Psilorhynchus nudithoracicus Tilak & Husain
|
publication ID |
https://doi.org/10.11646/zootaxa.3686.2.5 |
|
publication LSID |
lsid:zoobank.org:pub:B384D416-CF39-4FAB-B7CD-20C7E667E109 |
|
DOI |
https://doi.org/10.5281/zenodo.6158492 |
|
persistent identifier |
https://treatment.plazi.org/id/03BD6841-041D-FFDF-BD8B-FAF62003F818 |
|
treatment provided by |
Plazi |
|
scientific name |
Psilorhynchus nudithoracicus Tilak & Husain |
| status |
|
Psilorhynchus nudithoracicus Tilak & Husain View in CoL
Figs. 15–20 View FIGURE 15 View FIGURE 16 View FIGURE 17 View FIGURE 18 View FIGURE 19 View FIGURE 20
Psilorhynchus sucatio nudithoracicus Tilak & Husain, 1980: 35 View in CoL , figs. 1–3. Psilorhynchus gracilis Rainboth, 1983: 69 View in CoL , figs. 2–3.
Material examined: 97 specimens, including holotype and 32 paratypes of P. gracilis . Ganges: AMNH 43097, 5 (1 c&s) paratypes of P. gracilis , 20.0– 34.5 mm SL; ANSP 148729, 5 paratypes of P. gracilis , 28.1–37.1 mm SL; FMNH 94285, 5 paratypes of P. gracilis , 5, 30.5–38.1 mm SL; Bangladesh: Dinajpur, Mahananda River at Tetulia, in close vicinity of the Dak Bungalow, 26°28'60.0"N 88°19'60.0"E. KU 29143, 11 (3 c&s), 26.4–32.4 mm SL; Nepal: Jhapa, Mechi River at Bhadrapur, 26°32'17.9"N 88°6'6.1"E. KU 29461, 1, 35.0 mm SL; Nepal: Bardiya, Babai River at Chapang, 28°21'24.1"N 81°42'47.9"E. KU 29505, 1, 32.5 mm SL; Nepal: Nawalparasi, Tribeni, Narayani River, just downstream from Tribeni Barrage, 27°26'35.9"N 83°54'18.0"E. KU 28815, 7, 23.2–27.0 mm SL; Nepal: Banke, Bhurigaon, Rapti River at Madhuwaa/Kanchanpur, 28°4'12.0"N 81°50'48.1"E. KU 40285, 4, 15.2–38.0 mm SL; Nepal: Jhapa, Kankai River at Raj–Marg highway, 26°39'29.9"N 87°52'12.0"E. KU 40662, 3, 44.6–46.9mm SL: Nepal: Narayani, Chitwan, tributary of the Rapti River, Chitwan National Park, 27°34'20.7"N 84°29'47.0"E. UMMZ 205351, 15, 27.6–41.7 mm SL; Bangladesh: Dinajpur, Keratoya River at Bhajanpur, downstream from Indian border, 26°28'0.0"N 88°28'60.0"E. Brahmaputra: NRM 46908, 3, 26.1–45.6 mm SL; India: Assam, Sessa River close to Patiola Village, about 30 km SW of Dibrugarh, 27°18'47.0"N 94°49'46.0"E. UMMZ 205342, holotype of P. gracilis , 50.5 mm SL; UMMZ 205343, 26 (10 examined), paratypes of P. gracilis , 29.4–49.9 mm SL; Bangladesh: Rangpur, Jabuneswari River, downstream from Badarganj Ghat, 25°42’0.0”N 89°5’ 0.0E. UMMZ 205345, 5 (3 c&s) paratypes of P. gracilis , 34.5–40.0 mm SL; Bangladesh: Rangpur, Ghaghat River, 6.5 km E of Rangpur on Badargani Road, 25°45'0.0"N 89°28'60.0"E. UMMZ 205340, 18, 10.4–19.0 mm SL; Bangladesh: Rangpur, Dharla River at Kurigram, 400 m upstream from ferry ghat, 25°47'60.0"N 89°40'0.0"E. UMMZ 244920, 2, 49.0–50.0 mm SL; India: West Bengal, Panga River at Krishibagan, 26°28'22.0"N 88°42'8.0"E. Meghna: UF 180115, 5, 20.0–40.0 mm SL; Bangladesh: Dhaka. Sangu: UMMZ 205337, 2 paratypes of P. gracilis , 25.7–29.7 mm SL; Bangladesh: Chittagong, Chittagong Hill Tracts, Sangu River and small tributary creek ca. 2 km upstream from Bandarban, 22°13'0.0"N 92°11'60.0"E.
Diagnosis. Psilorhynchus nudithoracicus is distinguished from all congeners by its large and flap-like skin folds at the lateral corner of the mouth, which extend further posteriorly than the lower jaw cushion and are covered in large “head of cauliflower” papillae (vs. skin folds not reaching past posteriormost part of lower jaw cushion, non-papilliated or with small dome-like papillae). It is further distinguished from members of the P. nudithoracicus species group by the following combination of characters: two dorsal saddles anterior to dorsal saddle positioned at dorsal-fin origin (vs. one); L+2 row present, composed of 5–10 obvious dark brown blotches (vs. L+2 composed of few indistinct blotches in P. robustus ; absent in P. melissa and P. tenura ); most commonly 17+17 vertebrae (vs. 18+ 16 in P. melissa and P. robustus ; 18+ 18 in P. t e n u r a). It is further distinguished from P. melissa by having irregular dark brown or black markings on the dorsal fin (vs. dorsal fin marked with a dark brown or black band along distal edge), from P. robustus by having dorsal saddles posterior to the dorsal fin poorly developed (vs. dorsal saddles posterior to the dorsal fin well-developed), and by having the anteriormost lateral blotch equal in size or smaller than more posterior blotches (vs. anteriormost lateral blotch larger than more posterior blotches), and from P. tenura by a lower number of principal caudal-fin rays (9–10+9 vs. 9+8), a shorter caudal peduncle (caudal peduncle length 9–13% SL vs. 14–16% SL), the presence (vs. absence) of a short mandibular canal along the anguloarticular, and the presence (vs. absence) of L rows above and below the lateral line scale row.
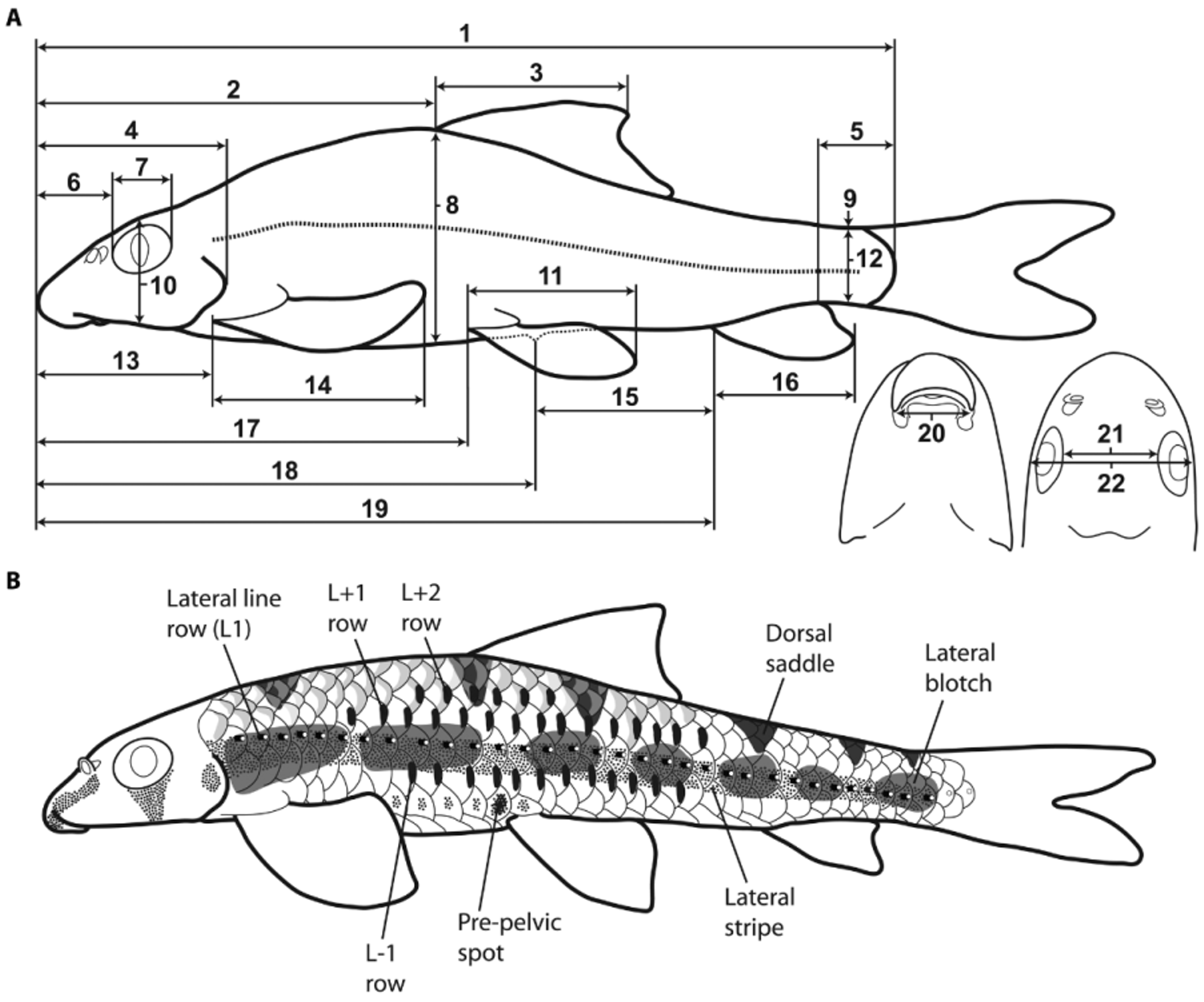
Description. General body shape as in Figures 15–17 View FIGURE 15 View FIGURE 16 View FIGURE 17 . Morphometric data are listed in Table 1 and select meristic characters in Tables 2 and 3. Body elongate, dorsal profile arched, rising moderately to dorsal-fin origin, sloping gently towards caudal peduncle. Body depth greatest at dorsal-fin origin, narrowest at base of caudal peduncle. Ventral profile moderately straight from lower jaw to anal-fin origin, weakly concave from anal-fin origin to caudal-fin base.
Head and eye large, pupil round. Mouth inferior. Snout moderate, less than half of head length. Snout rounded anteriorly, its ventral surface bordered by a deep longitudinal groove on each side. Rostral cap and upper lip fused, separated by a shallow groove; posterolateralmost part of rostral cap continuous around corner of mouth, contacting with skin fold at posterolateral corner of mouth; posterior margin of rostral cap covered in low, cobblelike papillae ( Fig. 19 View FIGURE 19 ). Lower lip portion of lower-jaw cushion broadly rectangular, anterior edge weakly rounded; superficial layer of lower-jaw cushion covered in large, pillar-like globular papillae, separated by deep grooves; smaller globular papillae, continuous with large pillar-like globular papillae on superficial layer of lower-jaw cushion, present on skin surface along ventral midline posterior to lower-jaw cushion, becoming increasingly sparse towards isthmus. Upper lip covered with low triangular unculi, ranging in height from ~5–15 μm; lower jaw unculi not examined. Skin fold around posterolateral corner of mouth large and flap-like, continuous anteriorly with posterolateralmost part of upper lip and rostral cap; outer edge of skin fold heavily papilliated with large cauliflower-like papillae; unculi and tubercles absent from surface of skin fold ( Fig. 19 View FIGURE 19 ). Posterior edge of rostral cap, lower lip, skin fold, and globular papillae all densely covered with tastebuds. Gill membranes joined to isthmus.
Pre-epiphyseal and post-epiphyseal fontanelle long and rectangular, separated by a narrow strut of frontal, dorsal to the epiphyseal bar. Five infraorbitals (IO1–5). IO1–3 plate like, IO1 largest of the series. IO4–5 narrow tube-like bones, composed of sensory canal ossification only. Cephalic sensory system well developed. Openings in anguloarticular portion of preopercular mandibular canal 2. Openings in preopercular portion of preopercularmandibular canal 6. Openings in nasal portion of supraorbital canal 2 or 3. Parietal portion of supraorbital canal open. Openings in parietal portion of temporal canal 2. Fifth ceratobranchial with a single row of four needle-like teeth. Hyoid bar with three branchiostegal rays of similar length and shape. Basihyal short, spatula shaped. Anterior swimbladder chamber surrounded by a thick peritoneal tunic, partially enclosed in a bony capsule formed anteriorly by lateral process of 2nd vertebral centrum and laterally by outer arm of os suspensorium. Posterior swimbladder chamber greatly reduced in size.
Dorsal-fin rays iii.9 (49) or iii.10 (2). Anal-fin rays ii.6 (44). Principal caudal-fin rays 9+9 (1), 10+8 (1) or 10+9 (39), dorsal procurrent rays 6 (2), 7 (3) or 8 (2), ventral procurrent rays 4 (1), 5 (4) or 6 (2). Pectoral fin rays iv.10 (6), iv.11 (11), iv.12 (4), v.10 (6), v.11 (16) or v.12 (3), pelvic fin rays ii.7 (48) or ii.8 (1). Paired fins horizontally placed, pectoral fins larger than pelvic fins. Pectoral fin not reaching vertical through dorsal-fin origin, reaching two to four scale rows anterior to pelvic-fin origin when adpressed. Pelvic-fin origin posterior to dorsalfin origin, insertion opposite second or third branched dorsal-fin ray. Well-developed unculiferous paired-fin pads present along ventral surface of four–five anteriormost pectoral-fin rays and two anteriormost pelvic-fin rays. Dorsal fin high, triangular in shape with weakly pointed tip; posterior margin weakly concave. Anal fin small, triangular in shape with weakly pointed tip; posterior margin weakly concave; not reaching caudal-fin base when adpressed. Caudal fin moderately forked, tips of upper and lower lobes weakly rounded.
Scales cycloid, large, with 6–8 well-developed radii over posterior field of scale body. 32 (6), 33 (26) or 34 (7) scales along lateral line, plus 1 (10) or 2 (22) on base of caudal fin. 3.5/1/2 transverse scale rows from dorsal-fin origin to pelvic-fin origin, 10 around caudal peduncle, 10 (16), 11 (27) or 12 (1) predorsal scales, 8 (1), 9 (33), or 10 (10) scales between anus and anal-fin origin. Ventral surface between paired fins without scales. Total number of vertebrae 34, consisting of 17+17 (6) or 18+16 (1) abdominal and caudal vertebrae.
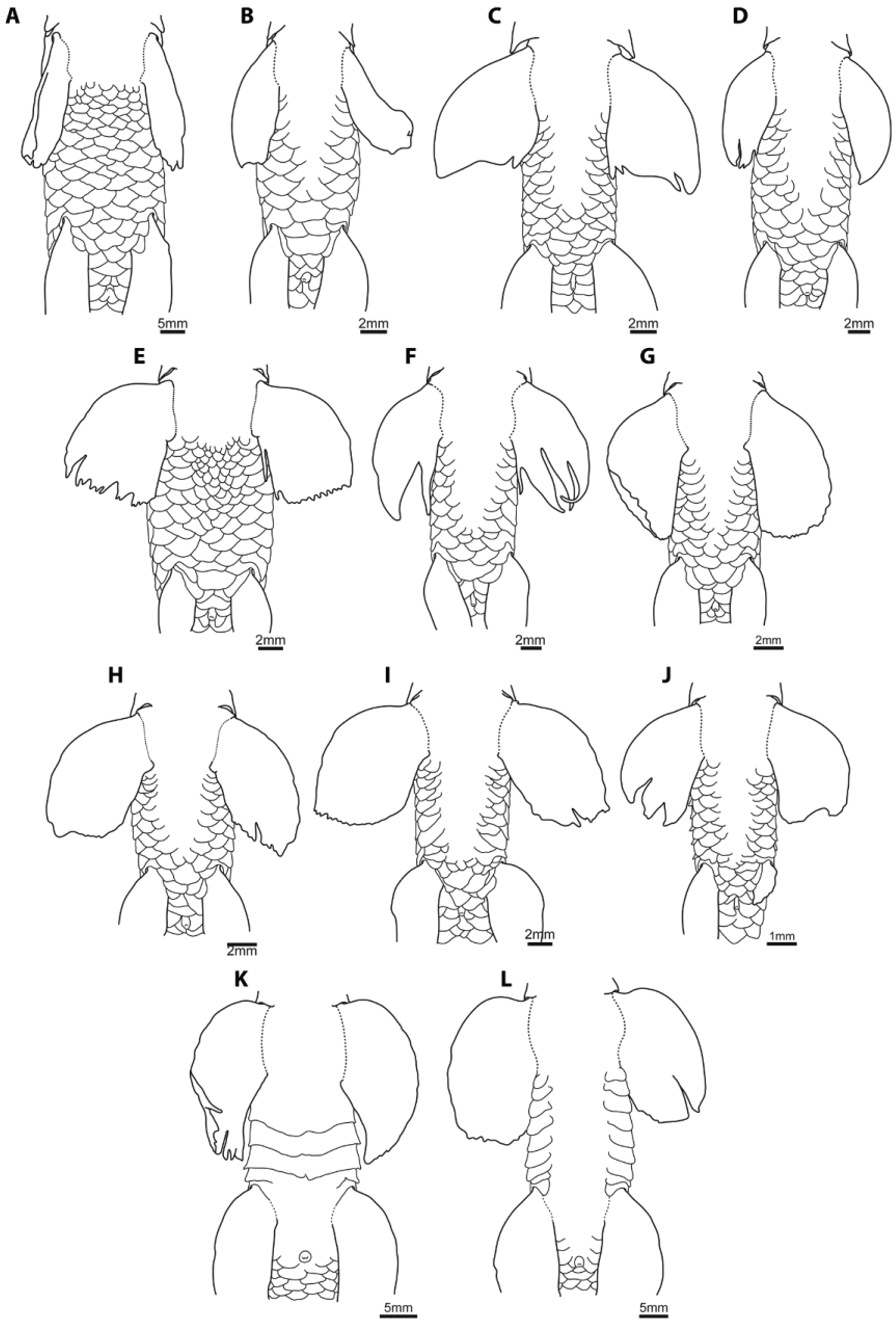
Small conical tubercles with hard keratinized tip distributed over lateral, dorsal and ventral surfaces of head, including snout and anteroventral region of rostral cap. Tubercles present on posteroventral region of rostral cap dagger-like, larger than those on other surfaces of head. Head tuberculation more strongly developed in males. Tight elongate cluster of tubercles present on lateral surface of head below orbit in males only. Scales situated posterior to occiput along dorsal midline and dorsolateral body surface with three or four small elongate tubercles, forming small keels orientated along antero-posterior body axis, in males only. Anteriormost rays of pectoral fin with small conical tubercles, multiple rows thick, present along dorsal surface; tubercles largest along anteriormost ray, gradually decreasing in size on more posterior rays. Remaining fins without tubercles in males. All females examined lack tubercles on body and fins.
Coloration. In alcohol body background color light cream ( Fig. 15–17 View FIGURE 15 View FIGURE 16 View FIGURE 17 ). Occiput dark brown. Dorsal surface between occiput and dorsal fin with three indistinct dark brown saddles, first situated anterior to midpoint between occiput and dorsal-fin origin, second situated at midpoint between first and third, third situated at dorsal-fin origin. Dorsal saddles posterior to dorsal-fin origin indistinct, numbering four or five; position variable except for first, situated below middle of dorsal fin, and last, situated at base of caudal fin. Dorsal saddles without contact to lateral blotches or lateral stripe, extending ventrally one (first four) or two (last four or five) scale rows on body side.
Flank with 7 to 10 (typically 8) dark brown lateral blotches arranged in a longitudinal row. Size and position of lateral blotches along flank highly variable, excluding first, situated posterodorsal to opercular opening, and last, situated at caudal-fin base. Lateral stripe poorly developed. Scales situated over dorsal surface of body bordered posteriorly with dark brown pigment, forming distinct reticulate pattern over dorsal surface, strongest anterodorsally.
Scales in lateral line scale row (L1) with dark blotch posteriorly, punctuated centrally by unpigmented lateral line canal (giving appearance of double dash line along lateral line scale row). L+1, L+2 and L-1 rows present in individuals over 30mm SL, length of rows increasing substantially with size. In large adult specimens (> 40 mm SL), L+1 row may extend along entire lateral side of body; L+2 shorter than L+1, restricted to middle of body, composed of 5–10 blotches; L-1 row intermediate in length between L+1 and L+2, rarely extending posterior to anal-fin origin. Unscaled base of pectoral fin and scales adjacent to pelvic-fin origin peppered with small dark brown melanophores, forming indistinct pectoral-base and pre-pelvic spots, respectively. Lateral surface of snout, region rimming ventral margin of orbit and skin over opercle densely scattered with dark brown melanophores.
Ventral surface largely devoid of pigment except for melanophores along anterior edge of rostral cap, small patch of brown melanophores situated beneath scales at anal-fin origin and a short line of melanophores posterior to anus, running along ventral midline from anus to 5th scale in scale row running between anus and anal-fin origin. Dorsal surface of anterior pectoral- and pelvic-fin rays marked with small melanophores, number and intensity increasing substantially with size. Dorsal fin with irregular apical dark brown blotch, formed by melanophores centered around fork of first branched ray. Anal fin hyaline. Caudal fin pigmentation highly variable. Majority of specimens with dark brown blotch over base of central caudal-fin rays and two dark brown marking on upper and lower lobes. In several specimens markings along lower lobe and blotch at base of caudal fin are confluent ( Fig. 17 View FIGURE 17 ).
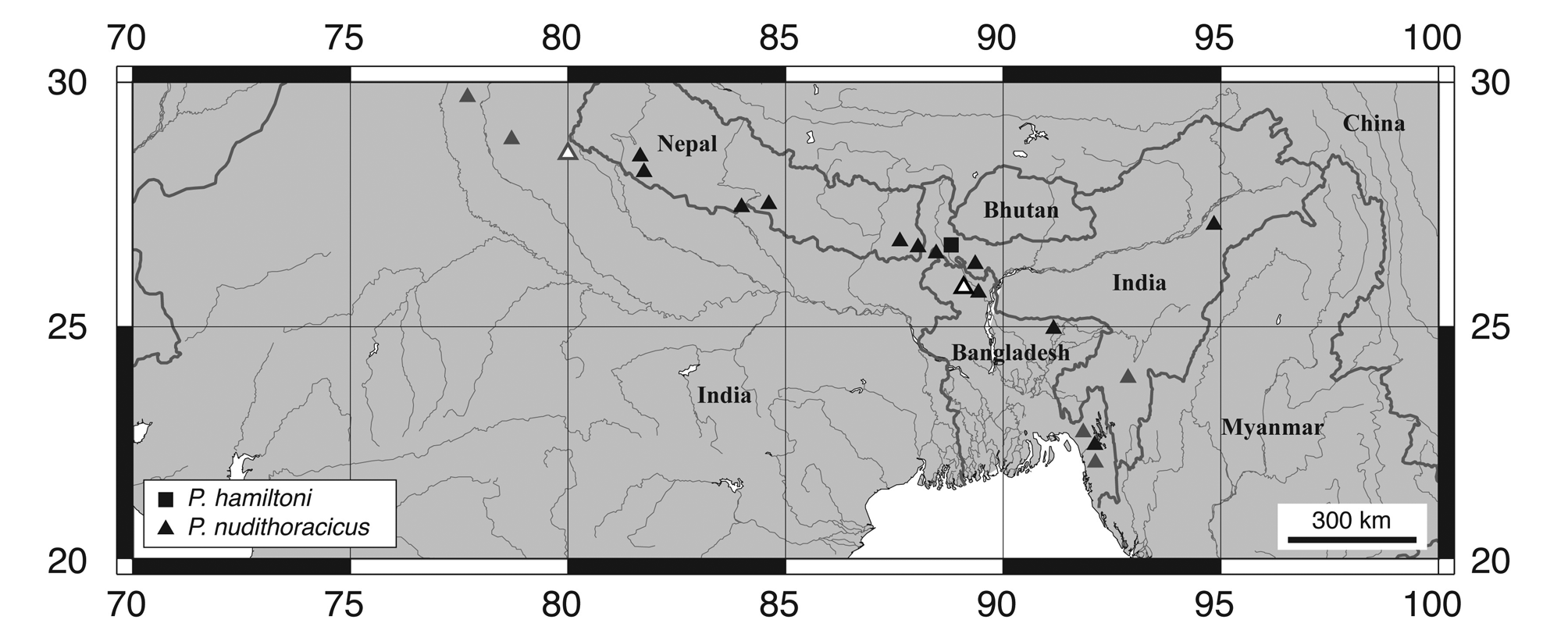
Distribution and habitat. Psilorhynchus nudithoracicus is known from the Ganges River drainage in Bangladesh, India (West Bengal and Uttar Pradesh) and Nepal, the Brahmaputra River drainage in Bangladesh and India (Assam and West Bengal), and the Meghna and Sungu River drainages in Bangladesh and India ( Rainboth, 1983; Rahman, 2005; Edds & Ng, 2007; Fig. 21 View FIGURE 21 ). Tilak & Husain (1980) describe the type locality (Bamrauli Canal; Pilibhit District, Uttar Pradesh) as a slow moving stream with a sandy bed. Rainboth (1983) reports collecting P. nudithoracicus over small pebbles, in shallow running water, over a “primarily sand” substrate.
Remarks. This species has been exclusively referred to as P. gracilis Rainboth, 1983 (e.g., Talwar & Jhingram, 1991; Menon, 1999; Conway & Kottelat, 2007; Edds & Ng, 2007; Conway, 2011). Rainboth (1983) described P. gracilis based on specimens obtained from Bangladesh, making explicit comparisons with P. balitora , P. homaloptera , P. pseudecheneis and P. sucatio (i.e., the four species of Psilorhynchus recognized as valid at that time). Rainboth (1983) also commented on other nominal forms of Psilorhynchus , including P. s. d a m o d a r a i, P. variegatus and P. h. ro w l e yi, but made no mention of P. s. nudithoracicus , suggesting that he was not aware of Tilak & Husain’s earlier work.
Though we have not had the opportunity to examine the type material of P. s. nudithoracicus , critical examination of Tilak & Husain’s (1980) description, combined with observations we have made on numerous specimens referred to as P. gracilis from Nepal, India and Bangladesh (including type material), failed to identify any differences between P. gracilis and P. s. nudithoracicus . Specifically, the characters listed as diagnostic for P. gracilis by Rainboth (1983) do not suffice to distinguish this species from P. nudithoracicus . Both species have overlapping lateral line scale counts (34–36 listed by Tilak & Husain for P. s. nudithoracicus [presumably including those scales on base of caudal fin] vs. 33–36 [35–37 including scales on base of caudal fin] listed by Rainboth for P. gracilis ), and identical numbers of unbranched pectoral-fin rays (4–5), lack scales on the midventral region between paired fins (see Fig. 2 View FIGURE 2 in Tilak & Husain, 1980; Fig. 12 View FIGURE 12 B), and exhibit two distinct spots along the dorsal midline between the occiput and dorsal-fin origin (see Fig. 1 View FIGURE 1 in Tilak & Husain, 1980; Figs. 15– 17 View FIGURE 15 View FIGURE 16 View FIGURE 17 ). We conclude, based on the evidence in hand, that both names apply to the same species. Accordingly, we treat P. gracilis as a junior subjective synonym of P. nudithoracicus . Examination of the type material of P. nudithoracicus , deposited in the collections of the Northern Regional Station of the Zoological Survey of India in Dehra Dun ( Tilak & Husain, 1980), will be an important next step to further test our conclusions.
Psilorhynchus nudithoracicus View in CoL , as understood herein, is a widespread species, occurring throughout much of the Ganges and Brahmaputra drainages in northeastern India and northwestern Bangladesh ( Rainboth, 1983), the Ganges drainage throughout southern Nepal ( Edds & Ng, 2007; T. K. Shrestha, 2008), and the Meghna and Sangu drainages in eastern Bangladesh ( Rainboth, 1983). Most of our observations on this species are based on Gangetic material from southern Nepal and northwestern Bangladesh and we have examined relatively few specimens from the Brahmaputra drainage of northeastern India ( 5 specimens; 2 from West Bengal and 3 from Assam) or the Meghna (5) and Sangu (2) drainages of eastern Bangladesh. Surprisingly, Menon (1999) makes no mention of P. gracilis View in CoL (sensu Rainboth, 1983) in his checklist of Indian freshwater fishes and the distribution of P. nudithoracicus View in CoL throughout northeastern India is currently unclear and notably patchy ( Fig. 21 View FIGURE 21 ). In addition to Assam, Uttar Pradesh, and West Bengal, P. nudithoracicus View in CoL has been reported from Manipur and Mizoram, by Vishwanath et al. (2007) and Kar & Sen (2007), respectively, based on material collected from the Barak River drainage. We have excluded the Manipur record of P. nudithoracicus View in CoL from our distribution map because Vishwanath et al. (2007) did not provide precise locality information, but include the record provided by Kar & Sen (2007), based on specimens collected from the Tuirial River. We have also recently examined photographs of P. nudithoracicus View in CoL from the Langkaih River, a tributary of the Tlawng River, in Mizoram. We have been unable to locate any records of P. nudithoracicus View in CoL from the Indian states of Bihar, Meghalaya, or Tripura in the literature but suggest that it is likely to occur in these states, given its presence in neighboring states or countries.
Tilak & Husain (1980) distinguished P. nudithoracicus from P. sucatio (in part), based on features of caudal-fin coloration, specifically a distinctive elongate black marking along the lower lobe of the caudal fin. We have observed such a marking only in relatively few individuals of P. nudithoracicus from southern Nepal ( Fig. 17 View FIGURE 17 ), but note that a similar marking is present in the specimen of P. nudithoracicus figured in Vishwanath et al. (2007; 91), the locality of which is unspecified. In the majority of specimens examined, the caudal fin is decorated with five dark brown/black blotches, with one located at the center of the caudal-fin base, and two on each lobe ( Fig. 15 View FIGURE 15 , 16 View FIGURE 16 ). There is also some variation in the extent of the L-1 row, which is restricted to the center of the body in the majority of material examined (including the holotype of P. gracilis ; Fig. 15 View FIGURE 15 ) but extends almost along the entire length of the body in the larger specimens that we have examined from eastern Assam ( NRM 46908; Fig. 16 View FIGURE 16 ). This is also the case in the individual of P. nudithoracicus figured by Vishwanath et al. (2007; 91).
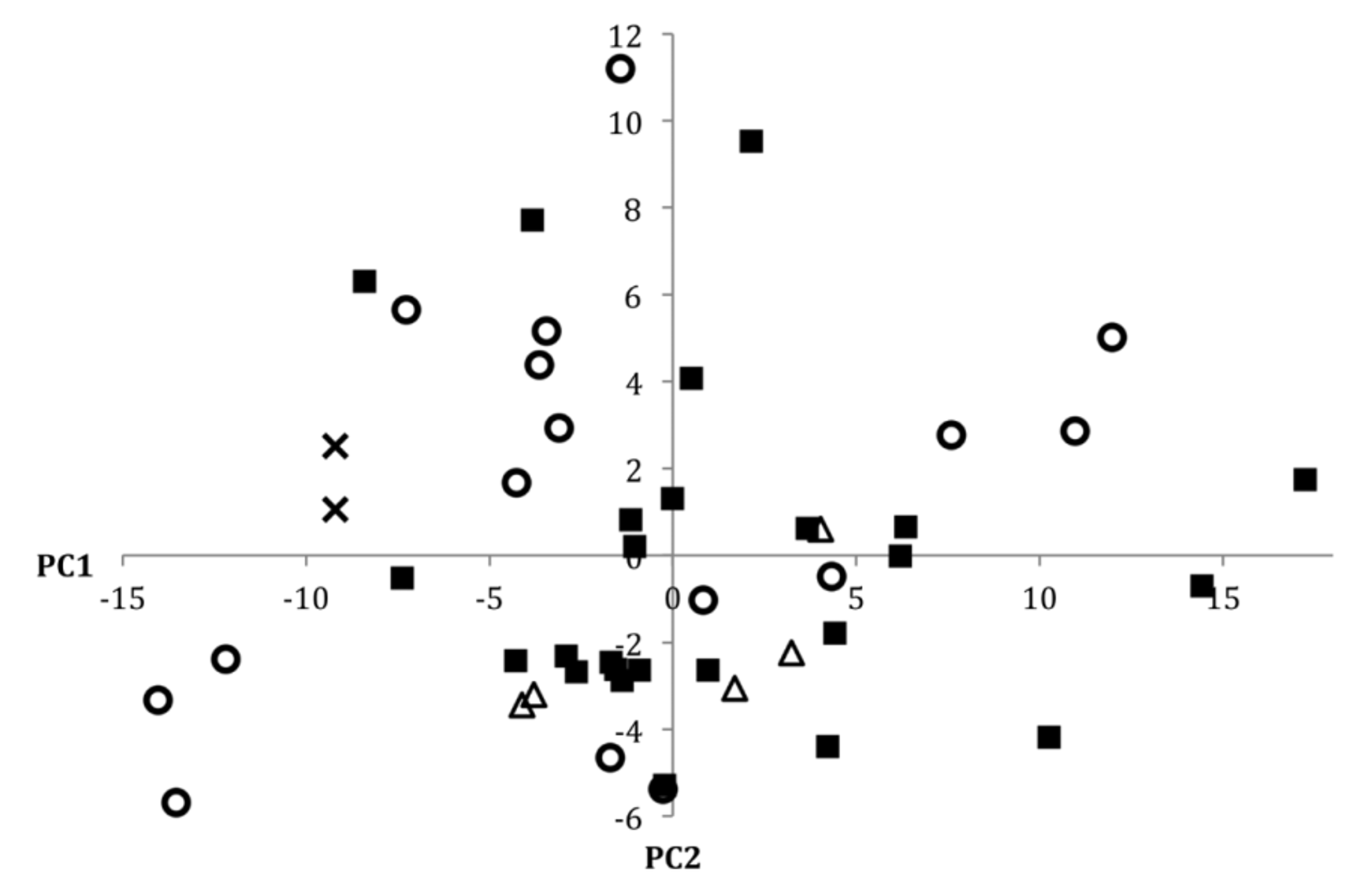
Despite variation in coloration, we were unable to identify any consistent meristic differences between populations of P. nudithoracicus from any of the four drainages for which we had the opportunity to examine material. In the size corrected principal components analysis of the morphometric data for P. nudithoracicus (including specimens from the Ganges, Brahmaputra, Meghna, and Sangu drainages), PC1 and PC2 accounted for 35% and 11% of the cumulative variation, respectively. For PC1 all measurements exhibited positive loadings, with particularly strong loading for caudal peduncle width (0.77). PC2 exhibited strong positive loading for mouth width (0.63), caudal peduncle length (0.55), and anal-fin length (0.37), and strong negative loading for snout length (-0.15) and caudal peduncle width (-0.14). In the scatterplot of PC1 vs. PC2 ( Fig. 22 View FIGURE 22 ) there is no obvious clustering of individuals by drainage, suggesting that there is little variation in the morphometric characters investigated between or within drainages.
Throughout its range, P. nudithoracicus is found in sympatry with several other members of Psilorhynchus , including P. balitora , P. hamiltoni , P. nepalensis and P. sucatio . Given its presence in the Barak River system of Manipur and Mizoram ( Kar & Sen, 2007; Vishwanath et al., 2007) and the Chittagong Hill Tracts of Bangladesh ( Rainboth, 1983), it may also be sympatric with P. amplicephalus , known to date only from the Barak River in Assam ( Arunachalam et al., 2007), and P. rahmani , known to date only from the Karnaphuli River drainage in the Chittagong Hill Tracts of southeastern Bangladesh ( Conway & Mayden, 2008b). We have outlined differences between P. sucatio and P. nudithoracicus under the remarks section of the former and focus here on differences between P. nudithoracicus and remaining syntopic (and putatively syntopic) species, all of which are members of the P. balitora species group (see below). Psilorhynchus nudithoracicus can be distinguished from all members of the P. balitora species group by having two dorsal saddles (vs. one) anterior to the dorsal saddle situated at the dorsal-fin origin, from all members of the P. balitora species group reported West of the Indo-Burma mountain range (viz. P. amplicephalus , P. balitora , P. hamiltoni , P. nepalensis and P. rahmani ) based on the modal number of scales in the lateral line scale row (32–34, most commonly 33 vs. 30–32, most commonly 31 in P. balitora ; 31–33, most commonly 32 in P. nepalensis ; 29–32, most commonly 30 in P. rahmani ; 34–35 in P. hamiltoni ; 35–36 in P. amplicephalus ), and from all members of the P. balitora species group reported west of the Indo-Burma mountain range, except for P. rahmani , by its higher number of caudal-fin rays (most commonly 10+9 vs. most commonly 9+ 8 in P. amplicephalus , P. balitora , P. hamiltoni ). The presence of a large post-epiphyseal fontanelle further distinguishes P. nudithoracicus from P. balitora and P. rahmani , and the absence of scales on the midventral surface between paired fins is perhaps the most useful character for separating P. nudithoracicus from P. balitora . When observations on skeletal material can be made, several osteological features serve to further distinguish P. nudithoracicus from members of the P. balitora species group, including: the presence (vs. absence) of a mandibular sensory canal (located on the anguloarticular); a higher number of pores in the preopercular sensory canal (6 vs. 4–5); and the shape of the anteriormost brachiostegal ray (similar in length and shape to more posterior basibranchial rays vs. greatly reduced, composed of articular head only, or absent).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Psilorhynchus nudithoracicus Tilak & Husain
| Conway, Kevin W., Dittmer, Drew E., Jezisek, Laci E. & Ng, Heok Hee 2013 |
Psilorhynchus sucatio nudithoracicus
| Rainboth 1983: 69 |
| Tilak 1980: 35 |