Loxodontomys micropus (Waterhouse, 1837)
|
publication ID |
https://doi.org/ 10.1644/837.1 |
|
persistent identifier |
https://treatment.plazi.org/id/03BF87B6-ED16-A076-FF17-BCA4FD7D1F6E |
|
treatment provided by |
Carolina |
|
scientific name |
Loxodontomys micropus (Waterhouse, 1837) |
| status |
|
Loxodontomys micropus (Waterhouse, 1837) View in CoL
Southern Pericote
Mus micropus Waterhouse, 1837:17 . Type locality ‘‘ Interior plains of Patagonia in lat. 50 u, near the banks of the
Euneomys micropus alsus Thomas, 1919:202 . Type locality ‘‘ Maiten, W. Chubut. 700 meters,’’ Chubut Province, Argentina (5 El Maitén; 42 u 039S, 71 u 109W).
Auliscomys micropus micropus: Gyldenstolpe, 1932:95 . Name combination.
Auliscomys micropus alsus: Gyldenstolpe, 1932:95 . Name combination.
Phyllotis [( Auliscomys )] micropus micropus: Ellerman, 1941:455 . Name combination.
Phyllotis [( Auliscomys )] micropus alsus: Ellerman, 1941:455. Name combination.
Phyllotis (Auliscomys) micropus fumipes Osgood, 1943:214 . Type locality ‘‘ Quellon , Chiloe Island, Chile,’’ (5 Quello´ n, Chiloe´, 43 u 079S, 73 u 359 W, X Regio´ n).
[ Phyllotis (Loxodontomys) ] micropus: Osgood, 1947:172 . Name combination.
Phyllotis micropus: Hershkovitz, 1962:391 . Name combination.
L [oxodontomys]. micropus: Massoia, 1983:129 . First use of current name combination.
CONTEXT AND CONTENT. Context as for genus. Three subspecies have been recognized, although their validity has not been tested by contemporary approaches (Cabrera 1961; Osgood 1943):
L. m. micropus (Waterhouse, 1837) . See above.
L. m. alsus (Thomas, 1919). See above.
L. m. fumipes ( Osgood, 1943). See above.
NOMENCLATURAL NOTES. Osgood (1947) described Loxodontomys as a subgenus of Phyllotis to include micropus , a taxon described by Waterhouse (1837). Based on phenetic analyses of karyotypic and morphological data, Simonetti and Spotorno (1980) transferred micropus from Phyllotis to Auliscomys . The generic status of Loxodontomys was supported by morphological (Braun 1993; Steppan 1993, 1995) and molecular-based phylogenies (D’Elía 2003; Smith and Patton 1999). Loxodontomys was included in the tribe Phyllotini by Olds and Anderson (1989), Braun (1993), and Steppan (1993, 1995). Morphological analyses indicate that Loxodontomys is a sister genus to the Reithrodon group ( Ortiz et al. 2000; Steppan 1993, 1995; Steppan and Pardin˜ as 1998). However, molecular-based phylogenies have shown that this group is not part of the phyllotine radiation and also that it is polyphyletic (D’Elía 2003; see also D’Elía et al. 2006a, 2006b; Engel et al. 1998; Smith and Patton 1999); in addition, molecular-based phylogenies place Loxodontomys within the phyllotine clade and sister to Auliscomys , in agreement with phenetic studies of morphological variation (D’Elía 2003; Smith and Patton 1999; Spotorno et al. 2001). Pine et al. (1979) called attention to the fact that the name Loxodontomys may not be properly available. However, Steppan (1995:84) consid- ers this not to be the case because a genus-group comparison was implicit in Osgood’s description. Here, we follow Steppan (1995) in considering that Loxodontomys Osgood, 1947 , is available.
The type of Mus micropus was taken by Charles Darwin during his navigation of the Santa Cruz River between 18 April and 5 May 1834 (Waterhouse 1839); no specific locality was recorded along the about 240 km traveled between the mouth of the river in the Atlantic Ocean and the upper course near the Andean Cordillera. Hershkovitz (1962:392) wrote ‘‘the type of micropus was taken … perhaps in the vicinity of the present locality of La Argentina, Province of Santa Cruz, southern Argentina.’’ However, we note that La Argentina (5 Estancia La Argentina, northern coast of Lago Argentino, 50 u 109S, 72 u 379W) is more than 70 km west of the most interior point reached by Darwin along the course of the Santa Cruz River (see Cueto et al. 2008).
Etymologically, the generic epithet is probably inspired in Loxodonta , from Greek loxo, meaning slanting, and odonta, meaning tooth, whereas the specific epithet is from the Greek micros, meaning small, and pous, meaning foot.
DIAGNOSIS
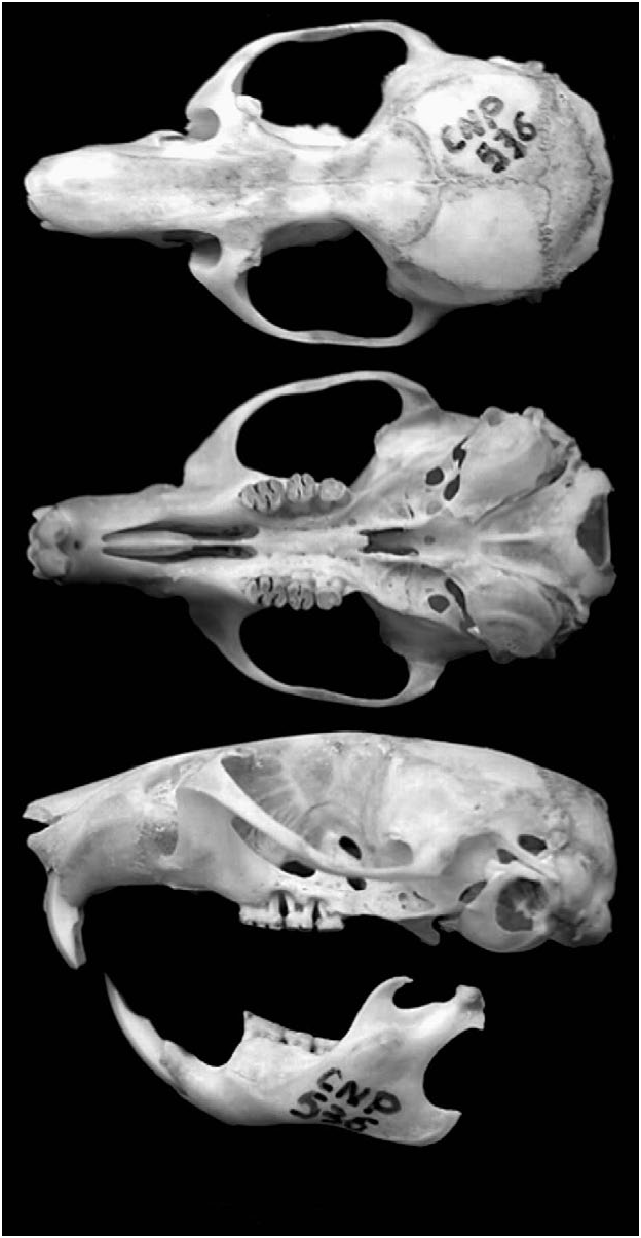
Some difficulties of identification may be encountered because some individuals of Loxodontomys micropus reach sexual maturity at a very young age (12 g; head and body 80 mm) compared with other adults of up to 100 g (head and body 150 mm). L. micropus ( Fig. 1 View Fig ) is distinguished from Phyllotis xanthopygus (yellow-rumped pericote) by its shorter tail and smaller ears ( Pearson 1995). L. micropus differs radically in external and cranial characters ( Fig. 2 View Fig ) from the slender, silky-furred, white-bellied, long-tailed, hairy-tailed Graomys griseoflavus (common pericote), which also has a divergent-sided supraorbital region ( Hershkovitz 1962). Species of Reithrodon have similar large body size, but they possess grooved incisors, a much shorter tail, and their hind feet have furry soles ( Pearson 1995). Species of Euneomys are paler, shortertailed, and have grooved upper incisors ( Pearson 1995). L. micropus is mainly distinguished from L. pikumche (pikumche pericote) by its karyotype. L. micropus has a diploid number (2n) of 34 (fundamental number [FN] 5 36–37) with telocentric autosomes except for 1 small pair that is metacentric, whereas L. pikumche has 2 n 5 32 (FN 5 34) with 4 pairs of metacentric autosomes. Also, L. micropus has hyperopistodont incisors, with tripartite (Yshaped) upper incisor dentine fissure, and M3, m2, and m3 each have 2 roots. L. pikumche has opistodont upper incisors with a short (comma-shaped) dentine fissure, and M3, m2, and m3 each have 3 roots.
GENERAL CHARACTERS
Loxodontomys micropus is a moderately large, heavybodied phyllotine. Fur is thick, lax, and lusterless. Ears are large. Feet are stout. Fifth hind toe is long, reaching to the end of the 1st phalanx of the 4th toe. Palms and soles are slightly scutulated and naked, except on heel. Tail is 75% of the length of head and body, sparsely furred, and with the terminal portion nearly uniform (Braun 1993; Hershkovitz 1962; Osgood 1943). The general color of L. micropus varies from gray to brownish, grizzled with brownish yellow and intermixed with dusky black dorsally, whereas the ventral surface is lighter, often washed with white or yellow ( Gyldenstolpe 1932; Mann Fischer 1978; Osgood 1943; Pearson 1958). The scale pattern of dorsal guard hairs is lanceolate (Chehe´bar and Martín 1989). Individual, local, and seasonal differences in L. micropus are slight. Geographic variation, if it exists, is not appreciable ( Hershkovitz 1962). The putative subspecies L. m. alsus is most similar to L. m. micropus , but almost without the strongly buffy suffusion, the general tone being more slate-gray above and clearer grayish with only a slight buffy wash on the venter ( Gyldenstolpe 1932; but see Hershkovitz 1962). According to Osgood (1943), L. m. fumipes is most similar to L. m. micropus , but has the upper side of all feet darker, somewhat brownish or sooty instead of whitish. Ranges of body measurements (mm) of types were: total length, 237–242; length of tail, 81–117; length of hind foot, 27–32; and length of ear, 19.3 ( Gyldenstolpe 1932; Osgood 1943). Means (mm) of total length (range, n) in adult male and female specimens, respectively, from northwestern Argentine Patagonia were: 133.1 (118–154, 20) and 131.5 (124–147, 25— Pearson 1983). Average measurements (mm) for 22 specimens from Aysén, southern Chile ( Kelt 1994), were: total length, 224.0; length of tail, 95.5; length of hind foot, 27.9; and length of ear, 20.5. The mean body mass (g) of 146 trapped individuals of all ages in northwestern Argentine Patagonia was 57.6, with a range from 10.5 to 105 g. Body mass of adult males varied from 50 to 103 g and they were not significantly heavier those of females (45–105 g), nor were males longer than females ( Pearson 1983). The mean body mass of 22 specimens from Ayse´n, southern Chile, was 62.67 g ( Kelt 1994).
The skull is robust. The interorbital region is narrow and the sides are slightly concave, with the borders square or raised. Hershkovitz (1962:477) stated that in L. micropus interfrontal fontanelles ‘‘are common, though rarely well developed ….’’ Only 1 specimen (American Museum of Natural History [AMNH] 14290, from Río Chico, Santa Cruz, Argentina) of 14 L. micropus examined by Gardner and Anderson (2001) had a fenestra. Nasals are broader than the interorbital constriction. Zygomatic arches are markedly expanded toward the posterior end. Zygomatic plate is narrow and high, with a rounded anterodorsal border and a straight or slightly concave anterior border. Premaxillo–maxillar suture is straight. Incisive foramina are well opened, ending at the level of the paracone and protocone of M1. Mesopterygoid fossa is distinctly narrower than the parapterygoid fossa. Posterior border of palatal bridge has a short, blunt median process. Internal carotid bounded by both auditory bulla and occipital. Sphenopal- lower (n 5 7) molars of L. micropus from Santa Cruz, Argentina (Pardin˜ as 1997), were: length of M1, 2.47; width of M1, 1.68; length of M2, 1.60; width of M2, 1.53; length of M3, 1.49; width of M3, 1.34; length of M1–3, 6.00; length of m1, 2.38; width of m1, 1.53; length of m2, 1.63; width of m2, 1.58; length of m3, 1.89; width of m3, 1.40; and length of m1–3, 5.94.
atine foramen is absent or nearly ossified. Squamosal fenestra is present between squamosal groove and masticatory–buccinator trough. Mandible is robust and short, with large coronoid process and marked capsular projection. Posterior border of mandibular symphysis is sharply angled (Braun 1993; Hershkovitz 1962; Steppan 1993, 1995). Average measurements (mm) of skull and mandible for 22 individuals ( Kelt 1994) were: greatest length of skull, 31.41; length of nasal bone, 13.07; length of maxillary diastema, 7.60; length of maxillary toothrow, 5.98; length of palate, 14.09; breadth across rostrum, 4.89; width of braincase, 13.79; zygomatic width, 17.83; width of incisors (measured at the alveolus), 3.48; length of mandibular diastema, 3.41; length of mandibular toothrow, 6.08; and greatest length of mandible, 16.79. Measurements (mm) for upper (n 5 9) and
DISTRIBUTION
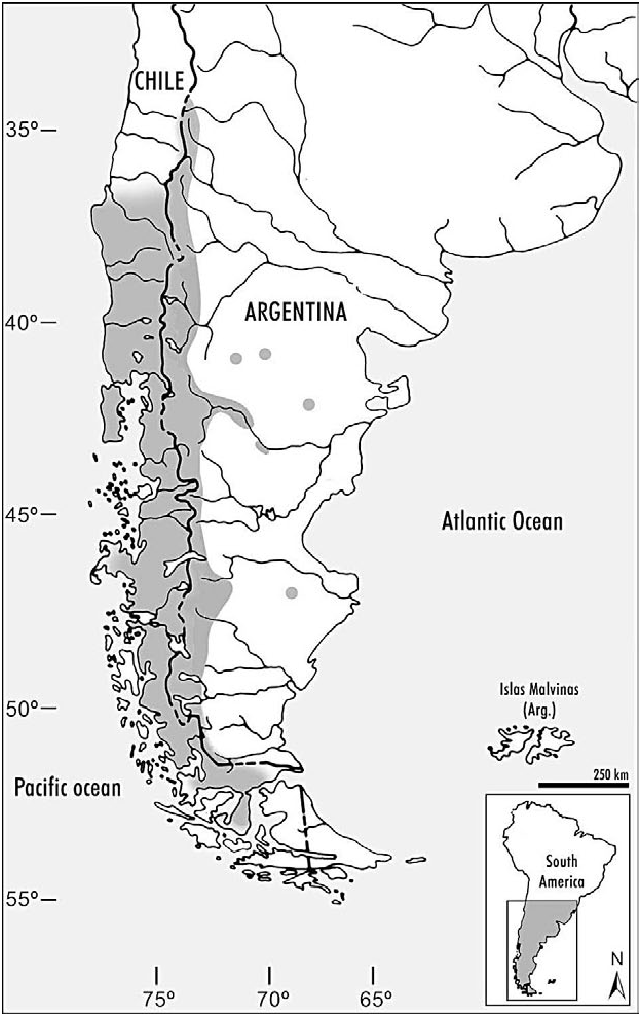
Loxodontomys micropus occurs in southern Chile and southwestern Argentina ( Fig. 3 View Fig ). In Argentina it ranges from about 35 u S south to southernmost Santa Cruz Province. In Chile it ranges along the Andes from about Ñuble Province (VIII Regio´n) south to the Strait of Magellan (Magallanes Province, XII Regio´ n), including Chiloe´ Island ( Hershkovitz 1962; Osgood 1943; Reise and Venegas 1987; Tamayo and Frassinetti 1980). Punta Arenas is the southernmost documented locality (Pine et al. 1979). L. micropus is absent on Tierra del Fuego Island ( Hershkovitz 1962; Osgood 1943; Pearson 1958). In Argentina, this species has been caught in the provinces of Neuquén, Río Negro, Chubut, and Santa Cruz ( Hershkovitz 1962). Specimens from Mendoza Province, Argentina, were referred to this species, mostly based on morphological characteristics ( Jayat et al. 2006; Pardiñas et al. 2008). The presence of L. pikumche (a species endemic to central Chile) in Mendoza, as has been suggested by Spotorno et al. (1998), needs adequate documentation. L. micropus is mostly restricted to forests, meadows, and mesic brushy habitats in the Andean foothills at elevations from sea level to 3,000 m. The known distribution of L. micropus has been considerably expanded to the east with the recent finding of several isolated populations in central basaltic plateaus and hills of Chubut, Río Negro, and Santa Cruz (Teta et al. 2002).
FOSSIL RECORD
Loxodontomys micropus was found in several archeological deposits of the latest Pleistocene–Holocene in the Argentinean and Chilean Patagonia (Andrade and Teta 2003; Pardin˜ as 1998, 1999a, 1999b; Pearson 1987; Pearson and Pearson 1993; Rebane 2002; Teta et al. 2005). Some of these records, especially those of the last 3,000 years, are extralimital to the current distribution of this species, suggesting that L. micropus has had a more continuous distribution in steppe areas during the cold-humid phases of the late Holocene (Pardin˜ as 1999b; Teta et al. 2005).
Some extinct presumed Phyllotini , such as Olympicomys and Ichthyurodon , from Plio–Pleistocene deposits of eastern Argentina, show similarities with Loxodontomys (Steppan and Pardin˜ as 1998). Ichthyurodon shares with Loxodontomys the tendency to exhibit involution in lower molars; whether this trait is a convergence or whether it indicates common ancestry is a debatable issue.
At least 3 extinct species are described under Auliscomys , a genus considered phylogenetically close to Loxodontomys . They are A. formosus , the oldest known South American sigmodontine (Pardin˜ as and Tonni 1998; Reig 1978), and A. fuscus and A. osvaldoreigi recently identified from upper Pliocene deposits (Quintana 2003). Although formosus seems to belong to Auliscomys sensu stricto (i.e., excluding micropus ), osvaldoreigi and fuscus can be clearly removed from this genus and arranged under Olympicomys and Panchomys , pending whether they are valid species or synonyms of O. vossi and P. steppani , respectively ( Pardiñas 1997; Pardin˜ as et al. 2002).
FORM AND FUNCTION
Loxodontomys micropus has 4 pairs of mammary glands: pectoral, postaxial, abdominal, and inguinal ( Gyldenstolpe 1932). Counts of vertebrae in L. micropus (7 specimens) yield 13 T, 6–7 L, and 28–29 Ca .
Dental formula is i 1/1, c 0/0, p 0/0, m 3/3, total 16 (Steppan 1993, 1995). Upper incisors are opisthodont, with orange frontal enamel and without grooves (however, 1 specimen from Punta Arenas, Chile, has the left upper incisor distinctly grooved according to Osgood [1943:213]). Molars are hypsodont with slightly alternating main cusps and flat crowns in adults (sensu Hershkovitz 1962). Mesolophs/lophids (including mesostyle/stylids) and enterostyle/stylids are absent, as in many phyllotines (see Steppan 1995). The molars of L. micropus have been selected as a good example of an ‘‘involution’’ process; that is ( Hershkovitz 1962:93 and figs. 17.C and 17.D): ‘‘the whole or part of a planed molar crown may be modified into a C-, or E-, or Sshaped pattern by varying degrees of penetration of each of the enamel folds.’’ M1 is trilophodont; main lophs are oriented at about 45 u; and folds penetrate to the middle line of the molar. The laminated procingulum is compressed anteroposteriorly and oblique in orientation, without evidence of an anteromedian flexus; the anteroloph appears as a short enamel spur. M2 is similar to M 1 in occlusal design. M3 is slightly smaller than M2, with a departing morphological pattern evidenced by a persistent free connection between hypoflexus and paraflexus producing a posterior continuous lamina; paracone is labially projected by a short paralophule. The m1 is elongated and trilophodont with a compressed procingulum, without anteromedian flexid, rotated lingually; a strong anterolabial cingulum connects the procingulum with protocone in adult individuals. The m2 shows an E or epsilon pattern. The m3 is simplified and slightly smaller relative to m2, with a clearly S or sigmoid pattern ( Hershkovitz 1962). Number of roots per molar is: M1, 3 (occasionally 4); M2, 3; M3, 3; m1, 4–3–2; m2, 2–3; and m3, 2 ( Pearson 1995).
Phallus morphology was studied for 3 specimens of L. micropus ; their description was given combined with those of 2 Auliscomys species (boliviensis and sublimis —see Spotorno 1986:120–121): ‘‘These phalli vary in shape from a barrel to an elongated form. The lateral processes are less developed than the central one, and distally, they have small lateral hooks. The proximal baculum is always long and generally slender, except micropus ; the base is notched in micropus and sublimis. Urethral processes are long [and unilobed, see Braun 1993] in micropus and sublimis, but not in boliviensis.’’ The distal bacular cartilage is tridigitate, with the medial digit longer than the lateral digits. The lateral digits have small lateral hooks (Braun 1993). The medial ventral prostates are 1.5–3 times as long as the lateral ventral prostates; the lateral ventral prostates appear unusually small and are closely pressed against the lateral surfaces of the tract; vesiculars are medially lobed along their greater curvatures; subterminal flexures are rounded and smooth (Voss and Linzey 1981).
ONTOGENY AND REPRODUCTION
The reproductive season of Loxodontomys micropus starts at the beginning of the spring and lasts until the end of summer ( Kelt 1994; Pearson 1983). In southern Chile, limited activity continues into early autumn ( Kelt 1994). All overwintered adults are in breeding condition by spring, and by the end of November a few of the young-of-the-year have large testes and vesicular glands ( Pearson 1983). In Chile, 21 of 25 males with descended testes were captured in November and December ( Kelt 1994). Breeding males had testes of 8 mm or longer and vesicular glands of 10 mm or longer ( Pearson 1983). All overwintered females were pregnant or parous in spring ( Pearson 1983). In northwestern Argentine Patagonia, pregnant females were caught early in November, and lactating females in mid-November ( Pearson 1983). In Chile, 9 of 13 parous females and 14 of 18 pregnant females were taken in November and December, whereas 13 postpartum females were collected in April ( Kelt 1994). By mid-February and March, few animals remain reproductively active, and in April most females were either nulliparous or lactating ( Kelt 1994). Pine et al. (1979) recorded pregnant females from Chile in January and March, and lactating females in mid-March. Two specimens caught in January had uterine swellings equaling 5 and 12 mm (Pine et al. 1979). In Argentina, average number of embryos was 4.01 (range 1–7, n 5 27 — Pearson 1983); in Chile the average was 4.8 (range 4–5, n 5 5 — Greer 1965; Pine et al. 1979). There was no relationship between the number of fetuses and the age of female ( Pearson 1983).
Juveniles were recorded in spring and in higher numbers and proportions during summer and autumn ( Pearson 1983). The young become independent at a very early age ( Pearson 1983). Both sexes were reproductively active after reaching 48 g of body mass ( Pearson 1983).
ECOLOGY
Population characteristics.— Population densities of Loxodontomys micropus in a uniform forest of Nothofagus antarctica at La Veranada, 43 km south-southwest of Bariloche ( Argentina), varied from 5.1 individuals/ha in May to 1.7–4.2 individuals/ha in November (Pearson and Pearson 1982). In a mixed forest of N. dombeyi and N. pumilio at Río Castan˜ o Overo, 44 km west of Bariloche ( Argentina), 0.9, 1.1, and 4.1 individuals/ha were recorded in the months of May, November, and April, respectively (Pearson and Pearson 1982). In small mammal communities of Nahuel Huapi National Park, Río Negro and Neuquén provinces, and adjacent areas of northwestern Argentine Patagonia, the probability of capturing L. micropus in different habitats was: forest, 0.08; shrub, 0.18; bush steppe, 0.02; bunch grass, 0.06; weeds, 0.17; turf, 0.02; rocky habitats, 0.02; and bare areas, 0.41 ( Pearson 1987, 1995). In Patagonia, sex ratio of a sample of 151 individual of all ages was insignificantly biased toward females (55%). The spring ratio was equal, but the autumn ratio was only 35% male based on 37 captures. Greer (1965) also reported an excess of females in populations from central Chile. Autumn populations consist mostly of young of the year; these will have matured to the old adult class by the next November, at which time a new cohort is entering the trappable population ( Pearson 1983). Early spring recruitment strongly skews the age structure toward very young animals, whereas by autumn these animals are mature. Two females that weighed 60 and 65 g when captured and released in May were recaptured 5.5 months later and had gained 14 and 13 g, respectively ( Pearson 1983).
Space use.— Loxodontomys micropus lives in forest if there is sufficient ground cover. In precordilleran shrubby steppes of northwestern Argentine Patagonia it has been caught in humid, dense grasslands or among bushes such as Berberis or the thorny Colletia spinossissima ( Monjeau 1989; Pearson 1983). In Chile, this species is found in bushy forests of Nothofagus pumilio and N. antarctica , and in N. dombeyi ( Fig. 4 View Fig ) and Araucaria araucana forests, with dense, low vegetation of Chusquea (Reise and Venegas 1987) . In Osorno (X Regio´ n, Chile), 1 specimen was caught in a mixed forest of Laurelia philippiana , Saxegothaea conspicua , and Nothofagus dombeyi (Pine et al. 1979) . In Ayse´n (southern Chile), L. micropus was found in meadows with lush grasses and loose soils and in open-canopied or 2nd-growth forests of N. dombeyi or N. antarctica ( Kelt 1994) . Along an elevational transect through temperate rain forests in Valle de La Picada (Osorno, Chile), L. micropus was taken more commonly in shrubby habitats of Nothofagus dombeyi and Drimys winteri at higher elevations ( Patterson et al. 1989, 1990).
Diet.— Loxodontomys micropus is primarily herbivorous. Stomachs may be filled with up to 10 g of chewed green leaves and fungi ( Pearson 1983). In individuals from Chile, stomachs were found to contain seeds (17%), fruits (28%), flowers (20%), other vegetal tissues (23%), and fungi (6%— Meserve et al. 1988). In humid, dense grasslands of northwestern Patagonia, stomach contents of L. micropus consisted mainly of grasses, such as Carex , Festuca argentina , Juncus , Poa pratensis , and Stipa ( Monjeau 1989) .
Diseases and parasites.— A considerable list of ectoparasites has been reported for Loxodontomys micropus . Those from individuals obtained in Argentina include 1 specific sucking louse, Hoplopleura auliscomydis (Anoplura: Hoplopleuridae ; described from specimens collected in Collon Cura, Neuqueń —Castro et al. 2001), and several fleas ( Siphonaptera ), Craneopsylla minerva wolffhuegeli ( Stephanocircidae ; on specimens from Estancia La Ensenada, Santa Cruz —Sa´nchez 2007), Ctenoparia topali ( Hystrichopsyllidae ; on specimens from Lago Ñorquinco, Neuque´n), Agastopsylla boxi gibbossa ( Ctenophthalmidae ; type host: L. micropus , type locality: Cerro Chapelco, Neuque´n), and Tetrapsyllus (Tetrapsyllus) tantillus ( Rhopalopsyllidae ; on specimens from Arroyo Chapelco and Lago Ñorquinco, Neuque´n—Beaucournu and Alcover 1989). From Chilean specimens, numerous fleas have been reported (Alarcón 2000, 2003; Beaucournu and Gallardo 1991, 1992) including Ctenoparia intermedia , C. jordani , C. topali (Hystrichopsyllidae) ; Agastopsylla boxi boxi , Chiliopsylla allophyla allophyla (Ctenophthalmidae) ; Barreropsylla excelsa , Sphinctopsylla ares (Stephanocircidae) ; and Ectinorus (Ectinorus) ixanus , Ectinorus (Ichyonus) onychius onychius , Listronius ulus , Tetrapsyllus (Tetrapsyllus) rhombus , and Tetrapsyllus (T.) tantillus (Rhopalopsyllidae) . Only 1 endoparasite, the nematode Stylestrongilus valdivianus (Trichostrongyloidea; Heligmonellidae ), has been recovered from L. micropus ; it infects the small intestine of Chilean specimens (Durette- Desset and Muru´ a 1979; see also Pe´rez-Ponce de Leo´ n et al. 2000).
Hantavirus antibody (Andes strain) was detected in 1 of 10 L. micropus caught in Bolso´n, northwestern Argentine Patagonia (Cantoni et al. 2001), and in 1 individual from Palmucho, BioBío, VIII Regio´n, Chile ( Ortiz et al. 2004; Pavletic 2000). There is another seropositive individual reported for Ayse´n, Chile (Cerda and Sandoval 2004). Apparently, these infections were produced by ‘‘spill-over’’ from the main reservoir of this virus, the sigmodontine Oligoryzomys longicaudatus (Muru´a and Padula 2004).
Interspecific interactions.— In forested areas of Argentina, Loxodontomys micropus is associated with the marsupials Dromiciops gliroides (monito del monte) and Rhyncholestes raphanurus (long-nosed caenolestid) and the sigmodontine rodents Abrothrix longipilis (long-haired akodont), A. olivaceus (olive-colored akodont), Chelemys macronyx (Andean long-clawed akodont), Geoxus valdivianus (Valdivian long-clawed akodont), Irenomys tarsalis (large-footed irenomysa), Oligoryzomys longicaudatus (long-tailed colilargo), and Rattus norvegicus (brown rat— Monjeau et al. 1997, 1998; Pearson and Pearson 1983). In the sub-Andean ecoprovince of Patagonia, at the forest– steppe ecotone, L. micropus was trapped syntopically with A. longipilis , A. olivaceus , C. macronyx , Eligmodontia morgani (west Patagonian laucha), and O. longicaudatus ( Monjeau 1989; Pearson 1987). Small mammals recorded in southern Chile with L. micropus include A. longipilis , A. olivaceus , C. macronyx , Ctenomys coyhaiquensis (Coyhaique tuco-tuco), D. gliroides , Euneomys chinchilloides (Tierra del Fuego euneomys), G. valdivianus , I. tarsalis , Mus musculus (house mouse), O. longicaudatus , Phyllotis xanthopygus , R. norvegicus , and Reithrodon auritus (hairy-soled conyrat— Kelt 1994, 1996; Patterson et al. 1989, 1990).
Throughout its range, L. micropus is preyed on by Tyto alba (common barn owl) and Bubo magellanicus (Magellanic horned owl—Donázar et al. 1997; Iriarte et al. 1990; Massoia 1983; Massoia et al. 1991, 1994; Massoia and Lartigau 1995; Massoia and Pardin˜ as 1994; Montserrat et al. 2005; Nabte et al. 2006; Pardin˜ as and Massoia 1989; Pillado and Trejo 2000; Teta et al. 2001; Travaini et al. 1997; Trejo and Grigera 1998; Udrizar Sauthier et al. 2005; Yan˜ ez et al. 1978). In addition, in northwestern Argentine Patagonia, L. micropus is a minor component of the diet of Caracara plancus (crested caracara—Travaini et al. 2001) and Geranoaetus melanoleucus (black-chested buzzard-eagle— Massoia and Pastore 1997; Trejo et al. 2006). Remains of this species also have been recovered in feces of Galictis cuja (lesser grison) and Lycalopex culpaeus (culpeo fox) from Neuque´n Province in Argentine Patagonia (Corley et al. 1995; Delibes et al. 2003). In Chile, L. micropus is an important prey item of Asio flammeus (short-eared owl) in agricultural landscapes of Osorno ( Martínez et al. 1998). In temperate rain forests of southern Chile, L. micropus is a component of the diet of Puma concolor (puma), Lycalopex griseus (gray fox), Lycalopex fulvipes (Darwin’s fox), Leopardus geoffroyi (Geoffroy’s cat), and Strix rufipes (rufous-legged owl— Elgueta et al. 2007; Johnson and Franklin 1991; Martínez and Jaksic 1996, 1997; Rau et al. 1991; but see Figueroa et al. 2006).
Miscellaneous.— In captivity, numerous adults can live amicably in the same cage ( Pearson 1983). Captives ate fungi of several kinds, including llao llao ( Cyttaria ); leaves of Vicia , Oenothera , and Mulinum spinosum ; and blossoms of dandelion ( Taraxacum officinale ), Colletia spinossissima , and Mulinum spinosum . They ate stems of Calceolaria , but not the blossoms ( Pearson 1983).
Loxodontomys micropus is mostly nocturnal, but may exhibit diurnal activity at times ( Kelt 1994; Pearson 1983). In humid, dense grasslands of northwestern Patagonia, L. micropus excavates tunnel systems, with numerous entrances and food chambers. Tunnels are 4 cm in diameter ( Monjeau 1989). L. micropus climbs skillfully ( Pearson 1983).
GENETICS
Specimens from 3 Chilean localities at Malleco, Valdivia, and Ayse´n have been karyotyped. Loxodontomys micropus has a diploid number (2n) of 34 and a fundamental number (FN) of 36 or 37 (Venegas 1974; Walker and Spotorno 1992). All autosomes are telocentric; except 1 small pair that is metacentric. The X chromosome is telocentric and the Y chromosome is a small submetacentric (Spotorno and Walker 1979; Walker and Spotorno 1992). Constitutive heterochromatin is confined to centromeric regions of almost all chromosomes; telomeric nucleolar organizing regions were detected in the following 4 chromosomal pairs: 3, 6, 9, and 11 (Walker and Spotorno 1992).
No published study has assessed the genetic variation of different populations of L. micropus ; unpublished preliminary results (C. Can˜ on et al., in litt.) based on cytochrome- b gene sequences show that L. micropus has little phylogeographic structure.
CONSERVATION
Loxodontomys micropus is categorized as of least concern in Argentina (Díaz and Ojeda 2000; Reca et al. 1996) and Chile (Mun˜ oz-Pedreros and Yáñez 2000; see also Baillie 1996; Johnson et al. 1990). Populations are protected in several national parks, reserves, and other protected areas of Argentina and Chile, including Epu Lauquen, Lanín, Los Arrayanes, Nahuel Huapi, Los Alerces, Perito Moreno, Los Glaciares, and Bosques Petrificados in Argentina (Heinonen Fortabat and Chebez 1997); and Alto Bío Bío, Nahuelbuta, Puyehue, and Torres del Paine in Chile ( Greer 1965; Johnson et al. 1990; Reise and Venegas 1987).
| T |
Tavera, Department of Geology and Geophysics |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Loxodontomys micropus (Waterhouse, 1837)
| Teta, Pablo, Pardiñas, Ulyses F. J., Sauthier, Daniel E. Udrizar & D'ElÍa, Guillermo 2009 |
Phyllotis micropus:
| HERSHKOVITZ, P 1962: 391 |
Phyllotis (Loxodontomys)
| OSGOOD, W 1947: 172 |
Phyllotis (Auliscomys) micropus fumipes
| OSGOOD, W 1943: 214 |
Phyllotis
| ELLERMAN, J 1941: 455 |
Phyllotis
| ELLERMAN, J 1941: 455 |
Auliscomys micropus micropus:
| GYLDENSTOLPE, N 1932: 95 |
Auliscomys micropus alsus:
| GYLDENSTOLPE, N 1932: 95 |