Caridina tupaia, Mazancourt & Marquet & Keith, 2019
|
publication ID |
https://doi.org/ 10.1080/00222933.2019.1612959 |
|
publication LSID |
lsid:zoobank.org:pub:17B62044-4D99-48EE-A665-3E5E7F1A864B |
|
DOI |
https://doi.org/10.5281/zenodo.3680512 |
|
persistent identifier |
https://treatment.plazi.org/id/1D42F67B-0F56-4D76-8772-32FBA80F9AE8 |
|
taxon LSID |
lsid:zoobank.org:act:1D42F67B-0F56-4D76-8772-32FBA80F9AE8 |
|
treatment provided by |
Valdenar |
|
scientific name |
Caridina tupaia |
| status |
sp. nov. |
Caridina tupaia View in CoL n. sp.
www.zoobank.org/ urn:lsid:zoobank.org:act:1D42F67B-0F56-4D76-8772-32FBA80F9AE8 ( Figure 5 View Figure 5 )
Caridina weberi – Edmondson 1935: 8 – 9 View in CoL , Figure 3 View Figure 3 (a – f) page 11, Figure 4 View Figure 4 (g) page 13; Marquet 1988 (partim); Marquet 1991: 129 – 130 (partim); Poupin 1998: 9 (partim); Keith and Vigneux 2002: 126 (partim), Keith et al. 2002: 44 – 45 (partim); Keith et al. 2013: 84 – 85 (partim)).
( Caridina cf. weberi – Page et al. (2007b) View in CoL : specimen voucher GU-993, sequence DQ478493 View Materials .
Caridina cf. weberi View in CoL sp. 3 – de Mazancourt et al. 2019).
Material examined
Type material. Holotype: 1 ♀, cl 4.1 mm, 23 October 2017, 17°49.719 ′ S 149°17.067 ′ W, Papehere river , Tahiti Island, French Polynesia, G. Marquet & P. Tiberghien coll., MNHN-IU -2018-260 (DNA: CA2058 ). GoogleMaps
Paratypes: 1 ♂, cl 3.9 mm, 11 July 2010, 21°14.806 ′ S 159°43.795 ′ W, Avana river , Rarotonga Island, Cook Islands, altitude 5 – 10 m, P. Keith, P. Gerbeaux & G. Marquet coll., MNHN-IU-2018-261 (DNA: CA1048 ); GoogleMaps 1 ♂, cl 4.1 mm, same data as previous, MNHN-IU-2018-262 (DNA: CA1046 ); GoogleMaps 1 ♂, cl 4.2 mm, same data as previous, MNHN-IU -2018-263 (DNA: CA1047 ); GoogleMaps 1 ♀ ovig, cl 3.8 mm, same data as previous, MNHN-IU -2018 – 264 (DNA: CA1049 ); GoogleMaps 1 ♂, cl 2.5 mm, 26 September 2017, 22°27.009 ′ S 151°21.061 ′ W, Puputa river , Rurutu Island, French Polynesia, G. Marquet & P. Tiberghien coll., MNHN-IU-2018-265 (DNA: CA2054 ); GoogleMaps 1 ♀, cl 3.5 mm, same data as previous, MNHN-IU -2018-266 (DNA: CA2055 ); GoogleMaps 1 ♀, cl 4.1 mm, same data as holotype, MNHN-IU-2018-267 (DNA: CA2059 ); GoogleMaps 1 ♀ ovig, cl 4.2 mm, 1 July 2001, Mahidi river , Huahiné Island, French Polynesia, P. Keith & E. Vigneux coll., MNHN-IU-2018 – 268; GoogleMaps 1 ♀, cl 4.5 mm, same data as previous, MNHN-IU-2018 – 269; GoogleMaps 1 ♀, cl 2.8 mm, 5 October 2017, 17°32.785 ′ S 149°48.484 ′ W, Afareaitu river , Moorea Island, French Polynesia, altitude 109m, G. Marquet & P. Tiberghien coll., MNHN-IU-2018-270 (DNA: CA2062 ); GoogleMaps 1 ♀ ovig, cl 3.9 mm, same data as previous, MNHN-IU-2016-11762 (DNA: CA2063 ); GoogleMaps 1 ♀ ovig, cl 6.2 mm, 10 July 2014, 13°54.543 ′ S 171°35.300 ′ W, Falefa river , Upolu Island, Samoa, altitude <50m, P. Gerbeaux coll., MNHN-IU-2016-11763 (DNA: CA1410 ); GoogleMaps 1 ♀ ovig, cl 5.5 mm, 8 July 2014, 13°53.642 ′ S 171°39.913 ′ W, Tuafaleloa river , Upolu Island, Samoa, altitude 100m, P. Gerbeaux coll., MNHN-IU-2016-11764 (DNA: CA1411 ); GoogleMaps 1 ♀ ovig, cl 4.4 mm, 8 July 2014, 13°53.788 ′ S 171°40.031 ′ W, Tuafaleloa river , Upolu Island, Samoa, altitude 250m, P. Gerbeaux coll., MNHN-IU-2016-11765 (DNA: CA1412 ); GoogleMaps 1 ♀, cl 5.0 mm, 12 July 2014, 13°43.741 ′ S 172°18.832 ′ W, Faleata river , Savaii Island, Samoa, altitude 195m, P. Gerbeaux coll., MNHN-IU-2016-11766 (DNA: CA1413 ); GoogleMaps 1 ♀ ovig, cl 5.0 mm, 7 July 2014, 13°51.900 ′ S 171° 41.196 ′ W, Ma ’ epu river, Savaii Island, Samoa, altitude 20 – 50m, P. Gerbeaux coll., MNHN-IU-2016-11767 (DNA: CA1918 ) GoogleMaps .
Comparative material. Type material
Caridina weberi De Man, 1892 .
Syntypes: 2 ♂, cl 4.4 – 4.5 mm and 1 ♀ ovig, cl 6.1 mm, Kotting , Flores Island, Indonesia, M. Weber coll., MNHN-IU-2015-1755.
Description
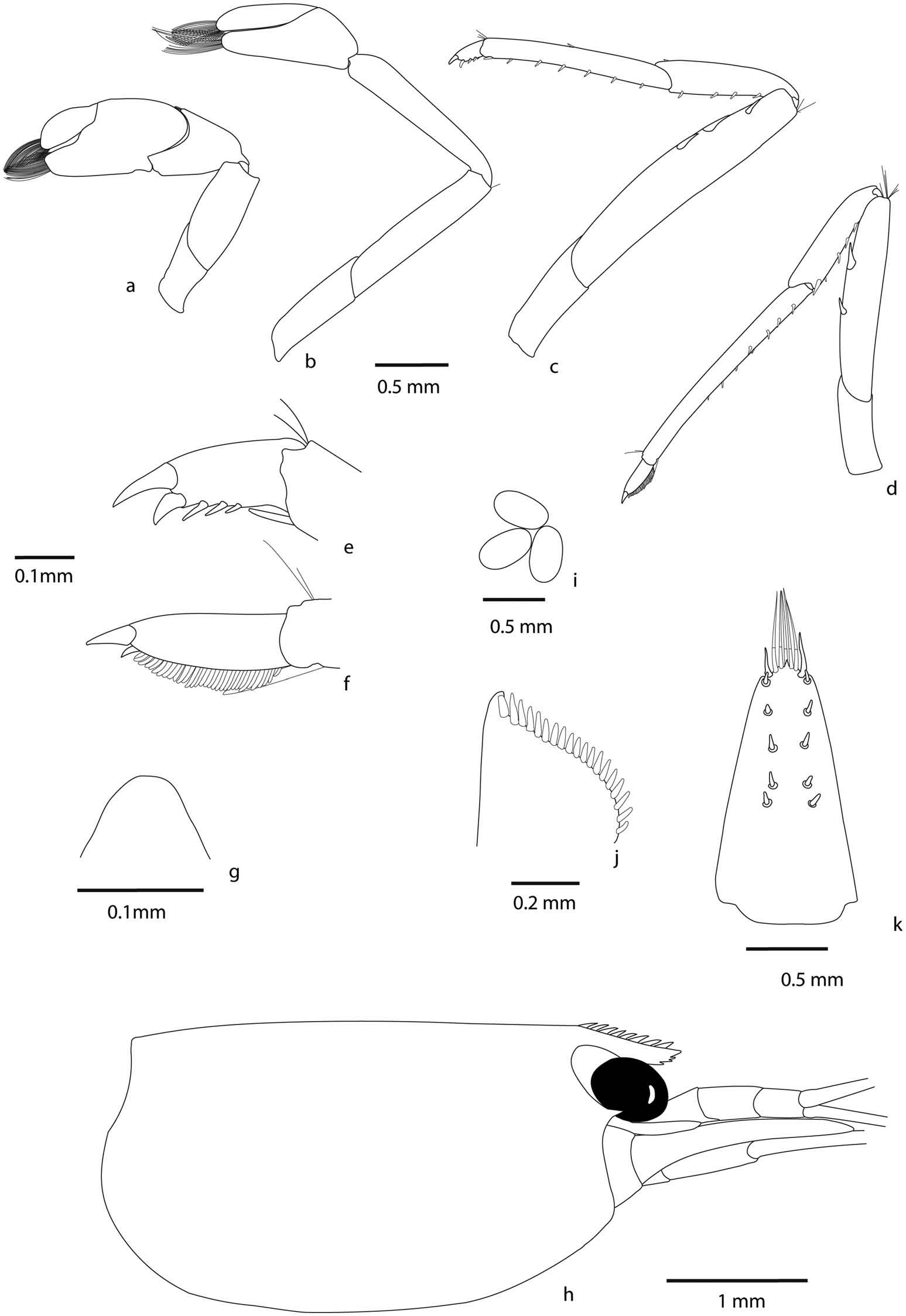
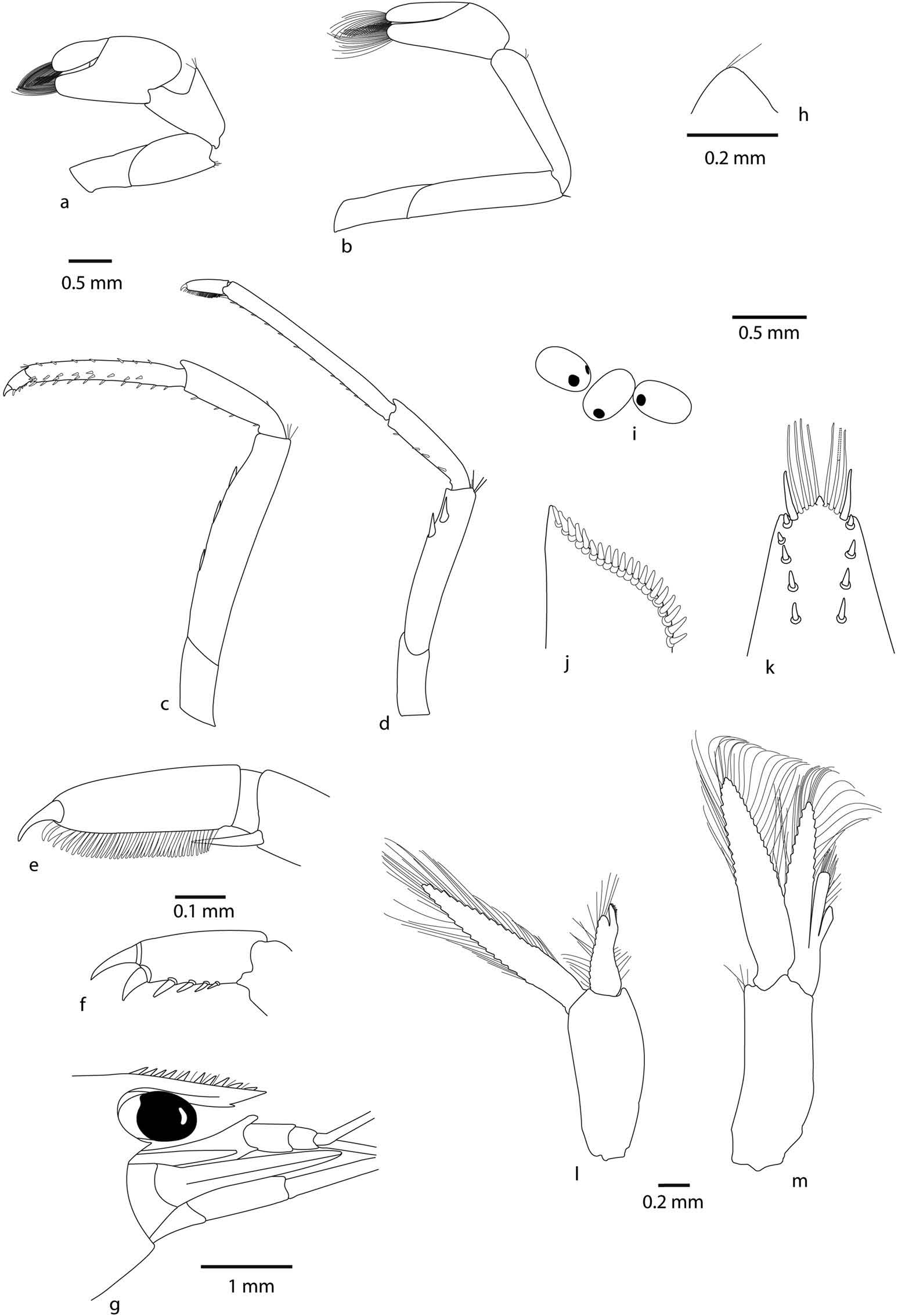
Cephalothorax. Rostrum ( Figure 5 View Figure 5 (g)): rarely straight, mostly bent down, short, 0.3 – 0.4 of cl, reaching to base or middle of the second segment of antennular peduncle, armed dorsally with 8 – 14 teeth, 0 – 3 of them situated on carapace posterior to orbital margin (mostly 0), ventral margin with 1 – 3 teeth. Rostral formula (0 – 3) 8 – 14/1 – 3. Suborbital angle indistinguishably fused with antennular tooth. Pterygostomian margin rectangularly rounded.
Eyes well developed, anterior end reaching to 0.77 length of antennular peduncle basal segment. Antennular peduncle 0.55 times as long as carapace. Anterolateral angle of basal segment reaching 0.20 length of second segment, second segment twice as long as third. Stylocerite reaching to 0.90 length of antennular peduncle basal segment. Scaphocerite reaching just slightly beyond the tip of antennular peduncle, about 3 times longer than wide.
Mouthparts. Left mandible, right mandible, first maxilla, second maxilla, first maxilliped, second maxilliped and third maxilliped typical of genus.
Pereiopods. Epipods on first four pereiopods.
Pereiopod 1 ( Figure 5 View Figure 5 (a)): chela about 1.9 – 2.1 times as long as wide, movable finger 2.2 – 3.3 times as long as wide, 0.7 1.0 times length of palm, carpus 1.3 – 1.7 times as long as wide.
Pereiopod 2 ( Figure 5 View Figure 5 (b)) more slender and longer than the first pereiopod with chela 2.4 – 2.8 times as long as wide: movable finger 3.8 – 4.9 times as long as wide, 1.3 – 1.7 times length of palm, carpus slender 4.7 – 6.1 times as long as wide.
Pereiopod 3 ( Figure 5 View Figure 5 (c, f)): stout, dactylus 2.6 – 4.2 times as long as wide including terminal spine with 5 – 7 spines including terminal spine, propodus with numerous spines on ventral margin, 7.6 – 10.6 times as long as wide, 3.4 – 5.2 times as long as dactylus.
Pereiopod 5 ( Figure 5 View Figure 5 (d, e)): dactylus 3.5 – 4.7 times as long as wide with 29 – 54 spiniform setae on flexor margin, propodus 11.6 – 16.0 times as long as wide, 3.7 – 4.4 times as long as dactylus.
Abdomen. Third abdominal somite with moderarely convex dorsal profile. Sixth abdominal somite about 0.46 times carapace length, as long as fifth somite, shorter than telson. Telson ( Figure 5 View Figure 5 (k)): 2.3 times as long as wide, with four or five pairs of dorsal spines and one pair of dorsolateral spines, posterior margin with median process, rounded with 6 – 10 intermediate plumose setae longer than lateral spines.
First male pleopod ( Figure 5 View Figure 5 (l)): Endopod extending to half length of exopod, elongate, with developed appendix interna near distal end of endopod.
Second male pleopod ( Figure 5 View Figure 5 (m)): Appendix masculina reaching 0.68 times length of endopod, appendix interna reaching about 0.40 times appendix masculina length Preanal carina ( Figure 5 View Figure 5 (h)): High, unarmed.
Uropodal diaeresis ( Figure 5 View Figure 5 (j)): With 16 – 20 spinules.
Eggs ( Figure 5 View Figure 5 (i)): Size 0.38 – 0.47 × 0.20 – 0. 30mm.
Habitat
This new species prefers fresh and well-oxygenated running waters from the lower course to the higher courses. Under the name of C. weberi , its microhabitat was intensely studied in Opunohu river (Moorea) with that of another sympatric species Atyoida pilipes (Newport, 1847) ( Resh et al. 1990). C. tupaia n. sp. were often found near leaf litter, tending to aggregate in on the bottom of pools where detritus accumulated. Both their leaf litter habitat and their ability to consume it in the laboratory indicate the possibility that this scraper increase rates of litter decomposition ( Williams 1999). Cheliped morphology and, consequently, differences in feeding methods are the main factors determining the microdistribution of A. pilipes and C. tupaia . A. pilipes is filter feeding in faster flowing habitats, while C. tupaia are scraping and sweeping detritus within and on leaf surfaces and also in other microhabiats on the stream channel bed ( Resh et al. 1990). C. tupaia , whose microhabitat is in pools and slow water, may be less able to grasp the substrate in high velocity water, while A. pilipes which is often found in the faster moving water of riffles may be better able to grasp the substrate ( Feldman 1996). These differences are likely a contributing factor in their distribution above and below barriers such as waterfalls and culverts, which require fighting current to move farther upstream. The climbing ability of these two species was tested by Falvo (2013) in rivers of Moorea (Opunohu and Afareaitu). C. tupaia had the highest success rate and A. pilipes was the fastest climber. The latter species is found at higher reaches of the streams than C. tupaia . These species exhibited different climbing behaviours. C. tupaia would consistently take breaks while climbing, only moving a few centimetres at a time, while A. pilipes would rarely stop once it started climbing. An examination of the distal portions of the pereiopods of A. pilipes and C. tupaia indicates that A. pilipes is more strongly built and its appendages may also be better suited for gripping substrate and with standing current, explaining its ability to reach higher altitudes.
Etymology
This new species is named in honour of Tupaia, the Polynesian pilot of James Cook, on board of H.M.S. Endeavour for its ability to cross long distances between Polynesian islands.
Colour pattern ( Figure 7 View Figure 7 (aı b))
The live general colour of the body is yellowish and slightly translucent. Numerous red spots are visible all over the body. Sometimes, a lighter longitudinal dorsal band is present stretching from the rostrum to the telson.
Distribution ( Figure 8 View Figure 8 )
Among the species studied here, this species seems to have the widest distribution in Polynesia (Upolu, Savaii ( Samoa), Rarotonga ( Cook Islands), Rurutu, Tahiti, Moorea and Huahine ( French Polynesia)).
Remarks
This new species was identified as C. weberi several times: Edmondson (1935), Adamson Adamson (1935, 1939), Marquet (1988), Marquet (1991), Poupin (1998), Keith and Vigneux (2002), Keith et al. (2002), Page et al. (2007b), Keith et al. (2013). Indeed, this species looks like C. weberi sensu stricto by its straight or bent down rostrum, 0.3 – 0.4 of cl, (vs 0.4 in C. weberi ) reaching to the base or middle of second segment of antennular peduncle with numerous dorsal teeth 8 – 14 (vs 10 – 19 in C. weberi ), but differs by the presence of 0 – 3 dorsal teeth situated on carapace behind orbital margin (vs 0 in C. weberi ). Also, its P1 movable finger is shorter 0.7 – 1.0 times length of palm (vs 1.2 – 1.3 in C. weberi ) as is its P1 carpus 1.3 – 1.7 times as long as wide (vs 1.9 – 2.0 in C. weberi ). Furthermore, the posterior margin of the telson shows always only one pair of lateral spines (vs two in C. weberi ).
This species is easily separated from C. marquesensis n. sp. by numerous characters (see previously).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Caridina tupaia
| Mazancourt, Valentin de, Marquet, Gérard & Keith, Philippe 2019 |
Caridina weberi – Edmondson 1935: 8 – 9
| Keith P & Marquet G & Gerbeaux P & Vigneux E & Lord C 2013: 84 |
| Poupin J 1998: 9 |
| Marquet G 1991: 129 |
| Edmondson CH 1935: 9 |