Iniistius bakunawa, Sorgon & Tea & Meren & Nañola Jr, 2023
|
publication ID |
https://doi.org/ 10.26107/RBZ-2023-0038 |
|
publication LSID |
lsid:zoobank.org:pub:D0F97218-433B-4C35-A59E-7CE257A00BFE |
|
persistent identifier |
https://treatment.plazi.org/id/7F848340-BCB3-403F-A6D6-CD41D4C8F928 |
|
taxon LSID |
lsid:zoobank.org:act:7F848340-BCB3-403F-A6D6-CD41D4C8F928 |
|
treatment provided by |
Felipe |
|
scientific name |
Iniistius bakunawa |
| status |
sp. nov. |
Iniistius bakunawa , new species
Eclipse-spot Razor Wrasse ( Figs. 1–3 View Fig View Fig View Fig ; Table 1)
Iniistius sp. ( Fukui, 2017): 184 (colour photograph of specimen from Panay Island, Philippines [reproduced here in Fig. 1A View Fig ; KAUM-I. 80684]).
Holotype. PNM 15676, 151.2 mm SL, purchased from public fish market in Loay, Bohol, Philippines, K.E.S. Sorgon et al., 20 April 2018.
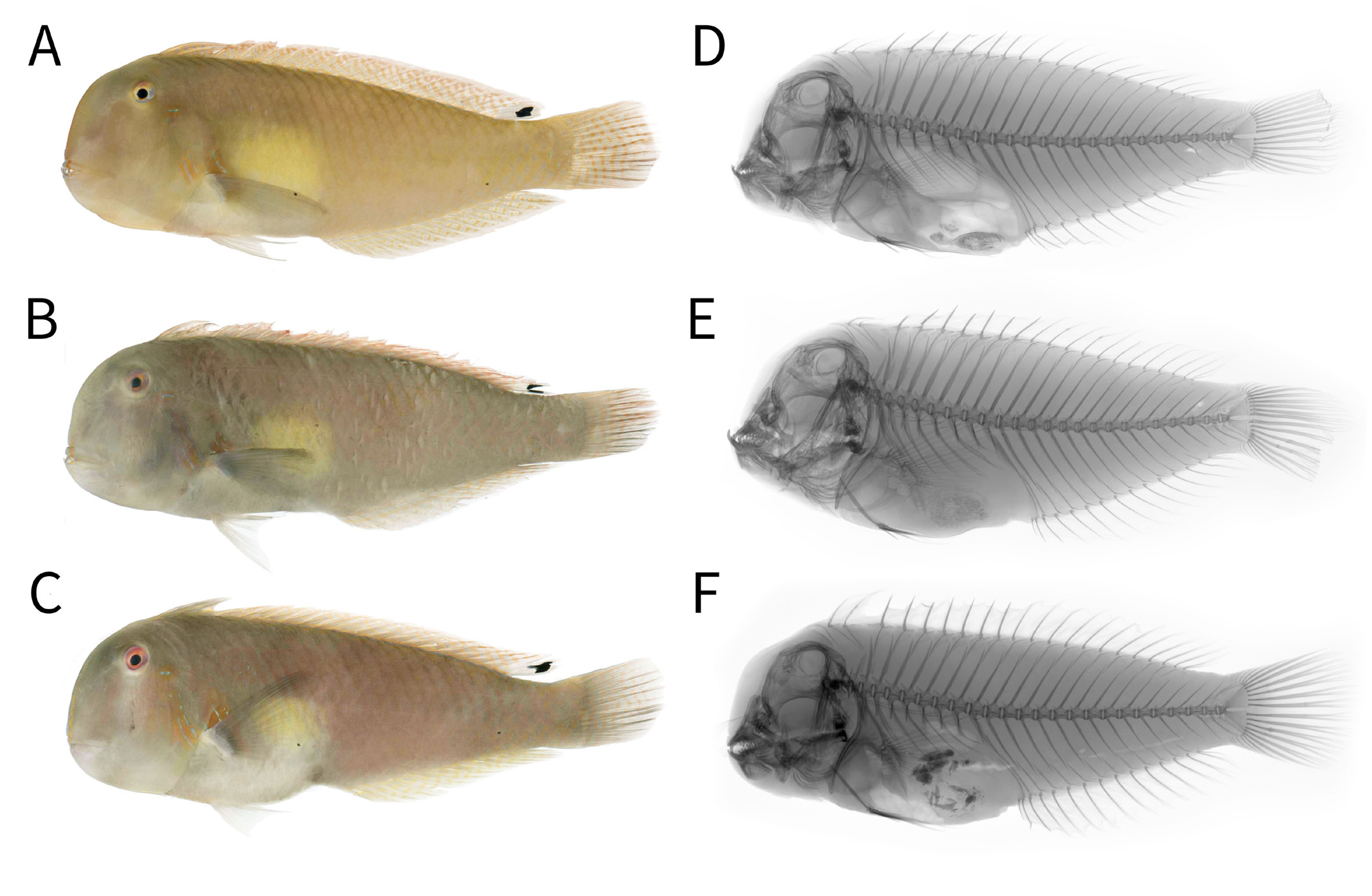
Paratypes. AMS I.50830-001, 151.9 mm SL, 129.6 mm SL, purchased from public fish market in Jolo, Sulu, Philippines, C.L. Nañola et al., 3 August 2019 ; CSIRO H 1488- 1, 129.8 mm SL, specimen trawled from northwest of Dampier Archipelago , Western Australia, 20°05′S, 116°09′E, 54 m, 26 September 1988 ( Fig. 2A View Fig ) GoogleMaps ; CSIRO H 1506- 1, 144.5 mm SL, specimen trawled from north of Dampier Archipelago , Western Australia, 19°39′S, 116°31′E, 57 m, 6 October 1988 ( Fig. 2B View Fig ) GoogleMaps ; KAUM-I. 80684, 172.0 mm SL, purchased from Tigbauan Market , Iloilo, Panay Island, Philippines, H. Motomura, 10 November 2015 ( Fig. 1 View Fig ) ; PNM 15711, 129.6 mm SL, same data as AMS I.50830-001 ; USNM 435404, 162.4 mm SL, purchased from public fish market in Daanbantayan , Cebu, Philippines, K.E. Carpenter et al., 29 January 2015 ( Fig. 3A View Fig ) ; USNM 437745, 155.1 mm SL, purchased from Estancia fish landing site, Iloilo, Panay Island, Philippines, J . T. Williams et al., 16 July 2015 ( Fig. 3B View Fig ) ; USNM 4357747, 158.8 mm SL, same data as USNM 4357745 ( Fig. 3C View Fig ) .
Diagnosis. A species of Iniistius distinct from all congeners based on the following combination of characters and live colouration details: 7 horizontal rows of scales on cheek; gill rakers 4–6 + 8–11 = 12–17; gill rakers short, bearing teeth; pored lateral line scales 19–20 + 4–6 = 23–26; 2 scales dorsoanteriorly on opercle; body yellowish to jade green; posteriormost dorsal fin with a large black centred white ellipsoid ocellus.
Description. Dorsal-fin rays IX,12, first 3–5 segmented rays unbranched, all remaining segmented rays branched (first 7 unbranched in PNM 15711), last ray branched to base; anal-fin rays III,12, all segmented rays branched except first ray unbranched (all rays branched in USNM 437745 and USNM 437747), last ray branched to base; pectoral-fin rays 12, uppermost two rays unbranched, first ray rudimentary; pelvic-fin rays I,5; principal caudal-fin rays 6+6, the upper and lowermost unbranched; upper procurrent caudal-fin rays 6; lower procurrent caudal-fin rays 5; lateral line interrupted, with dorsoanterior series of pored scales 20/19 (19–20) and midlateral posterior peduncular series 6/5 (4–6); first pored scale on posterior peduncular series often pitted; total number of scales in lateral line 26/24 (23–26); scales above lateral line to origin of dorsal fin 4; scales above lateral line to middle of dorsal fin 2; scales below lateral line to origin of anal fin 9; circumpeduncular scales 16; gill rakers 6 (4–6) + 11 (8–11) = 17 (12–17); branchiostegal rays 5; vertebrae 9+16.
Body depth 2.7 (2.5–2.8) in SL. Body extremely compressed, width 3.5 (2.9–3.3) in body depth; head length (HL) 3.0 (3.0–3.3) in SL; dorsal profile of snout nearly vertical, the remaining profile of head before and above eye strongly convex; snout length 3.0 (2.1–3.1) in HL; edge of snout a sharp ridge, this continuing through interorbital space to origin of dorsal fin; chin also with a sharp anterior ridge; eye set high on head at level of nostril, fleshy orbit diameter 5.2 (4.6–5.6) in HL; interorbital width 6.2 (5.2–6.5) in HL; caudal-peduncle depth 2.5 (2.3–2.8) in HL; caudal-peduncle length 4.2 (3.7–4.5) in HL.
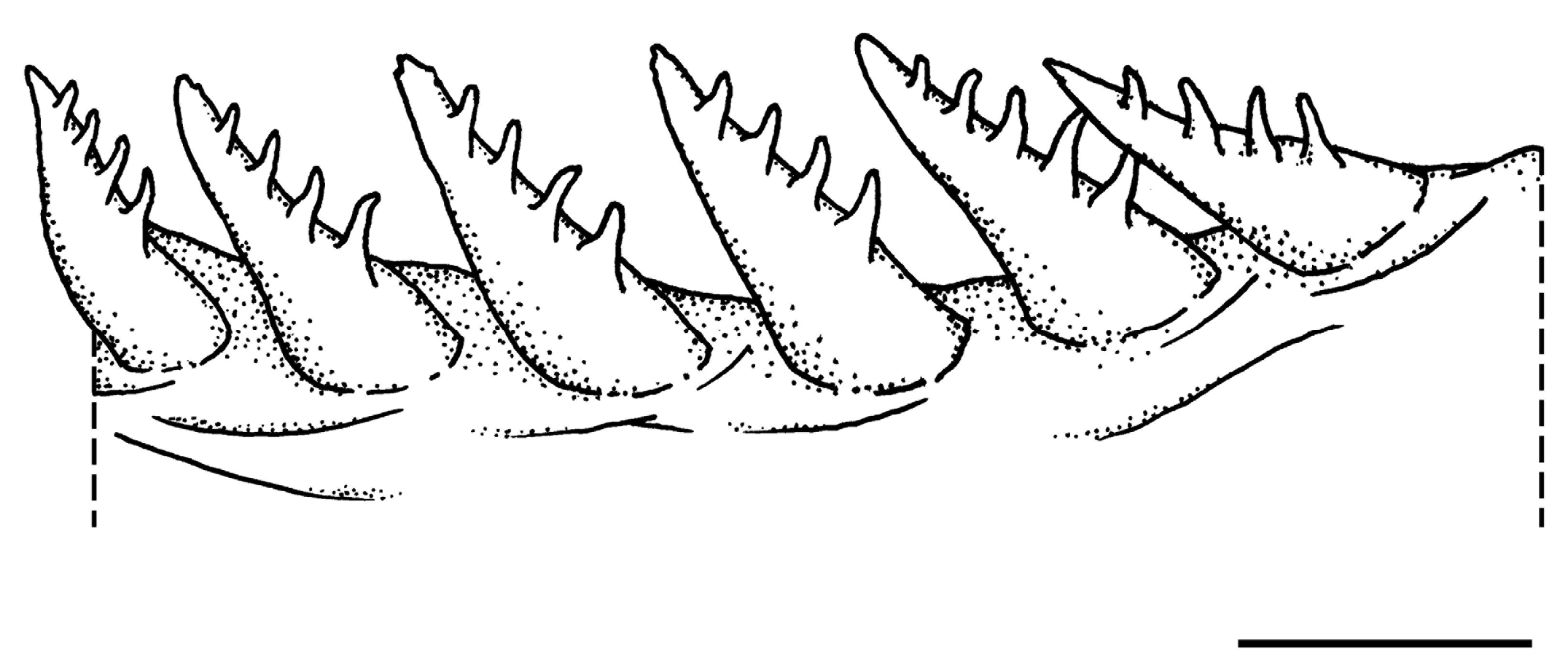
Mouth small, a little oblique, upper-jaw length 3.6 (3.3–4.1) in HL; a pair of long slender slightly incurved canines at front of each jaw extending well beyond lips with mouth closed, lower pair fitting inside the upper; upper pair of canines curving laterally, lower canines more forward-projecting and flaring only slightly to side; side of jaws with a row of 6–11 strong conical teeth. Tongue slightly pointed, set far back in mouth. Lips thin, the lower with a prominent flap on side of mandible. Gill rakers thick and short, less than half width of orbit, bearing teeth ( Fig. 4 View Fig ); anterior rakers on epibranchial of first gill arch with 2 teeth on each side, those on ceratobranchial with 4–5 teeth on each side.
Posterior edge of preopercle free to about an eye diameter below lower edge of orbit; ventral edge of preopercle free nearly to below posterior end of maxilla. Anterior nostril a tiny aperture, overlain by flap from anterodorsal edge; posterior nostril short, slightly oblique. Suborbital sensory canal with 6–7 short branches, each ending with single pore. Scales cycloid, thin and membranous; lateral-line scales with a single horizontal tubule ending in a posterior pore; scales on side of thorax about half height of scales on side of body; head naked except for 2 small scales dorsally at base of opercle and a broad zone of small scales on cheek from below eye to slightly below corner of mouth, these scales in about 7 small narrowly spaced rows (scales not in regular rows, so difficult to count); no scales basally on dorsal or anal fins; small scales basally on caudal fin extending nearly ⅓ distance to posterior margin; pelvic fins without an axillary scale; a single scale extending posteriorly from between base of pelvic fins, its length about ⅓ length of pelvic spine.
Origin of dorsal fin above posterior edge of orbit, predorsal length 3.4 (3.3–3.7) in SL; dorsal spines slender and flexible; dorsal fin incised between 2 nd and 3 rd dorsal spines, gap between base of 2 nd and 3 rd spines about 1.7 (1.6–1.8) times larger than gap between 3 rd and 4 th dorsal spines; first two dorsal spines flexible, the 1 st dorsal spine longest, 2.3 (1.9–3.4) in head length; 2 nd dorsal spine 2.8 (2.3–3.0) in head length; 3 rd to 9 th dorsal spines pungent; 3 rd dorsal spine 5.0 (4.4–7.7) in head length; 9 th dorsal spine 4.7 (3.7–5.3) in HL; penultimate soft dorsal ray longest, 2.8 (2.6–3.1) in HL; origin of anal fin below base of 1 st dorsal soft ray, preanal length 1.8 (1.7–1.9) in SL; 1 st anal spine 12.1 (9.4–14.6) in HL, the 2 nd spine 6.8 (5.6–8.4) in HL; 3 rd anal spine 4.5 (3.7–5.4) in HL; penultimate anal soft ray longest, 2.8 (2.5–3.3) in HL; caudal fin rounded, its length 4.5 (4.3–5.1) in SL; pectoral fins 4.7 (3.8–5.1) in SL; pelvic fins not reaching anus, pelvic spine 5.7 (4.4–6.1) in HL, 1 st soft ray longest, 5.4 (5.2–6.8) in SL.
Osteology. Vertebrae 9+16; first vertebral centrum about half width of subsequent centra, bearing a relatively short neural spine; second neural spine blade like and angled about 40° toward third neural spine, its length longer than the first but about ⅔ that of the third neural spine; third neural spine (third and fourth in CSIRO H 1506-1) with well-developed plate-like shelf with distal groove to accommodate associated pterygiophore; single supraneural, proximal to supraoccipital crest, inserted in preneural space; first dorsal pterygiophore bearing one supernumerary spine and one serial spine, inserted along with second pterygiophore in first interneural space; no pterygiophores in second interneural space; second interneural space narrow; remaining dorsal pterygiophores insert one per interneural space, except for 19 th interneural space with two pterygiophores (two pterygiophores in 18 th interneural space in KAUM-I. 80684); precaudal centra with thick, unornamented pre-zygapophyses (see Tatom-Naecker & Westneat, 2018); first anal pterygiophore bearing two supernumerary spines and one serial spine, inserted anterior to first haemal spine; subsequent anal pterygiophores each bear a serially associated segmented ray and inserted one per interhaemal space, except for two pterygiophores in 10 th interhaemal space (two pterygiophores in 11 th interhaemal space in CSIRO H 1506-1); ribs present on vertebrae 3 through 9; epineurals present on vertebrae 1 through 11–12 (to vertebrae 11 in CSIRO H 1488; to vertebrae 12 in CSIRO H 1506-1 and KAUM-I. 80684); preural 2 haemal spine autogenous, with prominent anterior flange; hypurals 1 and 2 undifferentiated from each other; hypurals 3 and 4 undifferentiated from each and from urostylar complex, distal portion with a broad shelf; hypural 5 absent; parhypural autogenous without apparent hypurapophysis ( Fig. 3D–F View Fig ).
Colour in life. Based on colour photographs of type specimens ( Figs. 1A View Fig , 2 View Fig , 3A–C View Fig ): head and body pale yellowish to jade green, becoming increasingly pale ventrally towards breast; posterior margin of scales on body very slightly darker in colour, appearing faintly crosshatched; anterior body behind opercle, anal-fin base and caudal-fin base with metallic blue spots; anteromedial portion of body behind pectoral fin with a pale, slightly metallic yellowish green blotch; iris orange suffused with purple, inner portion a yellow ring around pupil; predorsal ridge from upper lip to base of anteriormost dorsal-fin spine lined with metallic blue and orange; opercle and anteriormost body just behind free margin of opercle with two orange bands, their margins indistinct and very suffused, first from 5 o’clock position of orbit to edge of opercle at level horizontal to pectoral fin base, second from free margin of opercle to inner edge of pectoral fin axil; each orange band with a series of broken metallic blue spots and stripes arranged somewhat obliquely, these variable and sometimes absent or very attenuate; dorsal fin orange-yellow with metallic blue vermiculate markings, those anteriorly frequently broken into spots, becoming increasingly anastomosed and sinuous posteriorly; posterior most edge of dorsal fin with a large black centred white ellipsoid ocellus; caudal fin similar to dorsal fin, except blue sinuous markings arranged more vertically; anal-fin similar to dorsal-fin; pectoral fins translucent; pelvic fins greenish hyaline.
(Sygnathiformes; Centriscidae ; also known as shrimpfish). To maintain consistent terminology with other members of the Novaculini and to avoid confusion with the Centriscidae , we recommend razor wrasse as the preferred common name when referring to species in the genus Iniistius .
Colour in preservation. Body uniformly light tan; pectoral fin base and orbital rim pale cream, almost white; metallic blue markings on opercle and anterior body now dark tan; white portion of ellipsoid ocellus on posterior dorsal fin indistinct from rest of fin, black central portion remains; all fins translucent hyaline ( Fig. 1B View Fig ).
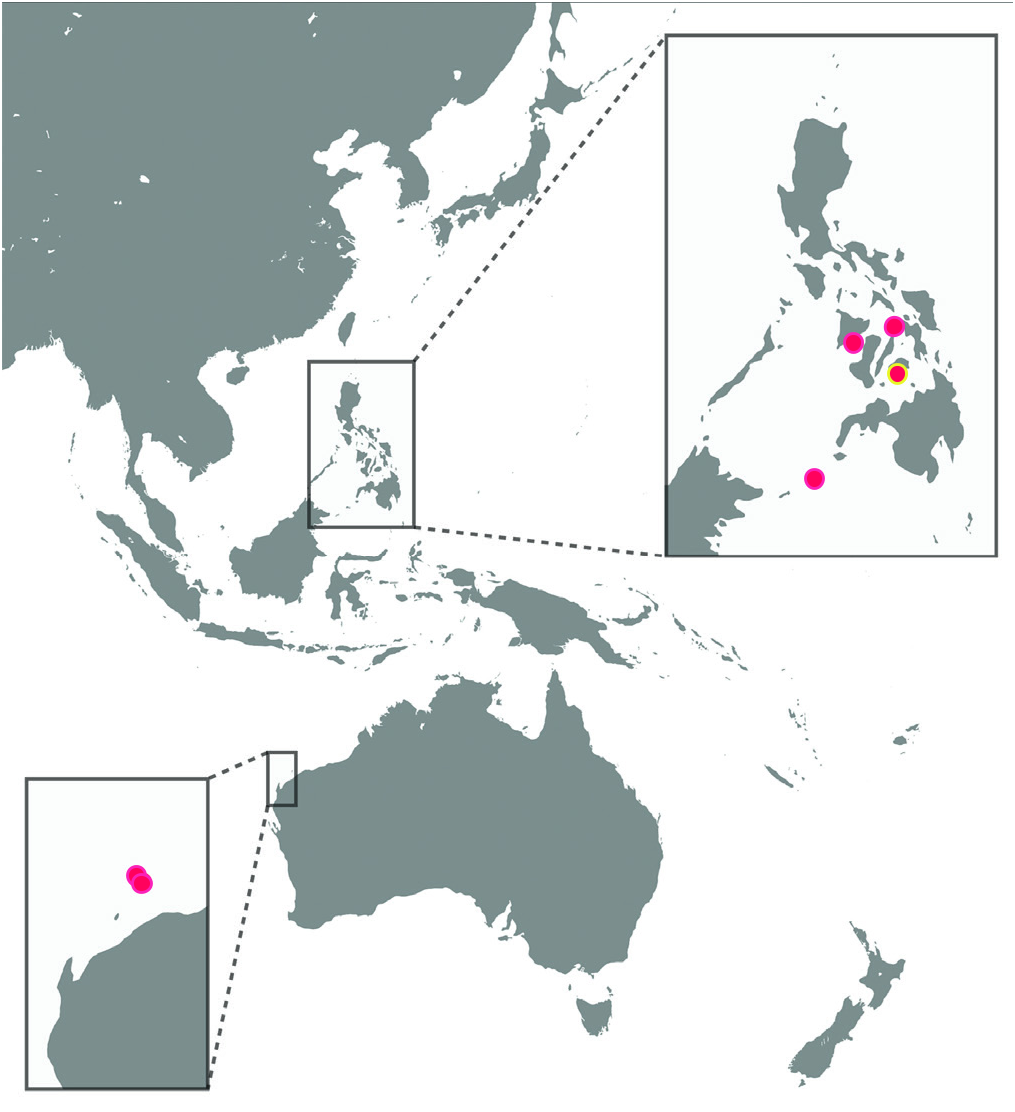
Habitat and distribution. Iniistius bakunawa is known only from seven specimens collected from artisanal fish markets in Panay, Cebu, Bohol, and Jolo in the Philippines, and two specimens trawled from the Dampier Archipelago, Western Australia at depths of 54–57 m ( Fig. 5 View Fig ). Although species of Iniistius are of some commercial interest, their preference for soft sediment and sand channels away from coral reefs means that they are often overlooked in faunistic surveys. These habitats are often overlooked by divers ( De Brauwer et al., 2017; De Brauwer & Burton, 2018), resulting in the patchy distribution records for many species of Iniistius and other novaculin genera. It is likely that the species occurs elsewhere in the Indo-West Pacific, particularly between Philippines and Western Australia.
Etymology. The specific epithet is given after Bakunawa, a serpentine or draconic figure in Visayan mythology believed to be responsible for causing an eclipse by devouring the moon. The common name is given after the black centred white ellipsoidal ocellus on the posterior dorsal fin. The name bakunawa is treated as a noun in apposition. Species of Iniistius are known by a variety of common names, including razor wrasse, cleaver wrasse, and razorfish. The first two names are sometimes used for other novaculin species in the genera Novaculops and Xyrichtys , whereas razorfish is sometimes used for Centriscus and Aeoliscus Comparisons. Randall et al. (2002) classified Indo-Pacific Iniistius into two groups. The first group, consisting of I. pavo and I. dea , is distinguished from congeneric species in having naked cheeks and the first two dorsal-fin spines greatly prolonged and separate from the rest of the fin. The second group consists of the remaining species and is diagnosed in having scales on the cheek, and in having the first two dorsal-fin spines not greatly prolonged and separated but still connected to the rest of the fin by a deep notch in the interspinous membrane. The degree of cheek squamation for this latter group varies from just a few (e.g., in I. aneitensis and I. cyanifrons ), to numerous scales arranged in rows.
Iniistius bakunawa belongs to the latter group with an anterior dorsal-fin notch and in having numerous cheek scales arranged in rows. It is, however, readily separated from all congeneric species in having the combination of 7 rows of cheek scales, teeth on its gill rakers, and a large concentric black and white ellipsoid ocellus on the posteriormost edge of its dorsal fin. Of the known species of Iniistius , only I. baldwini occasionally approaches this colouration pattern, with specimens sometimes having a small black spot in the same position as I. bakunawa (see BPBM 37237 specimen of I. baldwini ). This pattern, however, does not appear to be diagnostic for I. baldwini , with most specimens lacking this posterior dorsal-fin spot. Iniistius baldwini is readily separated from I. bakunawa in having a large black blotch on its dorsum below the sixth to ninth dorsal-fin spines, and in lacking iridescent blue markings on the opercle and pectoral fin axil.
Iniistius bakunawa also closely resembles I. bimaculatus and I. pentadactylus , with all three species having a pale yellow to jade green body colouration, sinuous markings on the median fins, and in having numerous small scales on the cheek arranged in horizontal rows. The three species differ in other aspects of colouration. In I. bimaculatus , the ventral half of the body is lined with numerous rows of bright orange spots, and there is a dark blotch situated in the middle of the body behind the pectoral fin base. In I. pentadactylus , the postorbital region is adorned with three to seven (usually five) scarlet scales arranged in an oblique series, and the posterior body is lined with rows of bright orange spots. The pale anteromedial patch of scales behind the pectoral fin typical of the genus is sometimes crosshatched with red, and immediately above there is usually a dark blotch. Iniistius pentadactylus further differs from I. bakunawa in lacking teeth on its gill rakers.
Remarks. Placement of the new species in the genus Iniistius is here regarded as provisional. The new species possesses several of the characters in agreement with Gill’s (1862) and Nguyen’s (1974) generic definition of Iniistius , such as in having the first two dorsal-fin spines separated from the remainder of the fin by a deep notch and in having no dorsal-fin pterygiophores in the second interneural space. It, however, possesses numerous rows of cheek scales. Randall & Earle (2002) proposed an additional character separating Iniistius from Xyrichtys : dorsal-fin origin less than half orbit diameter behind posteriormost edge of orbit. This character is true at least for our examined material of I. dea , I. jacksonensis , I. melanopus , I. opalus , I. pavo , I. pentadactylus , and I. bakunawa , new species (see list of material examined).
Two of the characters do not appear to be diagnostic for Iniistius . They are presence of cheek scales, and presence of an anterior dorsal fin notch. Aside from I. dea and I. pavo , all other species of Iniistius possess some degree of cheek squamation. If Iniistius (as currently defined) is indeed monophyletic, then the absence of cheek scales in I. dea and I. pavo can be considered apomorphic. In contrast, species of Xyrichtys (Atlantic and eastern Pacific) do not appear to possess cheek scales.
Presence or absence of an anterior-dorsal fin notch is variable in Iniistius . In Iniistius , the anteriormost first and second dorsal-fin spines are displaced forward from the remaining radial elements, resulting in the insertion of the first two pterygiophores in the first interneural space instead of the second. This displacement of the anterior dorsal-fin spines results in the second interspinous space being wider than all other interradial spaces along the dorsal fin. In most species of Iniistius , it is the widening of this space that results in the notching of the second interspinous membrane. The notch is, however, not always apparent, with some specimens having uninterrupted membranes across the anterior radial elements. Since notching of the interspinous membrane appears to be a variable consequence of the first and second dorsal-fin spines being displaced, we do not recommend its use as a diagnostic character. Displacement of the anteriormost first and second dorsal-fin spines is, however, consistent across our examined material of Iniistius and appears to be a synapomorphy diagnosing the genus. Notwithstanding the evaluation of the aforementioned characters, we exercise restraint in redefining Iniistius and Xyrichtys , in part due to lack of adequate comparative material, and that it goes beyond the scope of the current study.
Material examined. Iniistius dea (n=1): AMS I.19604-001, 231.3 mm SL, Sudbury Reef, QLD, Australia; Iniistius jacksonensis (n=6): AMS E. 1615, 155.1 mm SL, off Fraser Island , QLD, Australia ; AMS E. 2087, 117.6 mm SL, Cape Byron, NSW, Australia; AMS I.3978, 153.0 mm SL, off Cape Hawke, NSW, Australia; AMS I.34143-001, 165.4 mm SL, off Cape Hawke, NSW, Australia; AMS I. 21556-001, Middle Head, Sydney Harbour, NSW, Australia; AMS I.31249-001, 154.4 mm SL, Cape Banks, Botany Bay , NSW, Australia ; Iniistius melanopus (n=2): USNM 437839, 160.6 mm SL, purchased from Iloilo City “Super” fish market, Iloilo, Panay Island , Philippines , J. T. Williams et al., 18 July 2015; USNM 437893, 145.8 mm SL, purchased from Dumaguete Fish Market, Dumaguete City, Negros Oriental, Philippines , J. T. Williams et al., 21 July 2015; Iniistius opalus (n=1): AMS I. 50704-001, 141.5 mm SL, Cape Bossut, Kimberley, WA, Australia ; Iniistius pavo (n=1): AMS I.46345-001, 151.1 mm SL, Mauritius; Iniistius pentadactylus (n=10): AMS I. 37919-006, 45.0 mm SL, Vanua Lava, Vanuatu; AMS I. 37933-010, 6, 61.2–105.0 mm SL, Vanua Lava, Vanuatu; AMS I.37937-013, 2, 70.7–83.1 mm SL, Ambrym Island , Vanuatu ; AMS I.37937.013, dissected cleared and stained specimen, Ambrym Island , Vanuatu .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.