long, vertical penis is lodged along the G1 protopodite ( Figs. 15B View FIGURE 15 , 16C View FIGURE 16 , 18C View FIGURE 18 ).
The sternal location of the papilla (coxo-sternal condition) in Ethusidae is not to be confused with the coxal male gonopore. The coxo-sternal disposition is but a mechanism of penis protection, and yet Števčić (2005: 104) perplexingly characterised the male gonopores as “coxo-sternal” in Ethusidae as opposed to “coxal” in Dorippidae .
In both Dorippidae and Ethusidae (Dorippoidea) , the morphology of the penis, G1 and G2, and their interactions are strongly related to both the incomplete folding of the abdomen and the posterior thoracic curvature, leading to a dramatic change in the alignment of the arthrodial cavities, with a dorsal position of P4 and P5.
Molecular analyses ( Fan et al. 2004; Sin et al. 2009) support the recognition of the Dorippidae as monophyletic and the existence of several main clades. A reappraisal of dorippoid morphology has lead to the same results (Guinot & Lai, study in progress).
The different stages of the coxo-sternal condition observed among Dorippoidea attest to their long evolutionary history. See Monophyletic Heterotremata: Superfamily Dorippoidea ; Position of the Dorippoidea in the Brachyura ; Affinities between Dorippoidea and Hymenosomatoidea ; Concealment behaviour: Carrying behaviour: Families Dorippidae and Ethusidae ; Carcinisation and its outcomes: Cephalic condensation.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Dorippoidea
| GUINOT, DANIÈLE, TAVARES, MARCOS & CASTRO, PETER 2013 |
Ethusa
| Ng, P. K. L. & Guinot, D. & Davie, P. J. F. 2008: 59 |
| Stevcic, Z. 2005: 105 |
| Castro, P. 2005: 505 |
| Guinot, D. & Bouchard, J. - M. 1998: 651 |
| Manning, R. B. & Holthuis, L. B. 1981: 38 |
| Guinot, D. 1979: 45 |
| Guinot, D. 1979: 103 |
| Guinot, D. 1978: 249 |
| Guinot, D. 1977: ) |
| Balss, H. 1957: 1608 |
| Barnard, K. H. 1950: 388 |
| Bouvier, E. - L. 1940: 195 |
| Rathbun, M. J. 1937: 75 |
| Ihle, J. E. W. 1916: 113 |
| Miers, E. J. 1886: 326 |