Delomys altimontanus, Gonçalves, Pablo Rodrigues & Oliveira, João Alves De, 2014
|
publication ID |
https://doi.org/10.11646/zootaxa.3760.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:949EBA3E-5779-478C-9A6F-464E06908AAF |
|
DOI |
https://doi.org/10.5281/zenodo.5698102 |
|
persistent identifier |
https://treatment.plazi.org/id/03C4DA1F-FF96-9857-FF5A-F8BDFD18FB0A |
|
treatment provided by |
Plazi |
|
scientific name |
Delomys altimontanus |
| status |
sp. nov. |
Delomys altimontanus , new species
Delomys collinus: Bonvicino & Geise 1995 (not collinus Thomas 1917 ) Delomys dorsalis: Hershkovitz 1998 (not dorsalis Hensel 1972 )
Delomys sublineatus: Hershkovitz 1998 (not sublineatus Thomas 1903 )
Type material. The holotype, MN69746, is an adult male collected by Pablo R. Gonçalves, João A. Oliveira, Cibele R. Bonvicino and Maria Olímpia G. Lopes on 28 November 2004 (field number JAO 1558). The specimen was collected in a Sherman™ trap placed in a montane shrub dominated by bamboo at the margin of the Campo Belo stream, close to the Abrigo Rebouças. The specimen consists of a skin, skull and postcranial skeleton accompanied by a liver tissue sample preserved in ethanol and femoral medullar cells preserved in methanol/acetic acid solution. We designate as paratypes seven specimens collected in the localities of Abrigo Rebouças (MN69712, male) at Itatiaia -, Rio de Janeiro state, and Brejo da Lapa (MN60573-60576, 60584, 60585), municipality of Itamonte, Minas Gerais state.
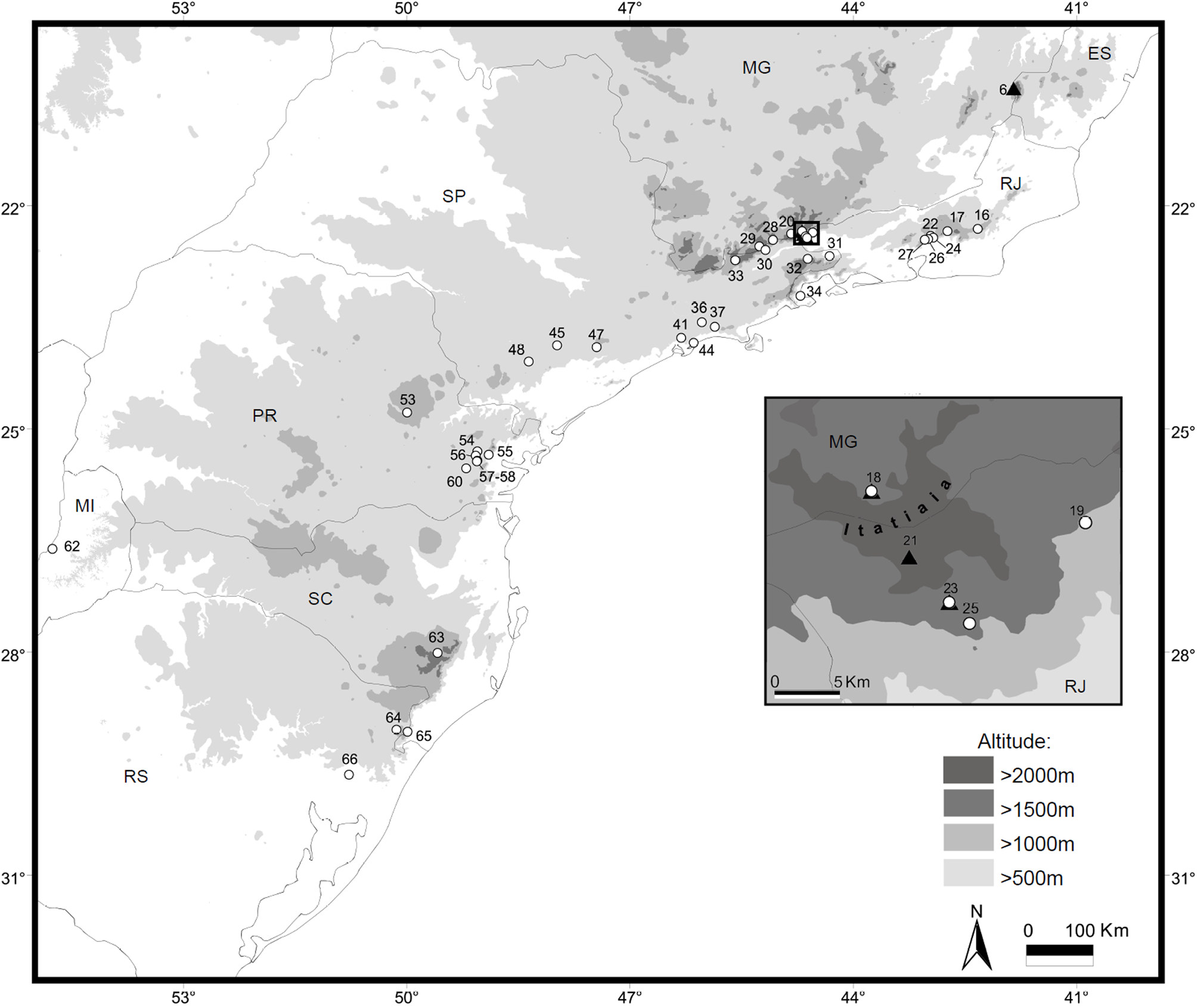
Type locality. Campos do Itatiaia, ( 22°23’26”S, 44°40’14”W, 2450m altitude), near Abrigo Rebouças, Parque Nacional do Itatiaia, municipality of Itatiaia, Rio de Janeiro state, Brazil (locality 21, Figs. 1 View FIGURE 1 and 12 View FIGURE 12 a).
Etymology. The specific epithet altimontanus refers to the restricted altitudinal distribution of the species, exclusively inhabiting the forested altiplano of the Mantiqueira mountain range in southeastern Brazil (from the Latin altus = high + montanus = mountain inhabitant).
Distribution. This species exhibits a disjunct distribution with reported populations apparently restricted to the highest altitudinal forested zones of 1800 to 2500 m in the Itatiaia and Caparaó mountain ranges, which are two different offshoots of the Mantiqueira mountain complex. Populations of Delomys that have been sampled in the intervening mountain chains that are part of the coastal Serra do Mar mountain complex, such as the Serra da Bocaina, Serra dos Órgãos and Serra do Desengano (in Rio de Janeiro state), or even in lower mountains of the Mantiqueira complex (Serra do Brigadeiro in Minas Gerais state), have been identified as either D. dorsalis or D. sublineatus ( Delciellos et al. 2012; Modesto et al. 2008; Moreira et al. 2009; Olifiers et al. 2007). These facts suggest that the current disjunct distribution of Delomys altimontanus sp. nov. is a relict of a formerly wider distribution rather than a sampling artifact across Southeastern Brazil.
It is probable that additional populations of D. altimontanus occur throughout the Mantiqueira range, especially in the mountains adjacent to Itatiaia that extend to the southwestern into São Paulo state (Campos do Jordão, Piquete) and Minas Gerais (e.g., Itamonte, Delfim Moreira, Passa Quatro). Nevertheless, available samples from these localities do not show the diagnostic morphological or genetic characters of D. altimontanus , rather representing D. dorsalis populations.
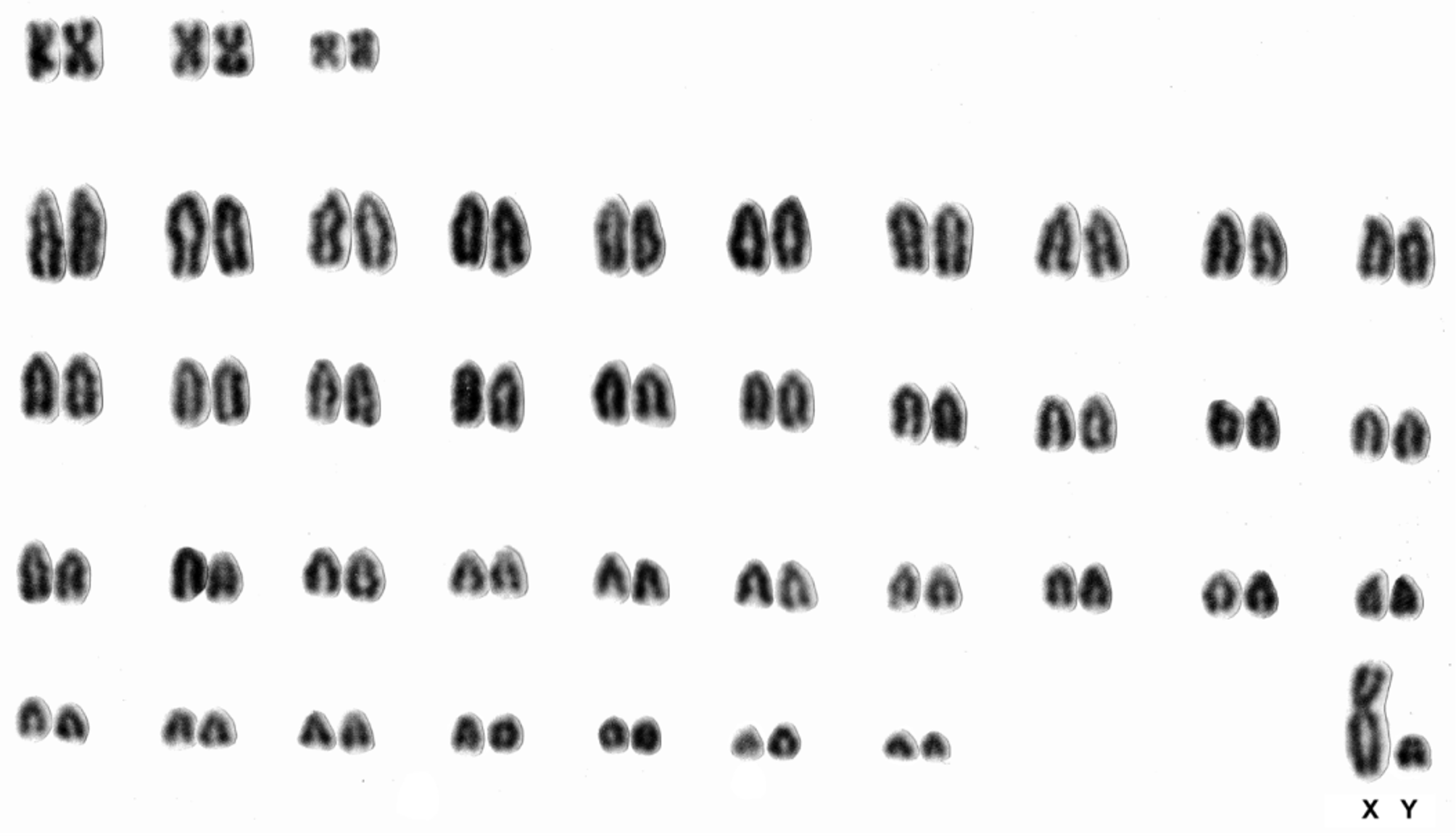
Karyotype. The karyotype of Delomys altimontanus ( Fig. 9 View FIGURE 9 ) exhibits 2n = 82 and FN=86, with an autosomal complement constituted by 3 pairs of biarmed chromosomes, 27 pairs of acrocentrics, a large-sized submetacentric X chromosome and a small metacentric Y chromosome, as previously described by Bonvicino & Geise (1995). Delomys altimontanus diverges from D. sublineatus (2n=72, FN=90) in both diploid and fundamental numbers, but shares the same diploid number with D. dorsalis (2n=82), from which it can be distinguished by the morphology of three small-sized biarmed pairs of autosomes. This karyological difference between Delomys altimontanus and D. dorsalis probably evolved through pericentric inversions involving the smallest pairs of autosomes.
Diagnosis. A medium-sized species of the genus Delomys with soft and long dorsal pelage, predominantly cinnamon-brown grizzled with yellow, but becoming brighter orange along the flanks; tail as long as head-andbody length and strongly bicolor throughout most of its length; skull with pronounced rostrum and elongated nasal tube projecting beyond the gnathic process; relatively longer and wider molar series than in other congeneric taxa; relatively narrow interorbit; supraorbital region exhibiting the fronto-parietal (coronal) suture collinear with the fronto-squamosal suture, without an area of dorsal contact between squamosal and frontal; incisive foramina with straight margins along the maxillary; mesopterygoid fossa equally wide at its anterior and posterior portions; mandible with wide, shallow and symmetrically excavated sigmoid notch, delicate coronoid process and reduced angular process; karyotype with 2n=82 and FN=86.
Description. Body pelage soft, dense and long, with guard hairs along the cervical region of the dorsum varying in length from 15 to 20 mm. General dorsal color is cinnamon-brown in adults, brighter and orangish along the laterals and gradually darker towards the mid-dorsum, generally forming a ill-defined median stripe in most specimens, which extends from the nape to the base of tail ( Fig. 10 View FIGURE 10 ). In old adults the sides of body are more rusty colored due to the presence of bright orange bands on hairs, while young specimens generally display a duller and grayer dorsal and lateral color with few traces of yellowish tone. Ventral pelage predominantly white, covered by whitish hairs with wide dark-gray bases frequently showing through. Head similar in coloration to the mid-dorsum, becoming brighter at the cheeks. Neck and chin region covered by smaller bicolored hairs, similar in coloration to those of the ventral region. Eyes large and surrounded by short dark hairs, forming a narrow eye-ring. Mystacial, superciliary, genal, submental, interramal and carpal vibrissae present. Mystacial vibrissae long, with the two longest reaching the distal tip of the pinnae when laid back. Pinnae large (Table 4) and sparsely covered with short hairs on its internal surface, but more densely furred on its dorsal surface.
Cheiridia dorsally covered by short whitish hairs, most of them with dark bases, specially at the proximal metacarpal and metatarsal regions, giving a general soiled or gray aspect to the dorsal surfaces of manus and pes. Hindfoot long and narrow. Ungual tufts long and conspicuous, completely enclosing the claws on digits I–IV, but reduced or vestigial on digit V. Middle digits (II–IV) are longer than the outer digits (I and V), with digit III slightly longer than digits II and IV. Digit I is longer than digit V, with its claw extending to the middle of the first phalange of digit II, while the claw of digit V reaches the first interphalangeal joint of digit IV. Plantar surface naked over the heel and much of the metatarsal region, being more squamate over the digits surface. Six fleshy plantar pads present (hypothenar, thenar and four interdigital pads).
Tail slightly longer to, or as long as, the head and body length (Table 4), sparsely covered by short hairs, and displaying a bicolor pattern throughout more than half of its length. The tail color is predominantly dark brown throughout its entire dorsum, but markedly whitish to buffy in the venter, throughout its first two proximal thirds, gradually intergrading to dark-brown towards the distal third. The brighter ventral color of the tail is provided by entirely whitish short hairs, which in the base of the tail span two to three scales in length. Towards the tail dorsum and the distal third of the tail venter, hairs with wide dark brown basal bands become more common, providing darker tones.
Six mammae present in lactating females, distributed in inguinal, abdominal and postaxial pairs (pectoral pair lacking), displaying the mammary formula of 0/2/2/2.
Cranium large and wide ( CIL = 31.4– 27mm, BB = 12.73– 11.7mm, Table 4), with a narrow and elongated rostrum, biconcave and narrow interorbit and globular braincase with smooth edges ( Fig. 11 View FIGURE 11 ). Nasals long (NL = 14.65– 11.75mm) and narrow, extending posteriorly beyond the fronto-premaxillary suture. The nasals taper more abruptly at their anterior midlength and then more subtly before converging at the fronto-nasal suture. Lacrimals squared and relatively large, contacting both the frontal and maxillary bones. Interorbital region relatively narrow and biconcave, exhibiting the typical “hourglass” morphology ( Voss 1993) with smooth edges and without supraorbital crested margins, even in older adults. Fronto-nasal suture slightly depressed, with frontal sinuses dorsally prominent. Fronto-parietal (=coronal) suture U-shaped, generally open angled in most specimens, but also sharply angled in some older adults, especially towards its lateral limits. Fronto-parietal and fronto-squamosal sutures collinear, as both the parietal and squamosal bones contact the frontal nearly at the same level along the postorbital wall, leaving no area for a contact between the squamosal and the dorsal surface of the frontal. Parietals wide and slightly expanded onto the lateral surface of the braincase near the squamosal root of the zygomatic arch. Interparietal moderately wide and semicircular, not contacting the squamosal laterally. Zygomatic plate broad and projected anteriorly to the base of the lacrimal capsule, forming a conspicuous zygomatic notch; its anterodorsal margin smoothly rounded. Posterior margin of the zygomatic plate situated anterior to the alveolus of M1.
Zygomatic arch laterally expanded, formed by a large jugal and non-overlapping maxillary and squamosal branches. Postorbital wall with small ridges near the squamosal root of the zygomatic arch, and a conspicuous buccinator-masticatory trough. Incisive foramina wide, with an inflated and expanded palatine process and reduced maxillary septum; lateral outlines of the incisive foramina are straight lined along their maxillary portions, being sharply bent only at their caudal ends, where they terminate anterior to the M1 alveolus. Palate surface flat, without expressive lateral troughs or posterolateral pits. Bony palate short, with the mesopterygoid fossa extending anteriorly between the molar rows; anterior margin of the mesopterygoid fossa biconcave in most specimens due to the presence of a sharp median process, but flat in a few specimens lacking this process, being equally wide at its anterior (near the level of sphenopalatine vacuities) and caudal portions (along the pterygoid processes) in specimens with intact pterygoids. Parapterygoid fossae shallow, at about the same level as the palate. Sphenopalatine vacuities reduced to narrow slits restricted to the presphenoid or completely ossified in a few specimens. Posterior opening of the alisphenoid canal and stapedial foramen large, sphenofrontal foramen and alisphenoid-squamosal groove conspicuous, conforming to the primitive carotid circulation pattern (Weksler 2006) or pattern 1 of Voss (1988). Alisphenoid strut absent, and buccinator-masticatory and accessory oval foramina confluent. Hamular process slender, delimiting a larger subsquamosal fenestra and smaller postglenoid foramen. Tegmen tympani present and connected to the posterior suspensory process of squamosal. Bullae globular, constituted by a large ectotympanic, which contributes to the wall of the carotid canal and restricts the exposed ventral surface of the periotic to a narrow lip. Mastoid completely ossified or with a narrow perforation on its posterodorsal limit.
Mandible high and without conspicuous masseteric crests ( Fig. 11 View FIGURE 11 ). Capsular process of lower incisive alveolus reduced to a slight elevation. Sigmoid notch wide, shallow and symmetrically incised; coronoid process reduced, not projecting beyond the dorsal limit of the condyloid process; coronoid and condyloid process connected by an elevated bony ridge that has about the same height as the coronoid process. Angular process reduced and not projected posteriorly beyond the limit of the condyloid process.
Incisors ungrooved, with yellow enamel bands. Upper incisors strongly opisthodont. Molars pentalophodont. Upper molar rows parallel sided, long (LM = 4.53–5.2 mm) and wide (BM1 = 1.34–1.64 mm) (Table 4). M1 with anterocone very asymmetrically divided by an anteromedian flexus, which penetrates the anterior margin of the procingulum at a point more lingually displaced in relation to the labial limit of the protoflexus, resulting in a much narrower anterolingual conule and a much wider anterolabial conule ( Fig. 7 View FIGURE 7 a). Anteroloph well developed and connected to the anterolabial conule in worn molars, forming an anterofossete which is confluent with the anteromedian flexus. Paraflexus and metaflexus deeply incised into the occlusal surface, penetrating beyond the medial limit of the protoflexus and hypoflexus. Median mure connected to the protocone. Mesoloph well developed, stemming from the median mure and reaching the labial cingulum. Paracone with a reduced paralophule contacting the mesoloph in older adults and delimiting a large medial enamel fossete and a shallow mesoflexus in older adults. Posteroflexus conspicuous, but frequently reduced to an enamel island in most adults or completely lost in older individuals. M2 squared and with narrow anteroloph and shallow protoflexus. M2 mesoloph as developed as in M1, also contacting the paralophule and delimiting a large medial enamel fossete. Median mure similar to that of M1. M3 small and triangular, with deep paraflexus and narrow anteroloph present. Mesoflexus and metaflexus reduced to enamel islands in adult individuals. Mesoloph present, but fully coalesced to the paracone at the labial margin. Hypocone reduced and hypoflexus shallow. Metacone, posteroloph and posteroflexus not identifiable in adult specimens.
Lower molar series longer and narrower than the upper series (length = 5.3–5.47 mm; width of m1 = 1.24–1.36 mm). Procingulum of m1 narrow, with anteroconid asymmetrically divided by a shallow anteromedian flexid ( Fig. 7 View FIGURE 7 d); anteromedian fossetid wide and fused to anteromedian flexid in younger individuals; anterolophid absent; protolophid well developed and diagonally projected to the labial margin, contacting the anterolabial conulid at its medial portion; protoflexid deeply incised into the occlusal surface; metaflexid subdivided into a shallow lingual fold and a small enamel island, which is separated from the protoflexid by the anterior murid; mesolophid well developed and projected lingually, isolated from the metaconid by a deep mesoflexid and connected to the entoconid by a lophulid; ectostylid present and well developed in most specimens, and in older adults resembling an ectolophid; posteroflexid deep and posterolophid wide; m2 similar to m1, with a variably present ectolophid; reduced anterolabial cingulum and shallow protoflexid as sole remnant structures of the procingulum; m3 about the same size as m2; anterolabial cingulum and protoflexid absent; mesolophid barely distinguishable in adult molars; entoflexid and posteroflexid as enamel islands; hypoflexid deep; ectostylid reduced and ectolophid usually absent.
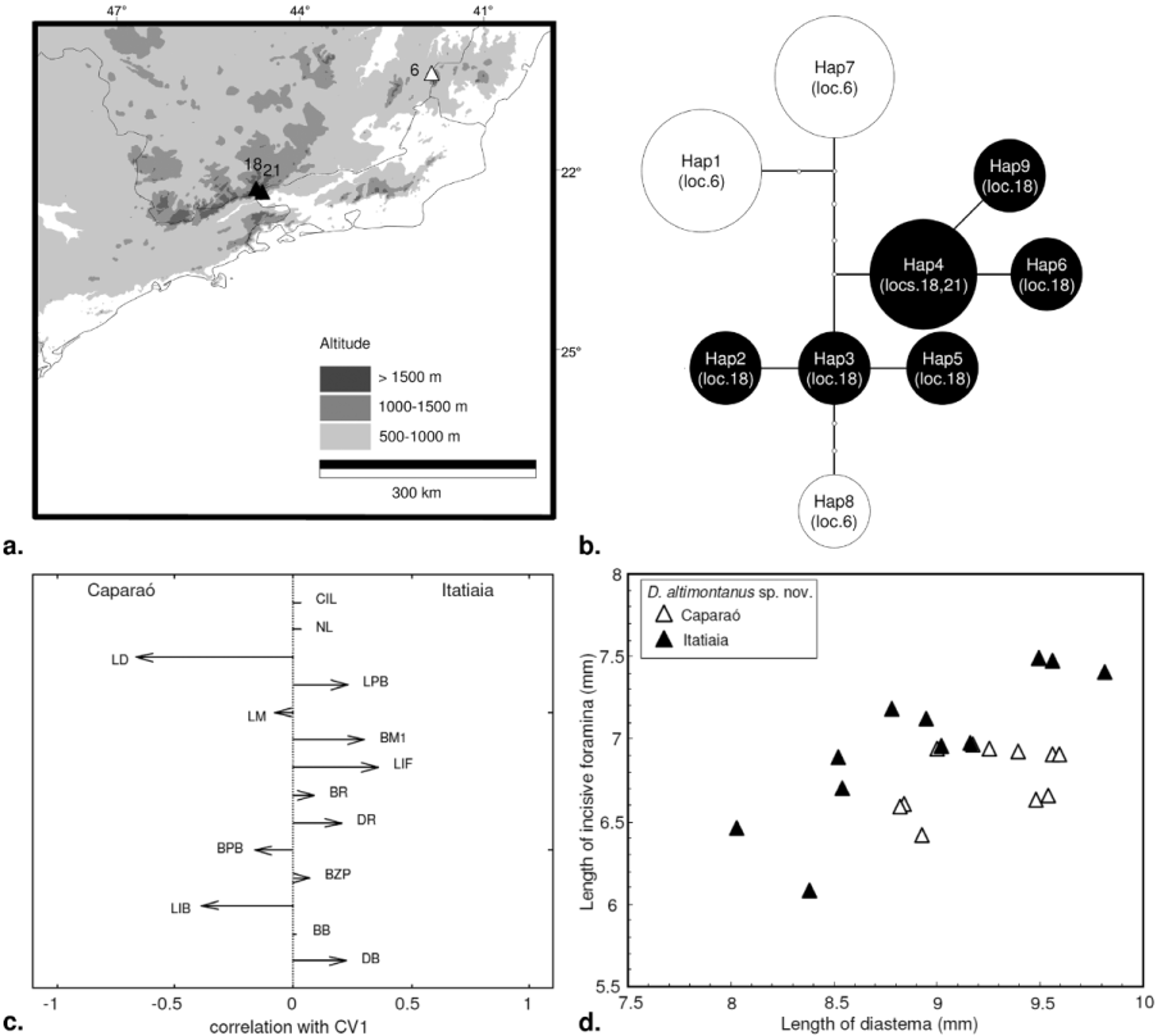
Variation in Delomys altimontanus . Nine cytb haplotypes were identified among the 15 sequenced D. altimontanus specimens from Caparaó Mt. (locality 6) and from two high-elevation localities in Itatiaia (localities 18 and 21) ( Fig. 12 View FIGURE 12 a). The genealogical relationships portrayed by the statistical parsimony network do not support the haplotypes from Itatiaia and Caparaó as two exclusive genetic groups, in spite of the large distributional gap (ca. 300 km) between these two populations ( Fig. 12 View FIGURE 12 b). Haplotype 8 from Caparaó, for instance, is connected to haplotype 3 from Itatiaia by three mutational steps rather than to the other two most frequent haplotypes from Caparaó (haplotypes 1 and 7), from which it differs by eight mutational steps. The six Itatiaia haplotypes are positioned in the center of the network, differing from one another by just a few mutational steps (one to six). The most frequent of these haplotypes (haplotype 4) is shared between two adjacent collecting sites (localities 18 and 21, Fig. 12 View FIGURE 12 b). The quantitative results of the AMOVA analysis show that most variation among haplotypes (69.3%) is due to differentiation within populations or within collecting localities rather than among the two disjunct populations (30.7%). Fixation indexes are also not statistically different from zero (Φ SC= 0.4861, p=0.1212; Φ CT= 0.3069, p=0.3118), suggesting a low level of geographic differentiation in D. altimontanus .
Despite the low level of geographic structure suggested by molecular variation in D. altimontanus , a Canonical Variates analysis of craniometric characters revealed that the Caparaó population differs from Itatiaia by having a somewhat longer rostrum (LD) and wider interorbit (LIB), whereas the Itatiaia population has slightly wider molars (BM1) and longer incisive foramina (LIF) ( Fig. 12 View FIGURE 12 c). All specimens from Caparaó could be correctly reclassified into their respective group, while 91.7% of the specimens from Itatiaia were correctly reallocated by minimum Mahalanobis distances. However, a bivariate plot combining the incisive foramina and diastema lengths shows that the segregation between the two samples is much more subtle, with at least four specimens completely overlapped ( Fig. 12 View FIGURE 12 d). The dorsal pelage of Caparaó specimens also tend to be more orangish along the flanks than in Itatiaia specimens. Nevertheless, the Caparaó sample we examined has a larger number of old adult individuals. Given that rostral length and brightness of the lateral pelage are both correlated with age, the age bias could not be discarded as a putative cause of these differences between samples.
Natural history. Little is known about life history aspects of D. altimontanus , most observations being based on habitat descriptions by collectors. At the Caparaó mountain range, Bonvicino et al. (1997) described the variation in small mammal species composition and abundance along the elevational gradient of 1000 to 2700 m. Delomys altimontanus (therein identified as D. collinus ) was trapped at elevations higher than 1800 m, where it was frequently associated with humid montane forests and shrubs, and corresponded to 14.3 to 27.1% of the small mammals captured at these high altitude habitats. No captures were recorded at lower altitudinal zones (1400 to 1000 m), where submontane secondary forests predominated. Bonvicino et al. (1997) did not report any other species of Delomys at the Caparaó mountain range. Nevertheless, nearby records at lower elevations throughout Espírito Santo (localities 5 and 7) and Minas Gerais states (localities 9 and 10) suggest that D. sublineatus may occur sympatrically with D. altimontanus in the Caparaó region, but probably occupying the lower altitudinal zones and exhibiting limited syntopy with its mountaintop congener.
At the Itatiaia mountain range, Delomys altimontanus appears to be the only Delomys species recorded above 2100 m, on the altiplano dominated by campos de altitude vegetation (locality 21, Campos do Itatiaia) and scattered high-montane forests and shrubs. At these high elevations, however, we captured specimens of D. altimontanus solely in forested habitats near streams and never on the open campos de altitude formation. At lower elevations on Itatiaia (2000– 1800 m), D. altimontanus is recorded in sympatry and syntopy with D. dorsalis (at localities 18 and 23, Brejo da Lapa and Abrigo Macieiras), where both species inhabit the humid montane forests characterized by the presence of the conifer Araucaria angustifolia and other cool-humid adapted austral plant taxa ( Brade 1956). No specimens of D. altimontanus have been genetically or morphologically identified at elevations lower than 1800 m, so the altitudinal intervals occupied by this species in the two mountain ranges are apparently similar.
Remarks. The holotype of D. dorsalis collinus Thomas, 1917 (BMNH 14.2.22.12, skin and skull) was collected in 1913 by James Peter Hill at about 1470 m in the Itatiaia mountain range. Two other specimens of Delomys were also collected by Hill at Itatiaia: an adult female of D. dorsalis (BMNH 14.2.22.13, skin and skull) with 6 mammae and without altitude information, and an adult male of D. altimontanus (BMNH 14.2.22.11, skull only) with the specific locality of “Maceiro” (=Macieiras) written in the label. This last locality refers to an old resting place (former “Abrigo Macieiras”), located at approximately 1880 m altitude along the dirt road (“travessia Ruy Braga”) that crossed the Itatiaia mountain range from the city of Itatiaia, in Rio de Janeiro state, to the city of Itamonte, in Minas Gerais state ( Brade 1956). As this road was the main travelling route between the southern Rio de Janeiro and Minas Gerais states in this region during the beginning of the 20th century, it is probable that J. P. Hill obtained the three specimens of Delomys along its margins. The road originally traversed the Itatiaia range along the Maromba valley, where many rural properties and (later in 1937) the administrative base of the National Park of Itatiaia were established at lower elevations ( 900–1200 m).
Hershkovitz (1998) identified both Delomys sublineatus and D. dorsalis collinus amongst the specimens from Caparaó that Bonvicino et al. (1997) previously identified as “ D. collinus ”. Nevertheless, the direct examination of this series and the inspection of the skulls and mandibles plates published in Hershkovitz (1998), confirms that all of Hershkovitz’ specimens represent D. altimontanus .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Sigmodontinae |
|
Genus |
Delomys altimontanus
| Gonçalves, Pablo Rodrigues & Oliveira, João Alves De 2014 |
Delomys dorsalis :
| Hershkovitz 1998 |
Delomys sublineatus :
| Hershkovitz 1998 |
Delomys collinus :
| Bonvicino & Geise 1995 |
dorsalis
| Hensel 1972 |
collinus
| Thomas 1917 |
sublineatus
| Thomas 1903 |