Protaphorura cykini, Parimuchová & Kováč & Žurovcová & Kadebskaya, 2017
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4350.1.12 |
|
publication LSID |
lsid:zoobank.org:pub:7B99CB67-1397-4319-B17F-83F22AB10CCA |
|
DOI |
https://doi.org/10.5281/zenodo.6033238 |
|
persistent identifier |
https://treatment.plazi.org/id/03C38339-2C48-2C5E-FF10-DA0AFAC8FA52 |
|
treatment provided by |
Plazi |
|
scientific name |
Protaphorura cykini |
| status |
sp. nov. |
Protaphorura cykini sp. nov. Parimuchová & Kováč
( Figs. 2–16 View FIGURES 2 – 10 View FIGURES 11 – 16 )
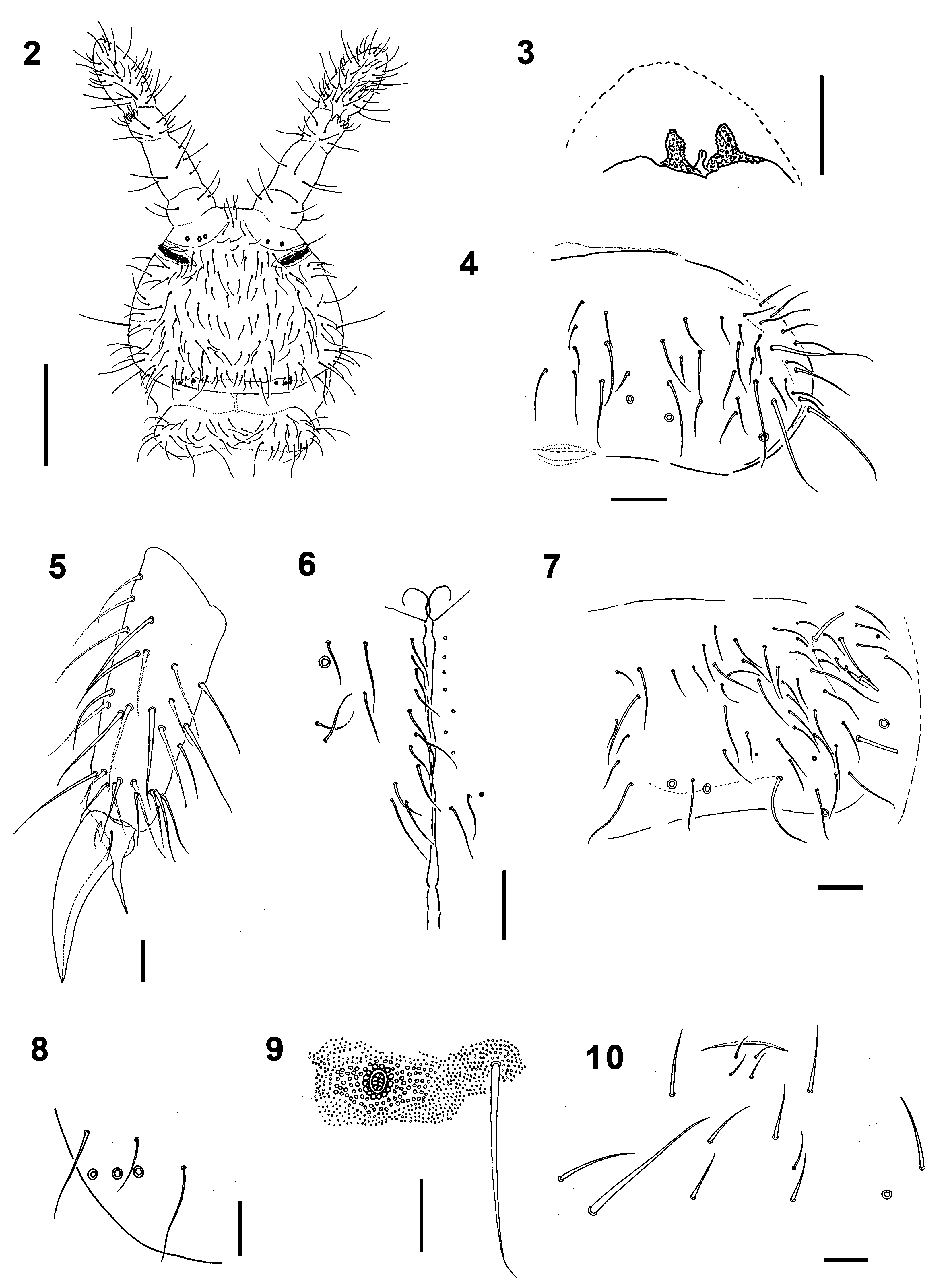
Diagnosis. PAO with 65–71 simple vesicles. Pso formula dorsally: (2)32/022/33343, ventrally: 1/000/0 0 0 0 0. Subcoxae 1 with 1,1,1 pso each. Antennae longer than head ( Fig. 2 View FIGURES 2 – 10 ). Subapical organite on Ant. IV with two papillae ( Fig. 3 View FIGURES 2 – 10 ; 12). VT with 12–15 distal and 2–3 basal chaetae on each side.
Type material. Holotype (female) and 12 paratypes (4 females, 8 males): Russia, Siberia, Irkutsk region, Pribaikalsky National Park , Primorsky Range, Okhotnichya Cave , collected by hand on mouldy excrements of pika ( Ochotona sp.) ( Fig.1a View FIGURE 1 , site 1), 12.vi. 2016, leg. O.I. Kadebskaya and A . V. Osintsev. Holotype and paratypes herein designated are deposited in IBE FS UPJS, Košice , Slovakia .
Other material. Russia, Siberia Irkutsk region, Pribaikalsky National Park , Primorsky Range, Okhotnichya Cave , 3 females and 1 male collected by hand on excrements of pika ( Ochotona sp.), ( Fig.1a View FIGURE 1 site 2) 4.x.2014, leg. O.I. Kadebskaya and P . Holúbek. Other material from type locality deposited in IBE FS UPJS, Košice , Slovakia .
Description. Body length 4.3–4.7 mm in males, 5.2–5.6 mm in females, body shape typical of genus ( Fig. 1c View FIGURE 1 ), with anal spines on distinct papillae. Colour white in ethyl alcohol.
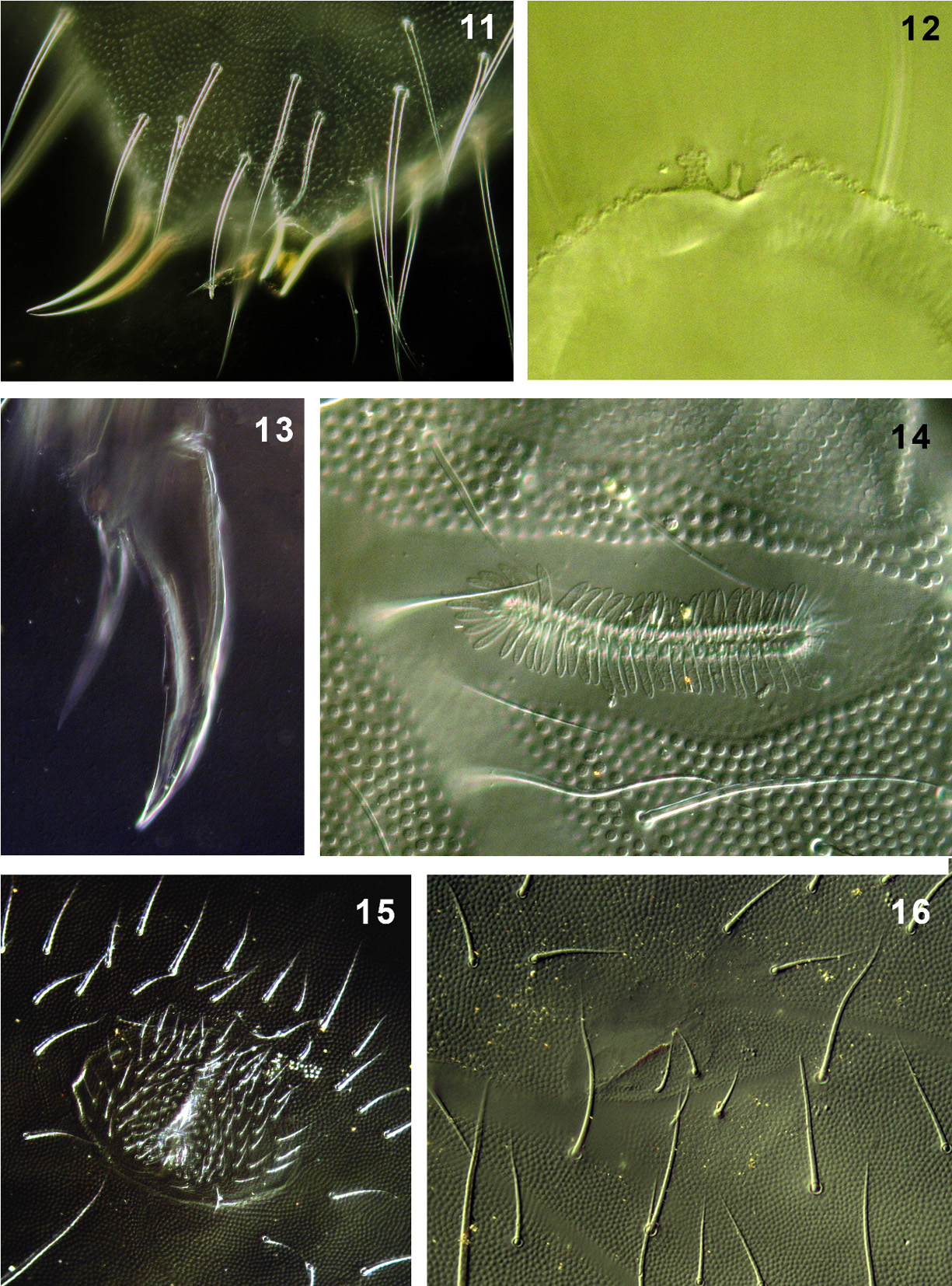
Granulation fine and uniform, coarser around pseudocelli ( Fig. 9 View FIGURES 2 – 10 ). Antennae slightly longer than head (head/ antenna ratio 1:1.1), area antennalis well marked ( Fig. 2 View FIGURES 2 – 10 ). Ant. I with 14–16 chaetae. AOIII with 5 guard chaetae, 5 papillae, 2 smooth sensory rods shorter than papillae and 2 morel-like sensory clubs of equal size. Microsensillum in ventro-lateral position, at level of last guard chaeta. Subapical organite of Ant. IV placed in cavity protected by two granulated papillae, one slightly forward the organite and the second behind it ( Fig. 3 View FIGURES 2 – 10 ; 12). Ms on Ant. IV in ventro-lateral position, in 1/2 of segment length.
PAO with 65–71 simple vesicles ( Fig. 14 View FIGURES 11 – 16 ). Labial palp of type A with 8 proximal chaetae. Basomedian field of labium with 4+4 chaetae, 9–12 postlabial chaetae with left/right asymmetry ( Fig. 6 View FIGURES 2 – 10 ). Maxillary outer lobe with simple palp, 1 basal and 2 sublobal chaetae. Pso formula dorsally: (2)32/022/33343, ventrally: 1/000/00000. Subcoxae 1 I–III with 1 pso each, psx absent. Ventral psx formula: 0/000/?111(2)00.
Dorsal chaetotaxy more-less symmetrical with macrochaetae well differentiated. Th. I with 18–22 chaetae on each side. Chaetae s on Abd. I–III short, s´missing ( Fig. 4 View FIGURES 2 – 10 ). Abd. IV as in Fig. 7 View FIGURES 2 – 10 . Abd. V always with chaeta s between pso a–b, ratio of chaetae M/ s= 7/5 ( Fig. 8 View FIGURES 2 – 10 ). Straight lines passing through the bases of K and K’ prespinal chaetae slightly convergent ( Fig. 11 View FIGURES 11 – 16 ).
Furca remnant with distinct, arched cuticular fold with 2+2 chaetae in two rows (1+1 placed on fold, 1+1 posterior to fold) ( Fig. 16 View FIGURES 11 – 16 ), arrangement of manubrial chaetae as in Fig. 10 View FIGURES 2 – 10 . Male ventral organ absent even in males with genital plate well developed ( Fig. 15 View FIGURES 11 – 16 ). VT with 12–15 distal and 2–3 basal chaetae on each side.
Legs. Tita of all legs with 11 chaetae in distal verticil (A+T), 7 chaetae and chaeta M in verticil B and 7 chaetae in verticil C. Two proximal chaetae Y (placed above verticil C) present ( Fig. 5 View FIGURES 2 – 10 ). Claw without lateral and inner teeth ( Fig. 13 View FIGURES 11 – 16 ). Ratio length/ width of claw ~2.2.
Etymology. The species is named after R. A. Cykin, an important personality in speleology of the Siberia.
Biology. Specimens of P. cykini sp. nov. were discovered in Okhotnichya Cave, aggregated on mouldy pika´s excrements ( Ochotona sp.), approximately 80 m from the entrance. The cave is mesotrophic with organic material (needles, leaf litter) accumulated near the entrance ( Fig. 1b View FIGURE 1 ), and deer´s and elk´s bones and excrements of small mammals ( Ochotona sp. and Microtus sp.) dispersed across the whole cave system. Fungi colonizing organic remnants and excrements probably serve as important food source for collembolans occupying the cave ( Fig. 1d View FIGURE 1 ). P. cykini sp. nov. displays troglomorphic traits, i.e. elongated antennae and claws and larger body compared to other congeners. It lives permanently in cold thermal conditions; in June 2016 the cave air temperature measured at collecting sites ranged from +1.1 to +1.9 °C and the floor ice was present on the bottom of the cave passages.
Along with the new species, the collembolan Oligaphorura schoetti was collected in Okhotnichya Cave in the same period.
Distribution. Known only from the type locality: Okhotnichya Cave, Primorski Range, Irkutsk Region, Siberia, Russia.
Discussion. The new species possess a unique combination of diagnostic features ( Table 1). With the body length from 4.3 to 5.6 mm, long PAO consisting of 65–71 simple vesicles and subapical organite of Ant. IV surrounded by two cuticular papillae, P. cykini sp.nov. is a well-defined species. Subapical organite protected by the papillae is characteristic of P. tschernovi from the Western Taimyr, Siberia, P. borealis from eastern Europe (Babenko & Kaprus´2014), and P. nikolai from the Primorsky Krai, Far East (Kaprus´ et al. 2016). In contrast to P. cykini , these three species have body length <3 mm and psx on ventral side of the head. P. borealis and P. nikolai differ from the new species also in number of pso on subcoxae 1 of all legs ( Table 1). Presence of 60 or more simple vesicles in PAO was documented mostly in large species such as P. macrodentata , P. borealis , P. armata multituberculata and also in P. aksuensis , the taxonomical status of the last two species being unclear. P. janosik is similar to new species in lacking psx on ventral side of head but differ in number of chaetae on Th. I tergum and in lack of cuticular papillae in subapical organite on Ant. IV.
Only few Palaearctic Protaphorura species have been described from caves, namely P. stalagmitorum ( Absolon, 1900) ; P. armata multituberculata ( Stach, 1934) ; P. teres ( Yosii, 1956) ; P. ombrophila ( Stach, 1960) ; P. triparallata ( Gisin, 1960) ; P. dallaii ( Nosek & Paoletti, 1981) ; P. brevispinata ( Yosii, 1966) ; P. septempapillata ( Palissa, 1986) ; P. janosik Weiner, 1990 ; P. aconae Arbea & Jordana, 1994 and P. zlatiborensis Lučić, Ćurčić, Pavković-Lučić & Tomić, 2008 . However, the first six species are considered as species dubia due to insufficient morphology data in their original descriptions ( Parimuchová & Kováč 2016).
Length PAO
Species Dorsal pso Ventral pso Psx SOp Th. I S-cox1 Distribution
(mm) vesicles
aksuensis ( Martynova, 1972) 1.6 33/022/33333?? 90??? Kyrgyzstan
armata multituberculata ( Stach, 1934) 4.2 32/022/33343?? 54–62??? Westphalia, Germany
borealis ( Martynova, 1973, in Martynova 2.5–2.9 32/022/33333 0/- 1/000/ 111101m 40–65 + 14–18 0 0 0 pso + 111 psx Eastern Europe to Alaska al. 1973) sensu Babenko & Kaprus´
2014)
janosik Weiner, 1990 * 2.9–4.3 33/022–3/33343 1/- 0/000/0(1)0(1)0(1)000 36–58 - 14–17 111 pso + 0 0 0 psx Western and Eastern Carpathians
macrodentata ( Hammer, 1953) 2.4–3.4 32/022/33342 1/000/0001 1/000/ 111001m 50–60 - 25–30 111pso + 111 psx Canada, Chukotka sensu Babenko & Fjellberg (2016) Peninsula
nikolai Kaprus ´, Weiner & Paśnik 2016 1.5–1.7 33/022/33342 1/- 1/000/100000 29–36 + 11–12 100pso + 0 0 0 psx Primorsky Krai, Far East
sayanica Kaprus´, Weiner &Paśnik 2016 2.7–2.9 32/022/33343 1/- 1/000/ 111101m 41–48 - 18–21 111pso + 111 psx southern Siberia
tschernovi ( Martynova, 1976) 1.8–2.3 32(3)/022/33343(2) 1/- 1/000/ 111101m 30–42 + 15–18 111pso + 111 psx Western Taimyr, Siberia sensu Babenko &Kaprus´(2014)
cykini sp.nov. 4.3–5.6 (2)32/022/33343 1/- 0/000/?111(2)00 65–71 + 18–22 111 pso + 0 0 0 psx Primorski range, Siberia based on recent data ( Parimuchová et al, 2017) of material from the type locality
Totally 22 troglobiotic collembolan species have been registered in southern karst regions of Russia ( Turbanov et al. 2016a, b, c), most of them distributionally limited to Crimea and Caucasus Mts that undoubtedly represent diversity hotspots of the cave fauna. The both regions are centered along the latitudinal band 42–45° N, corresponding by geographic position to the mid-latitude biodiversity ridge in Europe defined by Culver et al. (2006). Considering the number of biospeleologically surveyed caves in Russia, diversity of the cave-adapted Collembola in this country is still underestimated. Although, we cannot expect much addition by the further explorations in Siberian caves, since biodiversity of this vast region was repeatedly diminished during the cold Pleistocene glacial periods. The recent cave adapted fauna of Siberia probably evolved from the ancestors that occupied the soil or superficial subterranean habitats during the warmer Pleistocene periods. Accordingly, we consider the new species a glacial relict, supported by the morphological traits that do not show marked troglomorphisms.
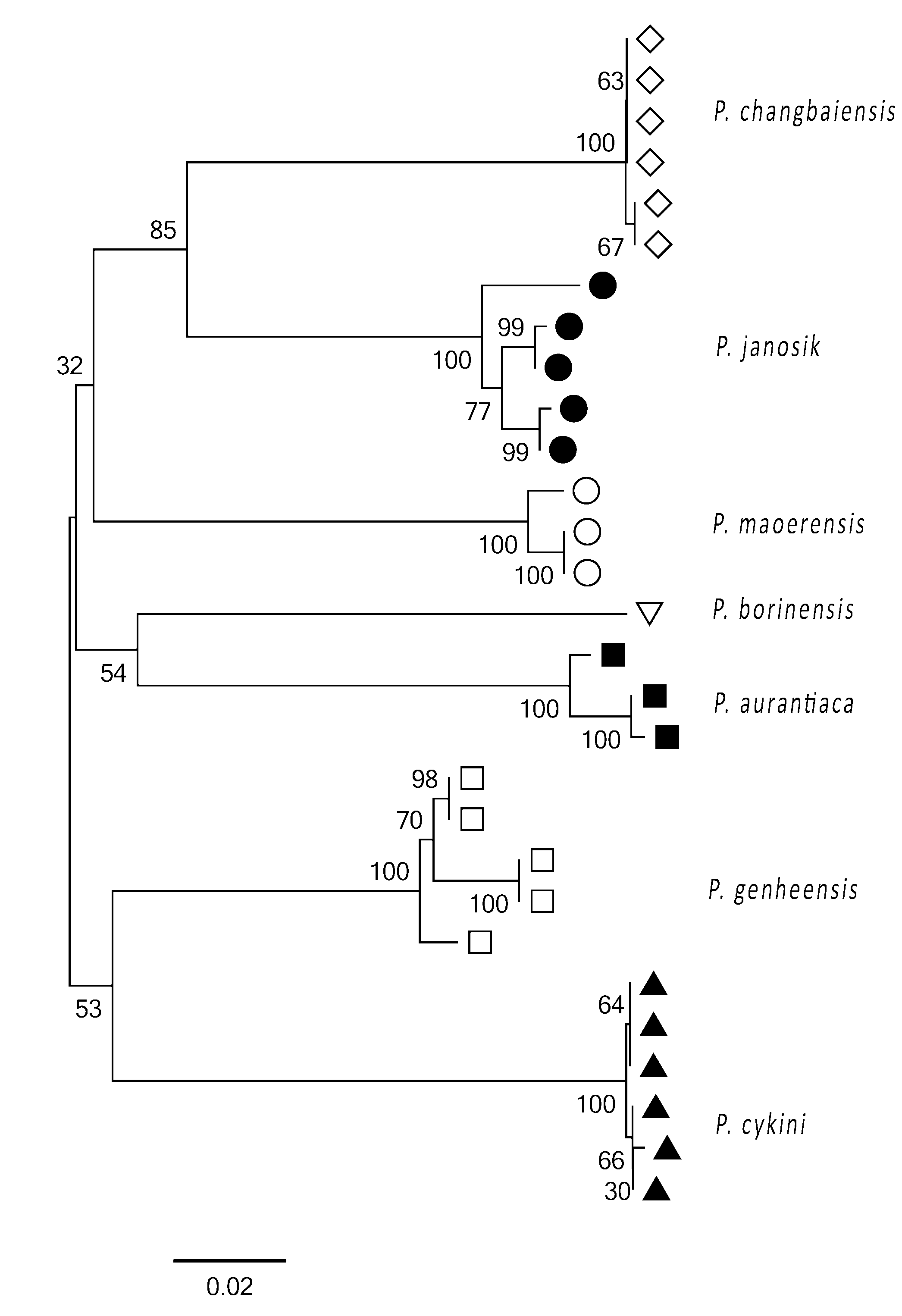
Molecular data. Obtained sequences are part of the COI gene at the 5´end corresponding to the position from 1564 to 2131 of the Drosophila yakuba mitochondrion (KF824900.1). We performed distance analysis by the Neighbor-joining distance tree ( Fig. 17 View FIGURE 17 ) based on the barcode sequences of the new species, P. borinensis Parimuchová & Kováč, 2016 and the species available in GenBank. Results strongly support the species differentiation. All the corresponding clusters have the highest bootstrap support, showing thus clear separation between the congeners ( Hogg & Hebert 2004). Clustering of species analysed follows neither their geographic distribution nor morphological characteristics ( Table 2). For example, interspecific K2P distances suggest that oligopseudocellar P. genheensis is the closest relative of P. cykini sp.nov. with the smallest genetic distance of 0.157 between the species involved in the analysis. P. janosik and P. borinensis , both from the Western-Carpathian caves, represent genetically well-separated species in spite of the low morphological differentiation ( Parimuchová & Kováč 2016).
interspecific intraspecific n 1 2 3 4 5 6
1 P. janosik 0.017 0.019 0.015 0.015 0.017 0.018 0.017 0.004 5 2 P. cykini sp.nov. 0.180 0.017 0.016 0.019 0.017 0.018 0.002 0.001 6 3 P. aurantiaca 0.184 0.192 0.018 0.019 0.018 0.017 0.011 0.003 2 4 P. genheensis 0.148 0.157 0.170 0.016 0.017 0.018 0.014 0.003 5 5 P. changbaiensis 0.142 0.201 0.193 0.170 0.017 0.019 0.001 0.001 6 6 P. maoerensis 0.166 0.180 0.192 0.171 0.178 0.017 0.008 0.003 3 7 P. borinensis 0.194 0.193 0.170 0.183 0.194 0.169 - - 1
| IBE |
Institut de Biologia Evolutiva, (CSIC-UPF) |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Protaphorura cykini
| Parimuchová, Andrea, Kováč, Ľubomír, Žurovcová, Martina & Kadebskaya, Oľga Ivanovna 2017 |
cykini
| Parimuchová & Kováč & Žurovcová & Kadebskaya 2017 |
janosik
| Weiner 1990 |
tschernovi (
| Martynova 1976 |
aksuensis (
| Martynova 1972 |
macrodentata (
| Hammer 1953 |
armata multituberculata (
| Stach 1934 |