Halicyclops uncus, Ueda & Nagai, 2009
|
publication ID |
https://doi.org/ 10.1080/00222930802585810 |
|
persistent identifier |
https://treatment.plazi.org/id/03BD3F5A-F86C-FFDC-C459-FF30904DFA10 |
|
treatment provided by |
Carolina |
|
scientific name |
Halicyclops uncus |
| status |
sp. nov. |
Halicyclops uncus sp. nov.
( Figures 4 View Figure 4 , 5 View Figure 5 )
Synonym
Halicyclops japonicus, Ishida (2002) , p. 41–42, figures 1a–e.
Type material
One female holotype ( NSMT Cr-18239), dissected and mounted on three glass slides, collected from the Kashima-gawa River estuary on 14 March 2002 , and eight undissected female paratypes ( NSMT Cr-18240) preserved in alcohol, collected from the Midori-kawa River estuary on 15 March 2002 .
Type locality
Kashima-gawa River estuary (33 ° 069370 N, 130 ° 049130 E) in Ariake Bay, Kyushu, Japan. The salinity was 2 psu .
Etymology
The specific epithet uncus , meaning ‘‘hook’’ in Latin, refers to the shape of the acute protuberance on the genital double-somite.
Description
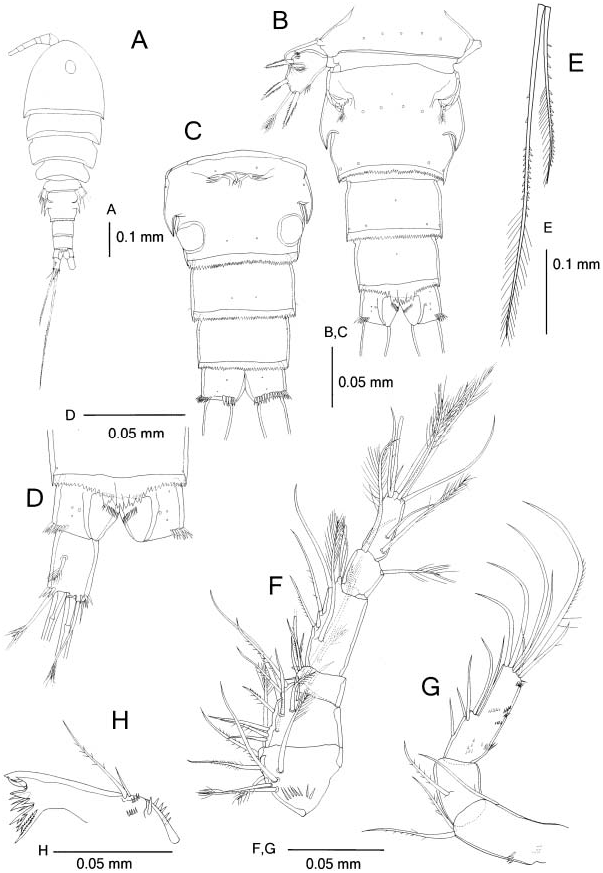
Female. Body ( Figure 4A View Figure 4 ) length 0.66–0.80 mm (holotype 0.69 mm). Prosome L/ W 1.7 –1.9 and 1.5–1.9 times longer than urosome. Forehead round in dorsal view. Genital double-somite ( Figure 4B View Figure 4 ) L/ W 0.8 –1.0, with backwardly directed hookshaped acute process on each side in dorsal view; tip of process not chitinized. Genital double-somite and subsequent two urosomites ( Figure 4B,C View Figure 4 ) with serrate distal frill; mid-dorsal part of frill of fourth urosomite ( Figure 4D View Figure 4 ) extending beyond anal operculum.
Caudal rami ( Figure 4D View Figure 4 ) L/ W 1.5 –1.7; proximal dorsolateral seta slightly shorter than ramus; medialmost terminal seta 0.4 times as long as ramus; medial median terminal seta 1.4 times longer than urosome and about 2.0 times longer than lateral median terminal seta. Middle terminal setae heterogeneously ornamented as follows: proximal half of lateral seta sparsely spinulose on the distal half of lateral margin; distal half of lateral seta spinulose on lateral margin and plumose on medial margin; proximal half of medial seta with two or three spinules on distal part of medial margin; distal half of medial seta spinulose proximally and plumose distally.
Antennule ( Figure 4F View Figure 4 ) with setal formula: I58, II512, III56+spine, IV56+ae, V 52, VI 59+ae; first segment with row of spinules ventrally; fourth segment L/W about 2.0.
Antenna ( Figure 4G View Figure 4 ) coxobasis two rows of spinules on medial and anterior surfaces, respectively; Enp2 L/W about 3.0, 1.6 times longer than Enp1, with several rows of short spinules scattered except on medial side.
Mandible ( Figure 4H View Figure 4 ) with two rows of spinules near palp and row of larger spinules proximally.
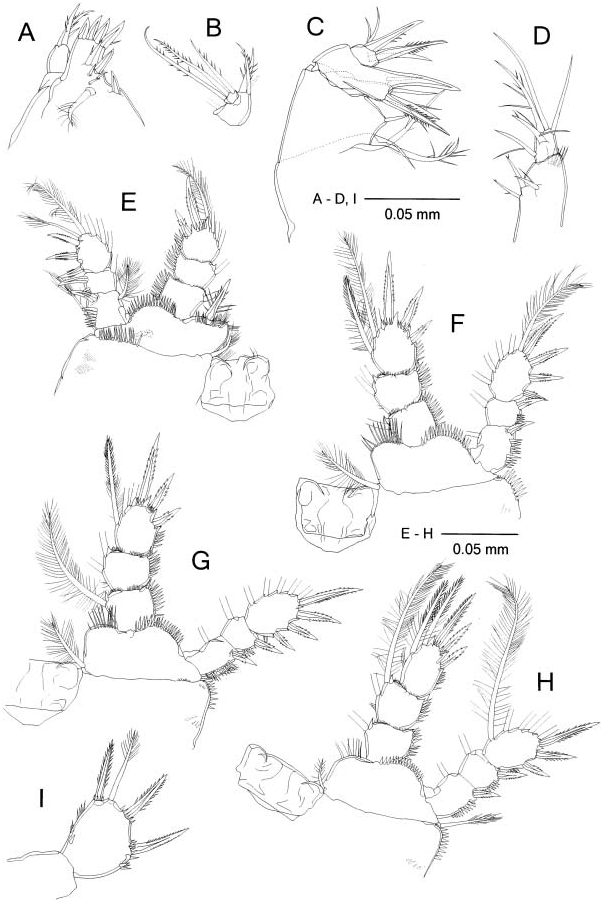
Maxillule ( Figure 5A View Figure 5 ) praecoxal arthrite medially with two strong spines, two thick setae and three spinules around midlength, and with four strong spines apically; palp ( Figure 5B View Figure 5 ) with one proximal and three apical setae on first segment (proximal and one apical setae missing in Figure 5B View Figure 5 ), and three setae on second segment.
Maxilla ( Figure 5C View Figure 5 ) basis and endopod with spine armed with long teeth on both sides; claw of basis naked.
Maxilliped ( Figure 5D View Figure 5 ) with distolateral row of long spinules on syncoxa; endopod L/ W 1.1.
P1–P4 ( Figure 5 View Figure 5 E–H) spine and setal formula of Exp3 as follows: III+1/1/3, III/ I+1/4, III/I+1/4, II/I+1/4; spinules on lateral margins of coxa, distal margins of basis and lateral margins of rami stronger than those of H. continentalis . P1 basis with medial spine reaching midlength of Enp2; spines on Exp1 and Exp2 naked; spines on Exp3 subequal and slightly shorter than segment. Proximalmost medial setae of P2– P3 Enp3 spiniform but more slender than apical spine at distal half. P4 Enp3 ( Figure 5H View Figure 5 ) L/ W 1.4; medial apical spine 1.2 times longer than segment and 1.5 times longer than lateral apical spine; medial setae spiniform at distal half.
P5 exopod ( Figure 5I View Figure 5 ) L/ W 1.3; length ratio of three spines to segment 0.7: seta about as long as segment; lateral and medial spinules thicker than those of H. continentalis .
Other diagnostic features as in H. continentalis .
Remarks
Halicyclops uncus sp. nov. belongs to the thermophilus species group, which is characterized by a well-developed process on each side of the genital double-somite in dorsal view and a 3.4.4.3 spine formula of the P1–P4 Exp3 ( Herbst 1983). Other members are H. thermophilus Kiefer, 1929 , H. spinifer Kiefer, 1935 , H. venezuelaensis Lindberg, 1954 , H. japonicus Ito, 1956 , H. latus Shen and Tai, 1964 , H. antiguanensis Herbst, 1983 , H. dedeckeri Brownell, 1983 and H. soqotranus Baribwegure and Dumont, 2000 . Karanovic (2008) considered H. spinifer , H. japonicus , H. latus , H. dedeckeri and H. antiguanensis as junior synonyms of H. thermophilus . The reasoning behind this was noted in his remarks and can be summarized as follows: according to the revision of the genus by Kiefer (1936), who created H. thermophilus , the nominotypical species of the group, and the second member H. spinifer , both provided in brief descriptions ( Kiefer 1929, 1935), the main differences between the two species seemed to be only the relative lengths of the lateral protuberance on the genital double-somite and of the medial spine on the female P5, which were longer in H. spinifer . However, specimens subsequently described as H. thermophilus or H. spinifer by others, such as Lindberg (1941), had different combinations of these characters or different shapes (spiniform or plumose) of setae on the P4 Enp3. The subsequent species of the group were generally created only by a difference in a single character, that is, the shape of the setae on the P4 Enp 3 in H. japonicus and H. dedeckeri , and the length of the medial spine on the P 5 in H. latus . However, the shape of the setae on the P4 Enp3 ‘‘could not be accepted as only distinguishing character in the genus Halicyclops , as this was also shown to be variable in H. venezuelaensis by D. Rocha (1995).’’
We do not agree with Karanovic (2008) based on three principal conclusions. First, Karanovic (2008) disregarded other important diagnostic characters of the genus, such as the serrate hyaline frill of the fourth urosomite, the L/W of the caudal ramus and that of the P4 Enp3. The caudal ramus of H. japnicus described by Ito (1956) is about twice as long as wide while that of H. thermophilus is almost as long as wide in Kiefer’s figure ( Kiefer 1929, 1936). The posterior margin of the fourth urosomite of H. latus is smooth, which was mentioned by Shen and Tai (1964) as a character distinguishing it from H. japonica . Disregard of the urosomal frill, which is serrate but not so developed at the mid-dorsal part of the fourth urosomite in H. thermophilus ( Kiefer 1936) , is also seen in the synonym list of Karanovic (2008), in which H. spinifer described by Pesce et al. (1996) bearing the well-developed middorsal frill is synonymized. Second, a difference in the shape of setae on the P4 Enp3 as a result of intraspecific variation is unlikely. Karanovic (2008) cited H. venezuelaensis described by Rocha (1995), of which the setae are different from those in the original description of the species ( Lindberg 1954), but failed to cite that Rocha had suggested the possibility of interspecific variation. Although there is currently no way to determine whether the characteristics in question are intra- or interspecific in origin, it is reasonable to regard them as interspecific by considering the following:
(1) as far as we are aware, the degree of variation of these setae within a population has never been recorded, not only in Halicyclops , but also other cyclopoid genera;
(2) specialized setae like spiniform ones on the swimming legs are hypothesized as apomorphies by Abiahy et al. (2006) and are therefore employed as an important diagnosis of a species;
(3) an extremely wide geographic range for a brackish-water Halicyclops species is unlikely because of the extreme barriers of marine, freshwater and land associated with brackish-water habitats.
If Karanovic’s synonymization of H. thermophilus is correct, it would be almost a cosmopolitan species, recorded from Java (type locality), Japan ( Ito 1956, as H. japonicus ), China ( Shen and Tai 1964, as H. latus ), Australia ( Pesce et al. 1996, as H. spinifer ), Somalia ( Dumont and Maas 1987), Uzbekistan ( Mirabdullayev and Getz 1996, as H. spinifer ), Madagascar ( Lindberg 1952), South Africa ( Brownell 1983, as H. dedeckeri ), North America ( Wilson 1958), West Indies ( Herbst 1983, as H. antiguanensis ), South America ( Reid 1985), and so on. Karanovic noted passive dispersal in ship ballast water as a partial cause for its current, very wide distribution. However, such a mode of introduction is very unlikely for the Japanese population ( H. japonicus ), of which the first record (the type locality of H. japonicus ) occurred from a brackish pond on a small island (1 km 2), about 50 km north of the Noto Peninsula, the middle mainland of Japan. In conclusion, it seems more likely that populations hitherto reported as H. thermophilus consist of distinct species.
The most distinguishing characteristic of the new species is a hook-shaped acute process on each side of the genital double-somite, by which the following species are distinguished: H. soqotranus ( Baribwegure and Dumont 2000) and H. venezuelaensis ( Lindberg 1954) , which have blunt and short processes, and H. antiguanensis ( Herbst 1983) , H. japonicus ( Ito 1956) and H. latus ( Shen and Tai 1964) , of which the processes are short and/or produced laterally rather than posteriorly and thereby not hookshaped. The new species is also distinctive by the following: caudal rami of the new species (L/ W 1.5) is longer than in H. antiguanensis [1.25 ( Herbst 1983)], H. dedeckeri [0.9 measured in figure from Brownell (1983)], H. soqotranus [1.0 measured in figure from Baribwegure and Dumont (2000)], and H. venezuelaensis [1.17 ( Lindberg 1954)]; posterior margins of the urosomites of H. latus are smooth ( Shen and Tai 1964); P2–P4 Enp3 of H. japonicus from Hegurajima Island in the Japan Sea ( Ito 1956) is obviously longer (e.g. P4 Enp3 with L/ W 1.9 in contrast to 1.4 in the new species) and has a normal plumose proximomedial seta (spiniform in the new species).
Of the eight other members of the thermophilus group, the remaining two, H. thermophilus and H. spinifer , are difficult to compare because the original descriptions were too brief and insufficient ( Kiefer 1929, 1935). However, subsequent descriptions from various collections around the world were not used for comparison because of the possibility of confusion with other species, because significant differences are apparent among these descriptions. For example, the L/W of the caudal ramus of H. spinifer is 1.22–1.27 by Kiefer (1936, as H. thermophilus spinifer ) from India, whereas it is about 1.0 in Pesce et al. (1996) from Australia, and the lateral spines on the P5 of H. thermophilus are much shorter in descriptions from Madagascar ( Lindberg 1952) and Somalia ( Dumont and Maas 1987) compared to that from Java ( Kiefer 1929). Therefore comparison of the new species should be made primarily with the original descriptions ( Kiefer 1929, 1935) and the subsequent redescription of his own specimen ( Kiefer 1936) even though the available information is limited. Their significant difference from the new species is seen again in the short caudal ramus, of which the L/Ws are about 1.2 for H. thermophilus [measured in the figure from Kiefer (1929)] and 1.22–1.27 for H. spinifer ( Kiefer 1936) . The two species are also distinguishable by the lateral process of the genital double-somite in dorsal view; the processes of H. thermophilus are very short and produced laterally ( Kiefer 1936) and those of H. spinifer are strongly chitinized at the tip ( Kiefer 1935).
The Halicyclops specimen described as H. japonicus by Ishida (2002) from a brackish lake in northernmost Honshu is identifiable to the new species by hookshaped lateral processes on the genital double-somite, caudal rami with the L/ W 1.5, serrate urosomal frills of which the mid-dorsal part of the fourth urosomite partly covers the anal somite. However, it is uncertain whether the Ishida’s H. japonicus specimen ( Ishida 2002) from Yakushima Island, south of Kyushu ( Ishida 1993) is identical to the new species or to that of Ito (1956), i.e. H. japonicus , because Ishida’s illustration shows that the process on the genital double-somite is short and produced laterally as in Ito’s description.
The new species was collected from estuaries of the Kashima-gawa (type locality) and Midori-kawa Rivers , where the salinity ranged from 4 to 11 psu. This species is probably endemic to the Japanese Archipelago because there have been no descriptions identical to the new species from East Asia ( Tai and Chen 1979) .
| NSMT |
National Science Museum (Natural History) |
| V |
Royal British Columbia Museum - Herbarium |
| VI |
Mykotektet, National Veterinary Institute |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Halicyclops uncus
| Ueda, Hiroshi & Nagai, Hidefumi 2009 |
Halicyclops uncus
| Ueda & Nagai 2009 |
Halicyclops japonicus
| Ishida 2002 |
H. soqotranus
| Baribwegure and Dumont 2000 |
H. antiguanensis
| Herbst 1983 |
H. dedeckeri
| Brownell 1983 |
H. dedeckeri
| Brownell 1983 |
H. antiguanensis
| Herbst 1983 |
H. dedeckeri
| Brownell 1983 |
H. latus
| Shen and Tai 1964 |
H. latus
| Shen and Tai 1964 |
H. latus
| Shen and Tai 1964 |
H. latus
| Shen and Tai 1964 |
H. japonicus
| Ito 1956 |
H. japonicus
| Ito 1956 |
H. japonicus
| Ito 1956 |
H. venezuelaensis
| Lindberg 1954 |
H. venezuelaensis
| Lindberg 1954 |
H. venezuelaensis
| Lindberg 1954 |
H. spinifer
| Kiefer 1935 |
H. spinifer
| Kiefer 1935 |
H. spinifer
| Kiefer 1935 |
H. spinifer
| Kiefer 1935 |
H. spinifer
| Kiefer 1935 |
H. spinifer
| Kiefer 1935 |
thermophilus
| Kiefer 1929 |
H. thermophilus
| Kiefer 1929 |
H. thermophilus
| Kiefer 1929 |
H. thermophilus
| Kiefer 1929 |
H. thermophilus
| Kiefer 1929 |
H. thermophilus
| Kiefer 1929 |
Halicyclops
| A. M. Norman 1903 |
Halicyclops
| A. M. Norman 1903 |