Crassostrea rhizophorae identity
|
publication ID |
https://doi.org/10.1016/j.jcz.2023.06.002 |
|
persistent identifier |
https://treatment.plazi.org/id/03BE87EB-FFEC-627F-FCB4-FAFBFD5FFD5F |
|
treatment provided by |
Felipe |
|
scientific name |
Crassostrea rhizophorae identity |
| status |
|
4.3. Crassostrea rhizophorae identity
4.3.1. Historical and taxonomical review
Crassostrea rhizophorae is a well-known concise species. However, earlier oyster studies have proposed other controversial names to address this entity, such as Ostrea arborea and O. parasitica . The name O. arborea was considered a synonym of C. rhizophorae for a long time, but it is now considered a nom. dubitum ( Huber, 2010).
Ostrea arborea View in CoL was included as a mangrove oyster species from Central America, later allied with O. rhizophorae View in CoL , in an evaluation of oyster specimens from Guadeloupe and Santo Domingo by Calonne and Humphreys (1797). Such decision was mainly motivated by the necessity to address a specific binomial name to a neglected group of mangrove oysters from Guadeloupe Island reported by several French writers, which they name “Huitres des paletuviers”, “Huitres d’ arbres” or “Huitres des mangroves” ( Beaurieu, 1764; D’ Herbigny, 1775; Davila´, 1767; Du Tertre, 1667; Lundy, 1667; Montcervelle, 1780; Poynz, 1753).
However, the conceptualization of mangrove oyster species from Chemnitz reflected an ecological approach to species. Much of his perception of oyster species is based on Martini (1774), his predecessor in conchology. For hierarchical organization of taxa, all tree oysters were assembled into one identity by host preference for affixation, regardless of any distributional and morphological differences. The tree oyster of Martini encompassed Antillean mangrove oysters: European tree oysters ( Beaurieu, 1764), Hebenstreit (1743), whose species reference is based on Martin Lister’ s Plate 175 Huddesford (1770), which corresponds to Ostrea parasitica Turton (1819) View in CoL = Ostrea edulis Linneaus (1758) View in CoL ; African mangrove oyster ( Adanson, 1757); and Indo-Pacific mangrove oyster ( Klein, 1753; Petiver, 1713; Rumphius, 1705; Smith, 1751). Consequently, O. arborea View in CoL virtually reunited all previously reported tree-affixed oysters, comprising the same variable morphological oysters as those verified in Gmelin’ s O. parasitica ( Lischke, 1869) View in CoL .
When Ostrea rhizophorae was described as a Caribbean mangrove oyster, the species was given a morphological description, type locality, and specific mangrove host tree ( Rhizophora mangle ) ( Guilding, 1828). The suggestion that both names refer to the same entity is biased.
Other historical considerations were base on the assumption that C. rhizophorae was an ecological variant of C. virginica ( Ahmed, 1976; Harry, 1985) or a possible subspecies ( Abbot, 1954) also restricted to the Caribbean region ( Arakawa, 1990; Gunter, 1951; Warmke and Abbott, 1975). C. virginica and C. rhizophorae were later considered separated species, but a segregation between Caribbean mangrove oyster from C. rhizophorae and C. gasar (sub C. brasiliana ) from Brazil made by Ranson (1967) had some implications on species identity in Brazil (see item 4.2.2). Such distributional criteria were disruptive for the reattribution of C. rhizophorae in the Atlantic Mesoregion. A reapplication of this distributional criterion, with the addition of some supposed dependable conchological and anatomical characteristics, was made in the Crassostrea revision in Brazil. C. mangle was described with the intention of identifying intertidal specimens somewhat more convergent to “ C. rhizophorae ” located in Brazil ( Amaral and Simone, 2014). Thus, the recurring issue of species identity related to C. rhizophorae is discussed.
4.3.2. Morphological and distribution review
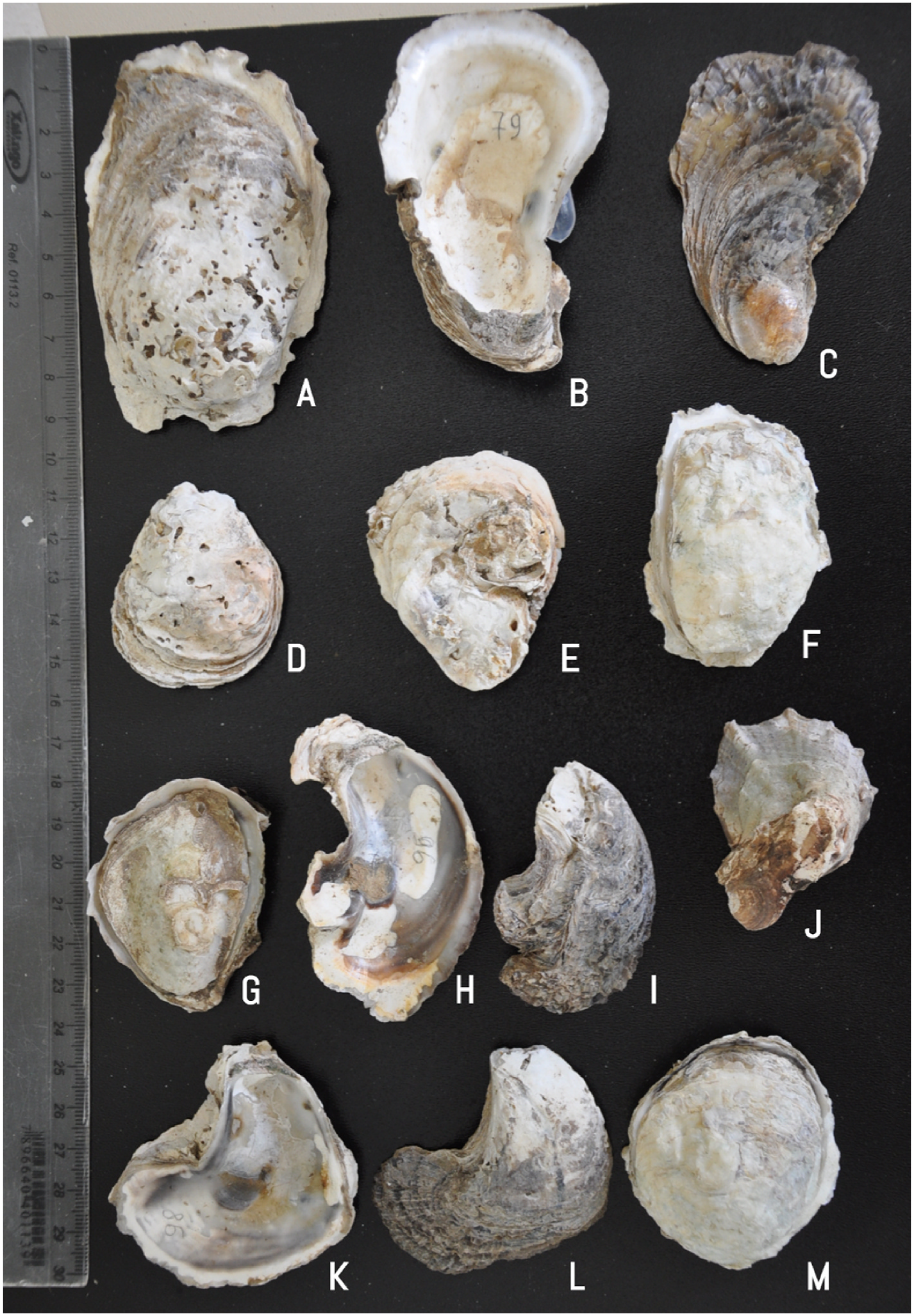
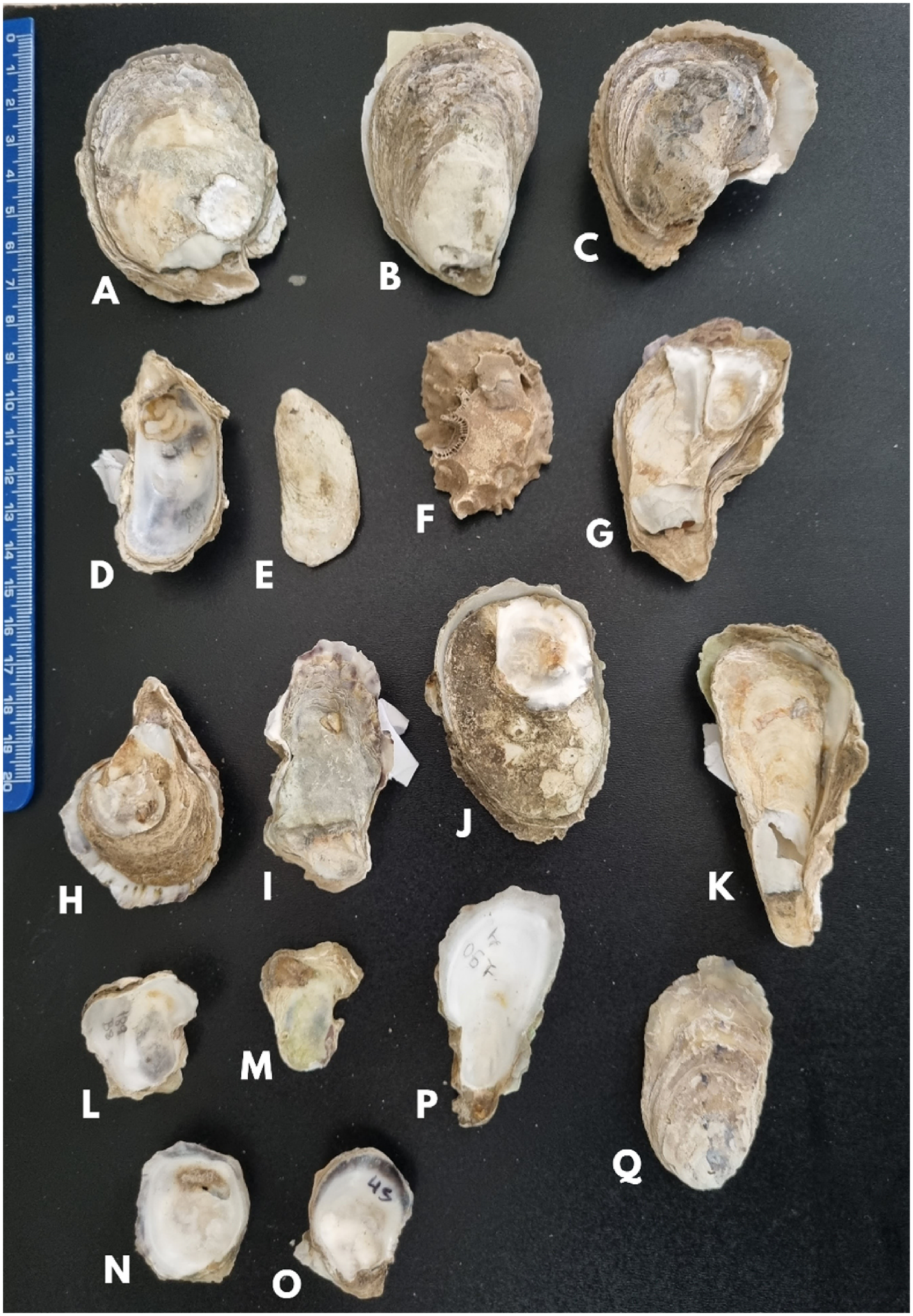
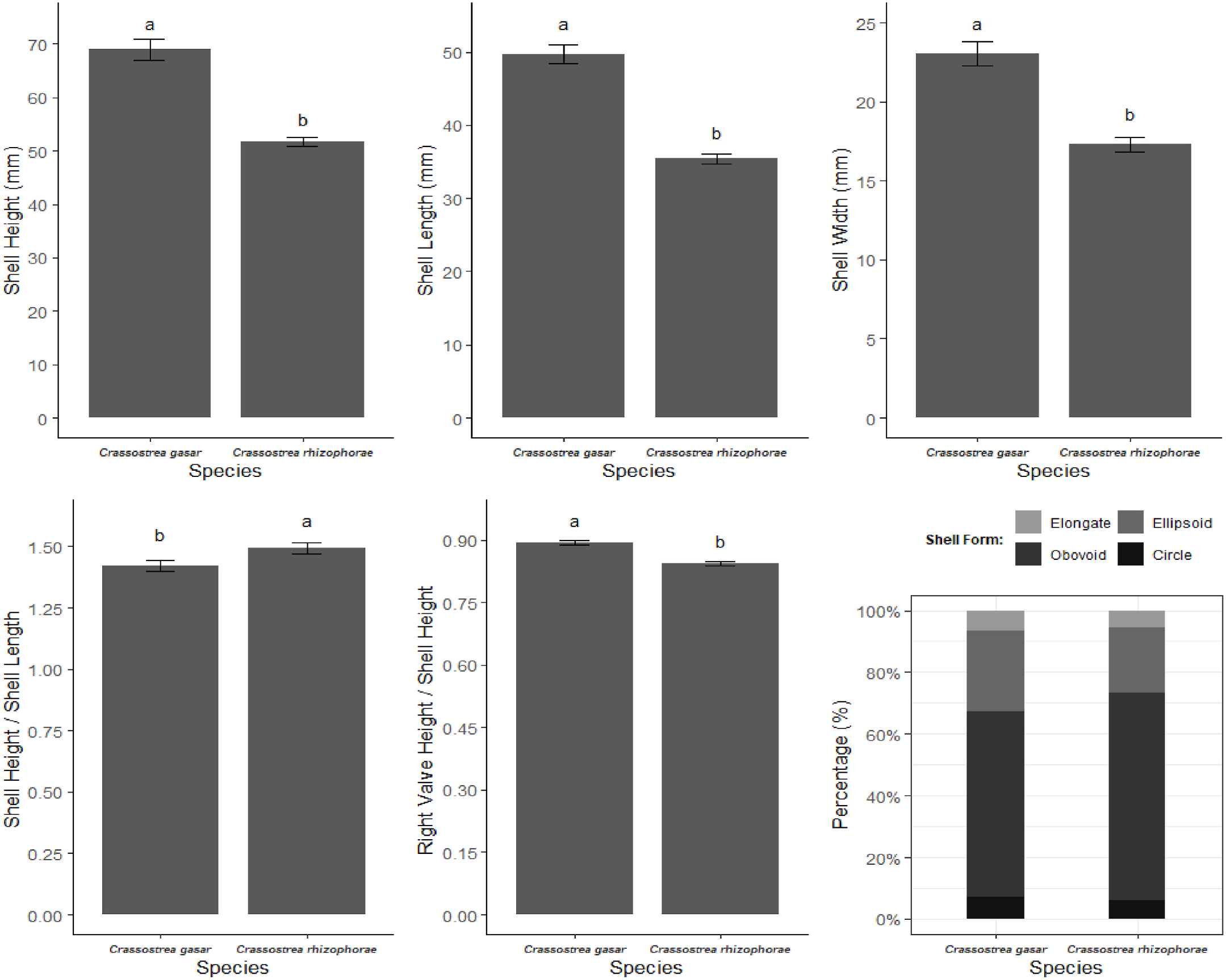
C. rhizophorae specimens are currently among small- and medium-sized oysters (shell height < 100 mm) with the majority of its population fitting under the Crassostrea -complex shells. There are some uncommon specimens with radiating longitudinal costae bearing some nodosities ( Fig. 18J View Fig and 19F View Fig ) (with more than 10 mm width), which constitute a unique pattern for C. rhizophorae in which it is possible to differentiate them from C. gasar .
Although the shell morphology of mangrove oyster species is polymorphic, for total species identification, a revision of the genus Crassostrea in Brazil proposed a new species, C. mangle , to separate the Brazilian specimens of C. rhizophorae from those of the Caribbean region. Morphological evaluation was performed based on a comparison of 50 specimens from a holotype location (Carriacou Island, Granada) with specimens collected in Brazil ( Amaral and Simone, 2014). We assume C. mangle is disruptive and has too many unsolved issues to be considered a cohesive species to address the Brazilian specimens of C. rhizophorae and we disagree with these authors. We present our arguments below.
First, the whole morphological definition of Crassostrea rhizophorae sensu Amaral and Simone (2014) (hereinafter named as Caribbean C. rhizophorae ) is rather intuitive and lacks profound connection with previous Caribbean studies reporting its morphological identity thorough the West Indies. Additionally, the distinction between C. rhizophorae s.l. ( C. rhizophorae and C. mangle ) and C. gasar ( C. brasiliana in Amaral and Simone (2014)) is problematic since the authors did not establish which taxonomic studies in the literature that reported on the morphological identity of Crassostrea species were used as reference for their own morphological delimitation of the species.
In Brazil, taxonomists have disputed the condition of recognizing either one or two native Crassostrea species before definitive molecular analysis ( Ignacio et al., 2000) (see revision in item 4.2.2 and Table 3). The recognition of the two Crassostrea species was first based on genetic evidence provided by Absher (1989) and Ignacio et al. (2000). Amaral and Simone (2014) used previous genetic evidence to confirm the presence of two native species in Brazil and not a classical morphological and anatomical delimitation of both species, lacking any at the time. Without a cohesive morphological criterion based on genetically traced Crassostrea specimens, conchological and anatomical descriptions of the species become artificial. Establishing a morphological delimitation between C. rhizophorae s.l. and C. gasar under such conditions is unlikely. Cumulatively, the separation of Caribbean C. rhizophorae and C. mangle (hereafter named Brazilian C. rhizophorae ) based on an unreliable morphological description of C. rhizophorae s.l. is mostly improbable.
Second, the conchological information that Amaral and Simone (2014) presented in Table 1 for C. gigas, Caribbean C. rhizophorae , and C. virginica , and used as a criterion for species delimitation is very similar to Arakawa’ s (1990) morphological interpretation these oyster species. The same condition occurred when we compared the conchological information of C. brasiliana from Table 1 of Amaral and Simone (2014) and C. brasiliana from Table 1 of Singarajah (1980). It is a common practice for authors to cite a study or revision used as the fundamental basis for their species interpretation, since it helps to understand the reviewer’ s point of view, especially in complicated taxonomic groups such as Crassostrea .
Amaral and Simone (2014) have established the color of the inner surface of shells, including its margin, the outer undulation aspect of the shells and the color of the muscle scar from C. gigas, Caribbean C. rhizophorae , C. virginica , and C. brasiliana in line with Arakawa’ s and Singarajah’ s interpretation of the species, respectively.
The morphological approach of Amaral and Simone (2014) on the morphological interpretation by the two authors raised two issues. First, Arakawa (1990) did not perform a taxonomic revision and did not offer a list of examined specimens, making it impossible to verify the morphology of the oyster specimens used in this study. Second, the description of C. brasiliana sensu Singarajah (1980) interprets the Brazilian C. rhizophorae as a synonym of the first species and C. paraibanensis as a distinct species from C. brasiliana , which is contradicted by later molecular findings. Therefore, the established morphological criteria for the species delimitation may not represent the real nature of oyster species found in Brazil.
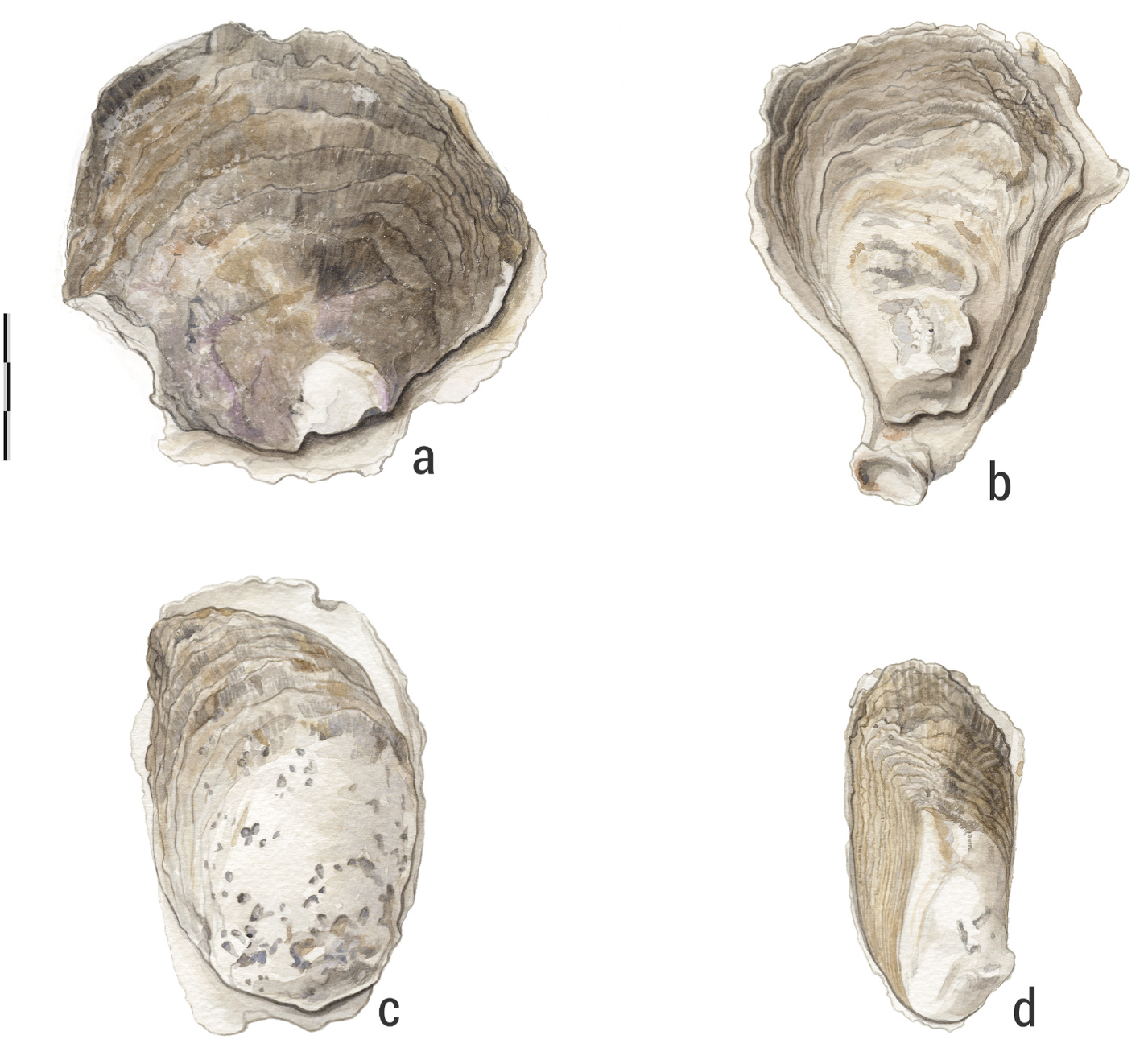
Third, according to Amaral and Simone (2014), the description of Caribbean Crassostrea rhizophorae is imprecise. The authors aimed to recover the morphology of the species based on the type of material; however, owing to the absence of holotype material deposited in the Natural History Museum of London (NHMUK), they performed fieldwork in the type of location to redeem a morphological reference to the species. Hanley (1854) created an illustration (Pl. I, Figs. 1 View Fig and 4 View Fig ) of this species based on Guilding’ s holotype and does not morphologically correspond with the specimens shown in Amaral and Simone (2014,
Figs. 3–4 View Fig View Fig ). Hanley’ s illustration of the species presents the shell’ s upper valves more or less flattened, becoming broader and scalier towards the ventral border. It also shows the upper valves on a dirty yellowish background with radiated lines or spots of brownish purple. The lower valve has loose concentric lamellae and some widely spaced radiant wrinkles, with purple radiated lines.
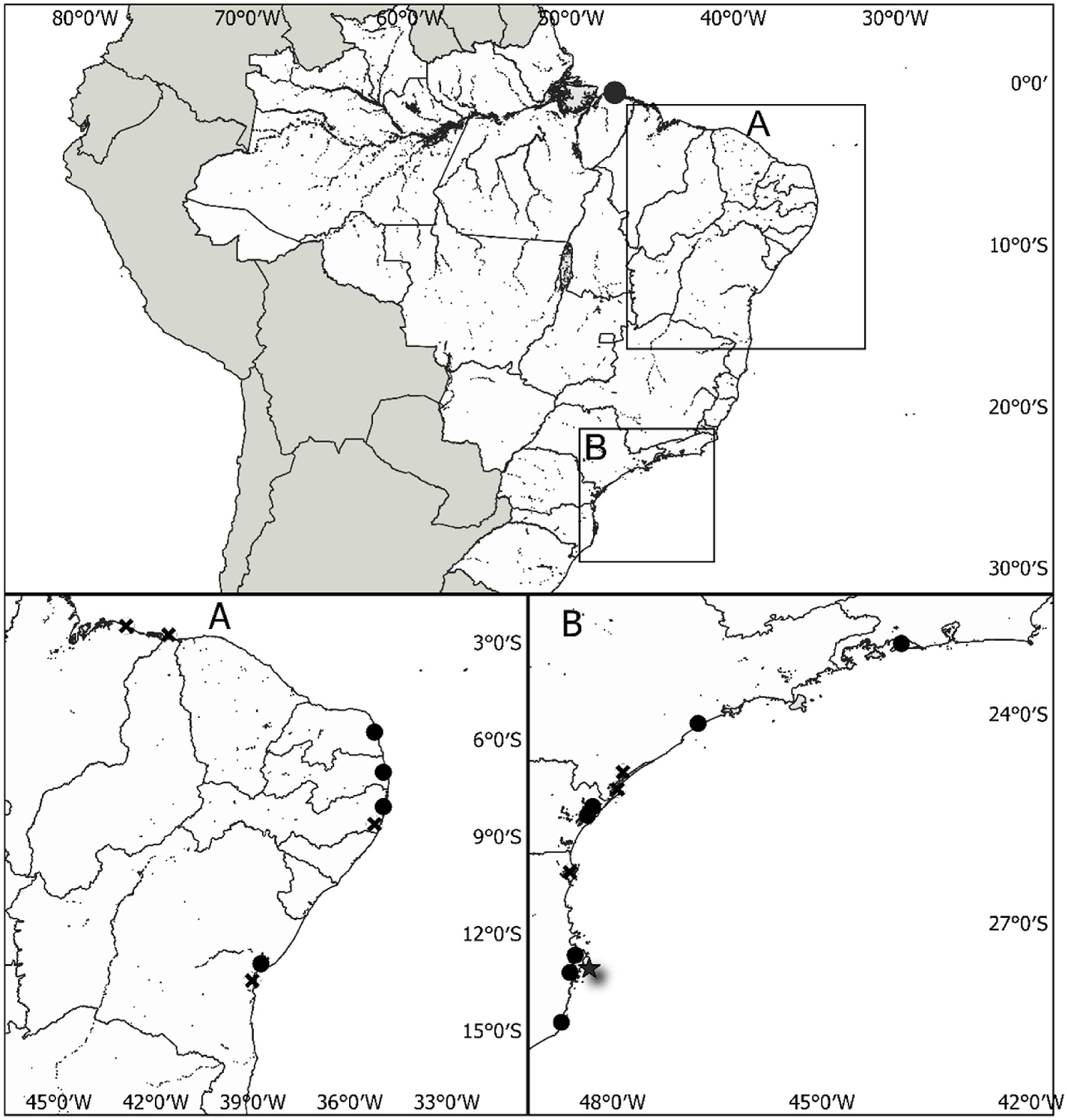
Moreover, The Caribbean Crassostrea rhizophorae ’ description includes only specimens from Granada, which do not represent all variants in the Caribbean region, as they have shell polymorphism owing to the environmental diversity of their habit ( Dall and Simpson, 1902; Gunter, 1950; Wakamatsu, 1973). The distribution of C. rhizophorae in the Caribbean region is wide and includes specimens located in Belize ( Ellison and Farnsworth, 1992), Honduras ( Hanley, 1856), Martinique ( Lamy, 1929b), St. Croix ( Nowell-Usticke, 1959), Antigua and Barbuda, Colombia, Costa Rica, Cuba, Dominica, French Guyana, Guadeloupe, Gulf of Mexico, Haiti, Jamaica, Panama, Puerto Rico, St. Lucia, Suriname, the state of Florida ( USA), Trinidad and Tobago, Venezuela and the Virgin Islands ( McLean, 1941; Newkirk, 1991), which the authors did not include in their revision.
Fourth, the characters used to separate species in the Identification Key are influenced by the authors’ bias. For example, Caribbean Crassostrea rhizophorae specimens have a “shallow” umbonal cavity, while C. gasar (sub C. brasiliana ) and the Brazilian C. rhizophorae have a “deep” umbonal cavity. Depth of umbonal cavity is a quantitative attribute, but no numerical assertions were made by the authors to confirm if such characteristic is different between species.
Modern taxonomy tends to avoid intuitive characteristics, especially in identification keys, because they need to be practical for use. Some subjectively appreciated features that are important for diagnostics in organisms are difficult to circumscribe ( Kendrick, 1964). However, in practice, many taxonomists are not particularly rigorous when it comes to transforming continuous data into discrete characters and arbitrarily choosing cutoff points between ranges ( Sokal and Sneath, 1963). Atypical specimen forms are generally excluded from comparisons because differences between individuals in a population are, in general, slight ( Quicke, 1993).
In addition, the authors also recognized the plasticity of the umbonal cavity size, in accordance with our results.
Fifth, when analyzing previous taxonomic studies involving Crassostrea rhizophorae , we found that some morphological accounts contradicting the authors’ findings were overlooked.
Caribbean Crassostrea rhizophorae View in CoL were defined as oysters with shallow umbonal cavities, but for Puerto Rican specimens (sub-erroneously C. virginica Gmelin, 1791 View in CoL ), the “lower valve has a strong, usually elongated beak, its hinge having a wide channel” which most certainly falls within the “deep umbonal cavity” character ( Dall and Simpson, 1902). The Venezuelan specimens also exhibited the deep umbonal cavities ( Ahmed, 1976, Fig. 7 View Fig ).
Muscle scar impressions in Caribbean C. rhizophorae View in CoL are mainly oval and reniform, but they can also be elongated, as perceived in Floridian specimens ( Galtsoff, 1964, Fig. 5 View Fig ; Huber, 2010, Fig. Crassostrea rhizophorae View in CoL ), and can be pigmented independently ( Castillo-Rodriguez and García-Cubas, 1984; Weisbord, 1964). The color of the inner valve of Caribbean C. rhizophorae View in CoL in the holotype location, which is supposed to be hazy purple and submargaritaceous, according to its protologue ( Guilding, 1828), was described as predominantly white with little brightness according to Caribbean C. rhizophorae View in CoL collected elsewhere (see Betanzos-Vega et al., 2016; Sowerby, 1870; Weisbord, 1964) or with reported Brazilian C. rhizophorae View in CoL specimens (see Absher et al., 2015; Ríos, 2009).
The edge of the inner surface of the left valve of Caribbean C. rhizophorae is predominantly white, and Amaral and Simone (2014, Table 1) include a possibility of a purple pigmentation. Brazilian C. rhizophorae is also plastic, with either purple ( Lamy, 1928) or white pigmentation ( Absher et al., 2015; Amaral and Simone, 2014; Ríos, 2009). Curiously, the evolutionary history behind the color of the margin of the inner shell seems to be scattered among oyster populations. In ancient times, an oyster in oyster beds with an inner purple margin were considered superior in quality, being seen as “calliblephara” or oysters with beautiful eyebrows ( Bostock and Riley, 1855; Philpots, 1890). Moreover, pigmentation in both muscle scar impression or in the inner valve is composed of melanin, and its intensity is associated, to a greater or lesser degree, with antioxidant responses from the adductor muscle and mantle of the oyster ( Hao et al., 2015); thus, it is an unreliable trait for species distinction.
The description of the Caribbean C. rhizophorae alludes to specimens whose shell size is estimated to be 80 mm in length in the species description and 60 mm as a discussion item in Amaral and Simone (2014), but specimens in the Caribbean region can reach approximately 150 mm in length ( Humphrey, 1975; Warmke and Abbott, 1975), with most adult individuals ranging from 60 to 110 mm in length in the Caribbean region ( Yoo and Ryu, 1984). In contrast, genetically traced Brazilian C. rhizophorae specimens rarely reach 90 mm ( Lazoski et al., 2011), but Amaral and Simone (2014) estimated the maximum size at 120 mm.
Amaral and Simone (2014) implied that the shells of C. rhizophorae s. l. lack outer undulations therefore they could be distinguished from C. virginica . However, several taxonomic studies showed different perspective. The shells of C. rhizophorae s.l. have fewer plicated shells than those of C. virginica ( Castillo-Rodriguez and García-Cubas, 1984; Galtsoff, 1964; Gunter, 1951; Mattox, 1949; V´elez, 1991; Weisbord, 1964), reflecting a prominent character state, rather than an exclusive one. Some specimens of C. virginica , though uncommon, have smooth or merged scale ornamentation ( Ahmed, 1976, Figs. 6 View Fig , 42; Huber, 2010,
Fig. C. virginica ) (this study, Fig. 20 View Fig ).
Early reports on Brazilian specimens of C. rhizophorae showed convergent descriptions with Caribbean C. rhizophorae ( Ihering, 1907; Lamy, 1928), together with Hanley’ s (1854, Pl. I, Figs. 1 View Fig and 4 View Fig ) illustrations and, consequently, with Guilding’ s protologue of the species.
Sixth, our sequence analysis, as well as, other studies using both Caribbean (Lap`egue et al., 2002; Lazoski et al., 2011; Lohan et al., 2015) and Brazilian specimens of Crassostrea rhizophorae showed a high degree of genetic correlation between them, indicating that the separation of both species is unwarranted.
Seventh, Crassostrea rhizophorae s.l. is not always associated with mangroves, as the authors mentioned, although that may possibly be regarded as its normal habit ( Sowerby, 1870). Thus species is also found on rocky coasts and other intertidal saline zones, as reported in several studies ( Absher, 1989; Ahmed, 1976; Boehs et al., 2019; Galv˜ao et al., 2013; Gunter, 1951; Ignacio et al., 2000; Lopes et al., 2018; Nascimento, 1991; Weisbord, 1964). In fact, this study observed that C. rhizophorae has a wide range of habitats and areas of occurrence, from mangroves and estuaries to rocky coasts, affixed in mangrove roots or rocks, and in intertidal zones, where water salinity varies between 12 and 35 g L 1.
The suggestion that mangrove areas are the only possible habitat for mangrove oysters is lessening, overlooking the wide range of coastal locations in which they occur ( Bacon, 1986).
Eighth, our genetically determined specimens of Crassostrea cover a more polymorphic range than expected, reflecting the morphological continuum presented in Fig. 2 View Fig of Lazoski et al. (2011). The evaluation of oyster specimens from zoological collections may not be sufficient to cover all variations among the same Crassostrea species, especially owing to the lack of genetic identification, along with the putative identification of species based solely on morphology.
Brazilian specimens of C. rhizophorae were defined by Amaral and Simone (2014) as shells without outer undulations and ornamentation, a deep umbonal cavity and an unpigmented inner surface of the left valve. However, our genetically traced Brazilian C. rhizophorae specimens covered a wide range of morphological variations, such as oysters with outer undulations on both valves ( Fig. 18C, I, J, L View Fig ; 19F View Fig ), shallow umbonal cavity ( Fig. 18K View Fig ; 19N, O View Fig ), and pigmented edges in the inner surface of the left valve ( Fig. 18H, K View Fig ).
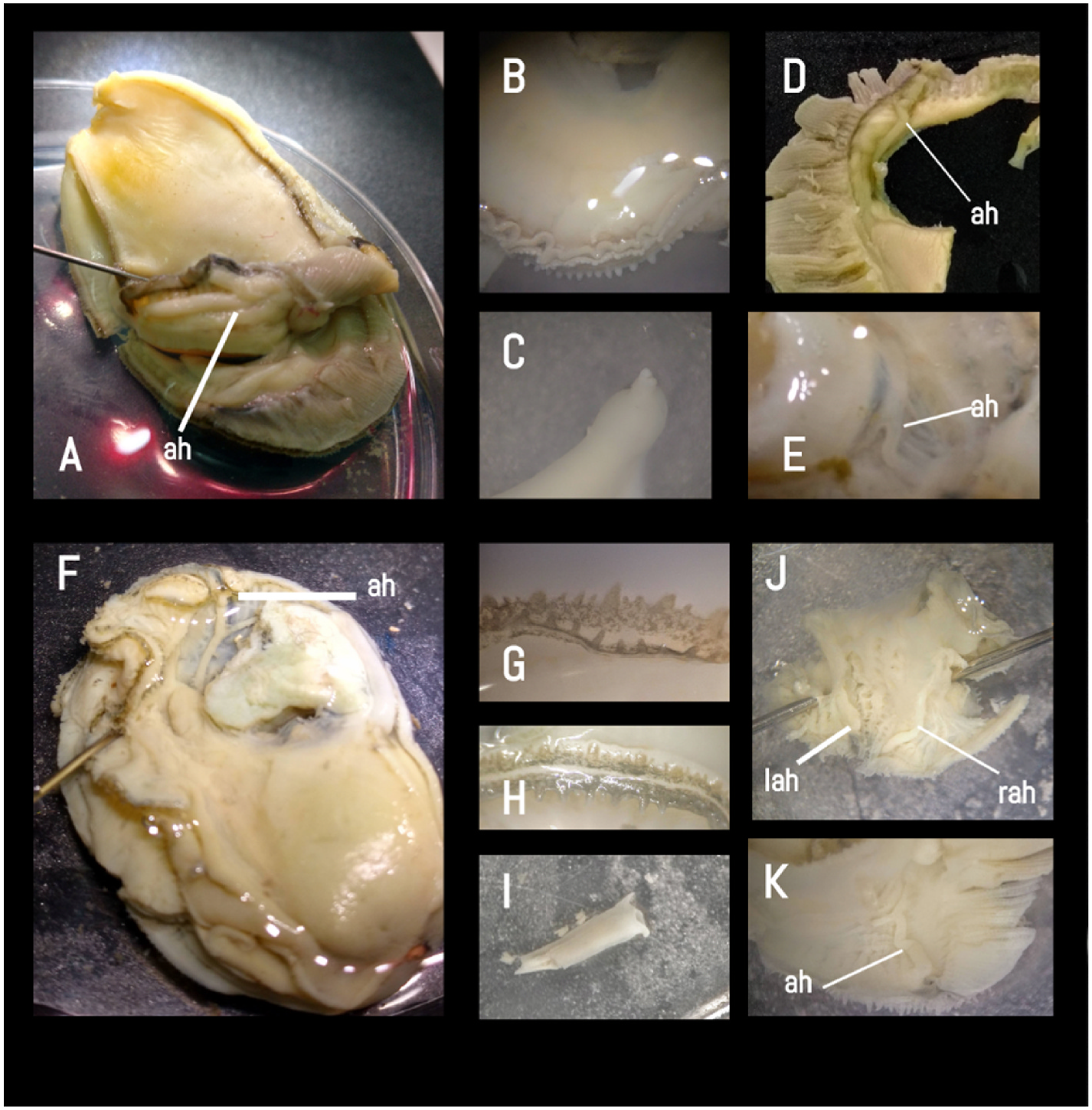
Ninth, from an anatomical perspective, Caribbean C. rhizophorae was separated from Brazilian C. rhizophorae owing to the conformation of the accessory heart. While Caribbean specimens have both lobes with a one-branched accessory heart, Brazilian specimens have only the right lobe with a three-branched accessory heart ( Amaral and Simone, 2014). However, based on genetic tracing, the accessory heart in our specimens of C. rhizophorae demonstrated different conformations, varying from both lobes with a one-branched accessory heart ( Fig. 14J View Fig ) to both lobes with three branches ( Fig. 14F, K View Fig ), forming a ‘Y’, and sometimes with an accessory heart reduced in the right lobe.
Considering all the arguments about the morphological inaccuracy of Crassostrea mangle , along with a high genetic correlation between the Caribbean and Brazilian C. rhizophorae , we discourage the use of C. mangle to address the Brazilian specimens of C. rhizophorae .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Crassostrea rhizophorae identity
| Ferreira, João Paulo Ramos, Legat, Angela Puchnick, Lazoski, Cristiano, Freire, Thais Brito, Gomes, Carlos Henrique Araújo de Miranda & de Melo, Claudio Rodrigues Manoel 2023 |
Crassostrea rhizophorae
| sensu Amaral and Simone 2014 |
C. rhizophorae
| sensu Amaral and Simone 2014 |
Crassostrea rhizophorae
| sensu Amaral and Simone 2014 |
C. rhizophorae
| sensu Amaral and Simone 2014 |
C. rhizophorae
| sensu Amaral and Simone 2014 |
C. rhizophorae
| sensu Amaral and Simone 2014 |
O. rhizophorae
| Rios 1970 |
Ostrea parasitica
| Turton 1819 |
C. virginica
| Gmelin 1791 |
Ostrea arborea
| Chemnitz sensu Morch 1785 |
O. arborea
| Chemnitz sensu Morch 1785 |
Ostrea edulis
| Linneaus 1758 |