Zanha africana, Radlk
|
publication ID |
https://doi.org/ 10.1016/j.phytochem.2016.01.008 |
|
DOI |
https://doi.org/10.5281/zenodo.10515255 |
|
persistent identifier |
https://treatment.plazi.org/id/03C0879E-2B16-0A47-FFC6-4CF1FBF91525 |
|
treatment provided by |
Felipe |
|
scientific name |
Zanha africana |
| status |
|
2.1. Identification of nor-hopanes in Z. africana View in CoL
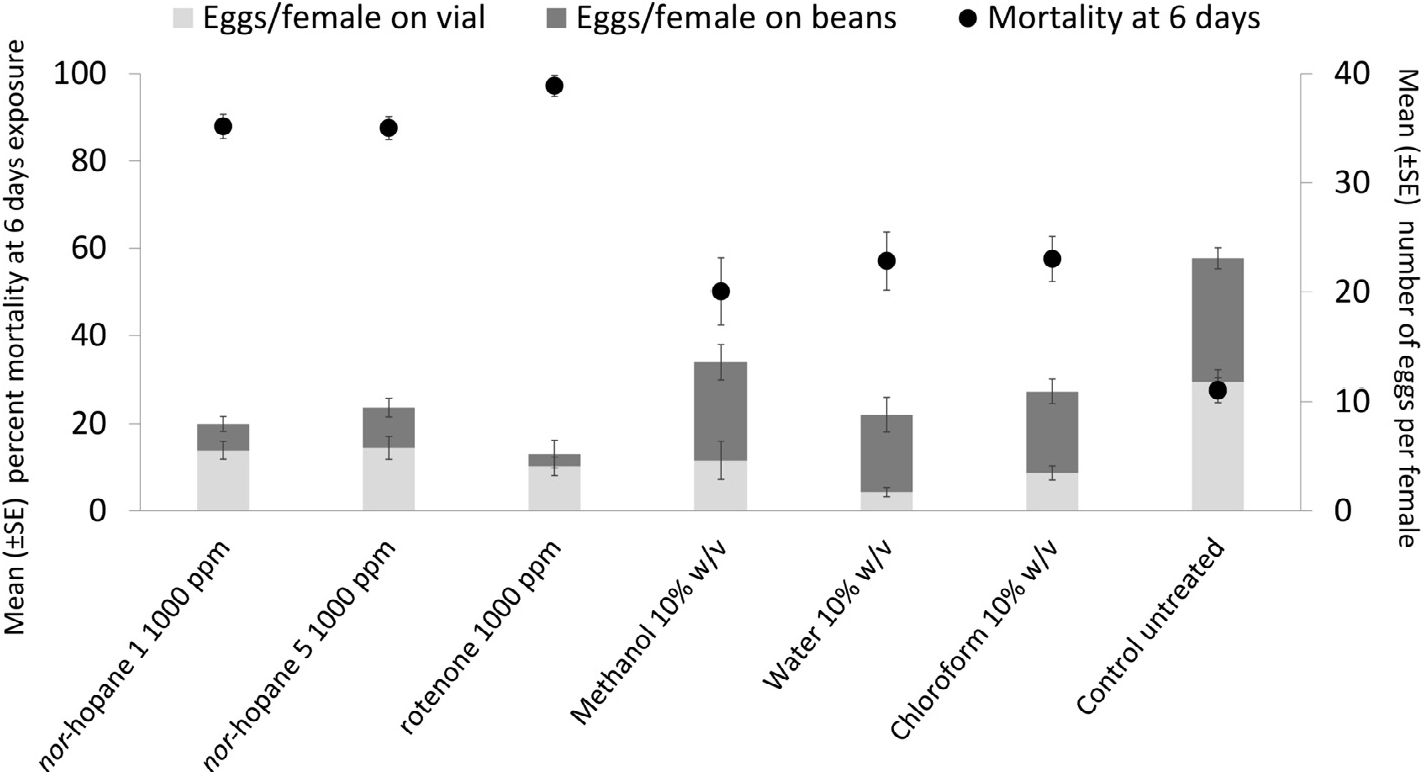
The chloroform extract of Z. africana root bark was shown to be toxic to a bruchid beetle, Callosobruchus maculatus (L.), in bioassays described in detail below (Sections 2.2 and 3.1). This extract was analysed using LC-UV-MS/MS and indicated the presence of numerous non-polar peaks with similar UV spectra. Seven compounds, 1–7 ( Fig. 1 View Fig ), were isolated using semi-preparative HPLC and characterized using spectroscopic techniques.
Full assignment of the 1 H and 13 C NMR spectra of 1 in CDCl 3 was obtained using COSY, HSQC and HMBC data ( Tables 1 View Table 1 and 2 View Table 2 ). The 13 C NMR assignments of 1 showed a good match with those for 3β,6β- dihydroxy-7β-[(4-hydroxybenzoyl)oxy]-21oi H -24-norhopa-4(23),22(29)-diene ( Chávez et al., 1997). Good agreement was also found between the 1 H NMR assignments and a partial dataset given by the latter authors, with the exception of the assignments of H-9 and H-13 which required revision ( Table 1 View Table 1 ). A second NMR dataset for 1 was acquired in MeOH- d 4 because of the improved resolution of the multiplet structure of several key resonances including H-3 and H-6. A series of 1D site selective ROE experiments indicated that 1 had the same relative configuration as the published structure ( Fig. 1 View Fig ). In particular, the oi- configuration of H-21 was confirmed by an ROE correlation with 28-Me. Other key ROE correlations were between 28-Me and 27-Me, 27-Me and H-7 (confirming the β- configuration of the 7-(4-hydroxybenzoyl)oxy group), H-7 and H-5, H-6 and H-5 (confirming the β- configuration of the 6-OH group), H-5 and H-3 (confirming the β- configuration of the 3-OH group) and 25-Me and 26-Me. The optical rotation for 1 of u D = +45.7 (c 0.54, MeOH) had the same sign as the literature value of u D = +10 (c 0.79, CHCl 3).
The molecular formula of 2 established by HRESIMS as C 36 H 50 O 6 differed from that of 1 by the inclusion of one additional oxygen atom. Full assignment of the 1 H and 13 C NMR spectra of 2 was carried out in both CDCl 3 and MeOH- d 4. Comparison of the latter with the analogous assignments for 1 indicated that 2 possessed an oxygenated methine in place of a methylene group. In the COSY spectrum (CDCl 3), the oxygenated methine (δ H 4.30) correlated with H-9 (δ H 1.65) and 12- CH 2 (δ H 1.81 and 1.59). Similarly, in the HMBC spectrum acquired in CDCl 3, correlations were observed from δ H 4.30 to C-8 (δ C 48.4), C-9 (δ C 54.2), and C-10 (δ C 39.8). The additional hydroxyl group was therefore located at C-11. NOE connectivities observed between both 25-Me and 26-Me with H-11 indicated that this hydrogen atom was β- oriented. The significant downfield shift (Δ δ + 1.06 ppm) experienced by H-1β (δ H 2.89 in CDCl 3) was also consistent with an oi- configuration for 11-OH ( Isaka et al., 2011). Compound 2 was therefore 3β,6β,11oi- trihydroxy-7β-[(4-hydroxybenzoyl)oxy]-21oi H -24-norhopa-4(23),22(29)- diene.
The 1 H NMR spectrum of 3 was similar to that of 2 with the exception that H-11 (δ H 5.48 in MeOH- d 4) showed a significant downfield shift (Δ δ + 1.28 ppm) and a 3H singlet at δ H 2.03 was observed corresponding to an acetyl group (δ C 172.1 and 22.1). In the HMBC spectrum, H-11 correlated with the acetyl carbonyl group at δ C 172.1 and also with C-9 (δ C 52.9). In the COSY spectrum, H-11 correlated with both H-9 and 12- CH 2 as expected. The magnitude of the coupling constant J 9,11 of 11.3 Hz indicated a diaxial relationship between these hydrogen atoms such that H-11 was β- oriented. Compound 3 was therefore 11oi- acetoxy-3β,6β- dihydroxy-7β-[(4-hydroxybenzoyl)oxy]-21oi H -24-norhopa-4(23),22(29)- diene.
The main difference between the 1 H NMR spectra of 4 and 2 was that the former contained resonances corresponding to two 4- hydroxybenzoyl groups rather than one. The first set of resonances was assigned to the 4-hydroxybenzoyl group at 7-OH. The second was placed at 11-OH on the basis of the large downfield shift (Δ δ + 1.56 ppm) experienced by H-11 (δ H 5.76) and the long-range correlation between this proton and the remaining 4-hydroxybenzoyl carbonyl at δ C 167.3. As expected, H-11 correlated with H-9 and 12- CH 2 in the COSY spectrum. In common with 3, J 9,11 for 4 was also 11.3 Hz, confirming a diaxial relationship between H-9 and H-11 with the latter β- oriented. Thus compound 4 was 3β,6β- dihydroxy-7β,11oi- di[(4-hydroxybenzoyl)oxy]-21oi H -24-norhopa-4(23), 22(29)-diene.
A full set of 1 H and 13 C NMR resonance assignments was obtained for 5 using COSY, HSQC and HMBC data. Compound 5 was isomeric with 1 and could be readily identified as a 24- norhopadiene derivative. The difference between the two compounds resided in the structure of the E-ring. In the case of 5, the E-ring was a fused cyclopentene with an isopropyl group at C-21, whereas 1 featured an isopropylidene group attached to C-21 of a fused cyclopentane moiety. The multiplet structure and J -values for H-3, H-6, and H-7 (MeOH- d 4) were similar for 5 and 1 indicating that the configurations of these atoms were conserved between the two compounds. Thus 5 was 3β,6β- dihydroxy-7β-[(4-hydroxybenzoyl)oxy]-24-norhopa-4(23),17(21)-diene.
The molecular formula of compound 6 differed from that of 5 by the inclusion of one additional oxygen atom, and it was also isomeric with 2. Analysis of its 1 H and 13 C NMR spectra indicated that 6 was the 11oi- hydroxyl derivative of 5. Thus in the HMBC spectrum acquired in CDCl 3, H-11 (δ H 4.25) correlated with C-8 (δ C 48.5), C-9 (δ C 54.5), C-10 (δ C 39.8) and C-12 (δ C 36.6). Similarly in the COSY spectrum, H-11 correlated with H-9 and 12- CH 2. The relative configuration of 6 was examined in a series of 1D site selective ROE experiments ( Fig. 2 View Fig ). In particular, H-11 correlated with both 25-Me and 26-Me, as was also found with the isomeric 2, allowing the β- orientation to be assigned. Thus 6 was 3β,6β,11oitrihydroxy-7β-[(4-hydroxybenzoyl)oxy]-24-norhopa-4(23),17(21)- diene.
Comparison of the 1 H NMR spectra of 7 with 6 indicated that 2- CH 2, H-5 and 23- CH 2 were all downfield shifted, and the doublet of doublets resonance of H-3 was lacking. In the 13 C NMR spectrum, there were 3 rather than 4 resonances attributable to oxygenated methines and a new resonance at δ C 204.8 assigned to a carbonyl group. The location of the latter was readily established as C-3 from HMBC data, with correlations from 1- CH 2, 2- CH 2, H-5, and 23- CH 2 to δ C 204.8 detected in the spectrum. Compound 7 was thus 6β,11oi- dihydroxy-7β-[(4-hydroxybenzoyl)oxy]-3-oxo-24- norhopa-4(23),17(21)-diene.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.