Strongylophthalmyia Heller
|
publication ID |
https://doi.org/10.11646/zootaxa.4189.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:6AE6BFFF-C89E-4BBA-A2BE-CE648ECBD4D |
|
DOI |
https://doi.org/10.5281/zenodo.6070364 |
|
persistent identifier |
https://treatment.plazi.org/id/03C587D8-FF88-FFBE-5EBD-F779E7BC07C4 |
|
treatment provided by |
Plazi |
|
scientific name |
Strongylophthalmyia Heller |
| status |
|
Strongylophthalmyia Heller View in CoL
Strongylophthalmus Hendel, 1902: 179 . Type species: Chyliza ustulata Zetterstedt, 1847 , by original designation. [Preoccupied by Strongylophthalmus Mannerheim, 1853 (in Coleoptera View in CoL ).1]
Strongylophthalmyia Heller, 1902: 226 View in CoL . Replacement name for Strongylophthalmus Hendel, 1902 . Type species: Chyliza ustulata Zetterstedt, 1847 , automatic.
Lapropsila Meijere, 1914: 241 . Type species: Labropsila polita Meijere, 1914 View in CoL , by subsequent designation ( Hennig 1941b: 36). [Synonymized by Meijere 1916: 87.]
Previous studies. No single review has been done on Strongylophthalmyia previous to this study. Most papers involved descriptions of one or a few species from results of collecting expeditions or museum collection studies (e.g., Krivosheina 1981; Shatalkin 1981, 1993, 1996; Iwasa 1992, 1995; Yang & Wang 1992, 1998; Barber 2006; Papp et al. 2006; Iwasa & Evenhuis 2014; Galinskaya & Shatalkin, 2016). Shatalkin’s (1993, 1996) study of many Old World species are the closest to a review of the genus and were based upon a comparatively small collection of specimens, primarily from southern and eastern Asia.
Frey (1956) and Steyskal (1971) provided keys to most species known at the time of their publication. Other keys that have been published are more geographically or taxonomically limited and include Barber (2006; Nearctic); Hennig (1940; Taiwan) ; Frey (1928; Philippines); Iwasa (1992; Japan); Galinskaya & Shatalkin (2016; Vietnam and adjacent countries); Iwasa & Evenhuis (2014; Papua New Guinea); Krivosheina (1999; Far East Russia); Meijere (1914; Indonesia); Shatalkin (1993; Palearctic; 1996; S. crinita group); Yang & Wang (1998; China, incl. Taiwan) .
The genus has been treated in different families, but most commonly Psilidae and Tanypezidae . Hendel (1917)
1. Previous works have indicated the earliest published date for the coleopteran genus-group name Strongylophthalmus as from Motschulsky (1860). However, research conducted in this study has found that an earlier publication by Mannerheim (1853) listed Strongylophthalmus in synonymy and it was made available by subsequent usage as a valid taxon in Motschulsky (1860); thus, the name is authored by Mannerheim in 1853.
proposed the subfamily Strongylophthalmyinae [sic] within the Psilidae . Hennig (1958) was the first to treat it within its own family, Strongylophthalmyiidae , noting synapomorphies with Tanypezidae . Because of the monophyly and synapomorphies of the two family-groups, some authors (e.g., Griffiths 1972; McAlpine 1997) have treated the Strongylophthalmyiidae as a subfamily within the Tanypezidae ; while others have maintained Hennig’s (1958) separate family status (e.g., Steyskal 1977, 1987; Stackelberg 1988; McAlpine 1989; Evenhuis 1989, 1998; Iwasa 1999; Iwasa & Evenhuis 2014; Galinskaya & Shatalkin 2016).
Lonsdale’s (2013) phylogenetic analysis noted a number of synapomorphies for Strongylophthalmyiidae + Tanypezidae , including the wide prosternum reaching the proepisternum; calypter large, lobe-like; surstylus fused to epandrium [= non-motile surstylus of Hennig (1958)]; elongate band-like postgonite; and epiphallus plate-like, comprising two articulating sclerites. The autapomorphies he listed for Strongylophthalmyiidae include the absence of a suture between prosternum and proepisternum; postvertical bristle anteroclinate; lateral scutellar bristles absent; distiphallus long, two-segmented with sclerotized apical section; and a single developed spermatheca. My examination in this study shows the distiphallus of the S. ustulata group appearing to be two-segmented; however, that of the remaining species is unsegmented. Regarding the spermathecal character, this was based on examination of a Nearctic species. Hennig (1958) also noted a single sclerotized spermatheca based on his study of S. ustulata . My study here shows the single sclerotized spermatheca to be restricted only to species of the S. ustulata species group [a second spermatheca was noted in S. pengellyi and S. angustipennis by Barber (2006) but it is considerably reduced and not sclerotized]; the remainder of species in the genus have two well developed and sclerotized spermathecae
Molecular studies have not been specifically conducted to corroborate the putative monophyly of the family and relationships within the Diopsoidea, but Strongylophthalmyiidae have been included in a few molecular analyses: e.g., as part of a larger analysis of Diptera families ( Wiegmann et al. 2011) and as an outgroup of other taxa being analyzed ( Gibson et al. 2010). In the former study, the analysis recovered a Strongylophthalmyiidae + Tanypezidae clade with moderate support. In the latter study, a sister-group relationship with Psilidae + Micropezidae was shown in one most parsimonious cladogram, while a majority rule consensus cladogram showed Strongylophthalmyiidae having a sister-group relationship with Psilidae + Pallopteridae . In another study, which used a combination of morphological and molecular characters ( Gibson et al. 2013), Strongylophthalmyiidae was shown to be the sister to Psilidae + Pyrgotidae . Obviously much more study and more representative taxa are needed to provide more accurate phylogenetic placement of the Strongylophthalmyiidae with other taxa in the Schizophora.
I follow Lonsdale (2013) in maintaining a separate family status, based on the autapomorphies he listed as well as the presence of downward-projecting anterior lobes of the hypandrium (noted by Griffiths 1972 [as “?pregonites”]), presence of a dorsal bridge of the hypandrium (noted by Sueyoshi 2006), and the incomplete Sc (noted by McAlpine 1987).
Generic characters. Male. Lengths. Body: 2.0– 7.5 mm; wing: 2.0– 6.9 mm.
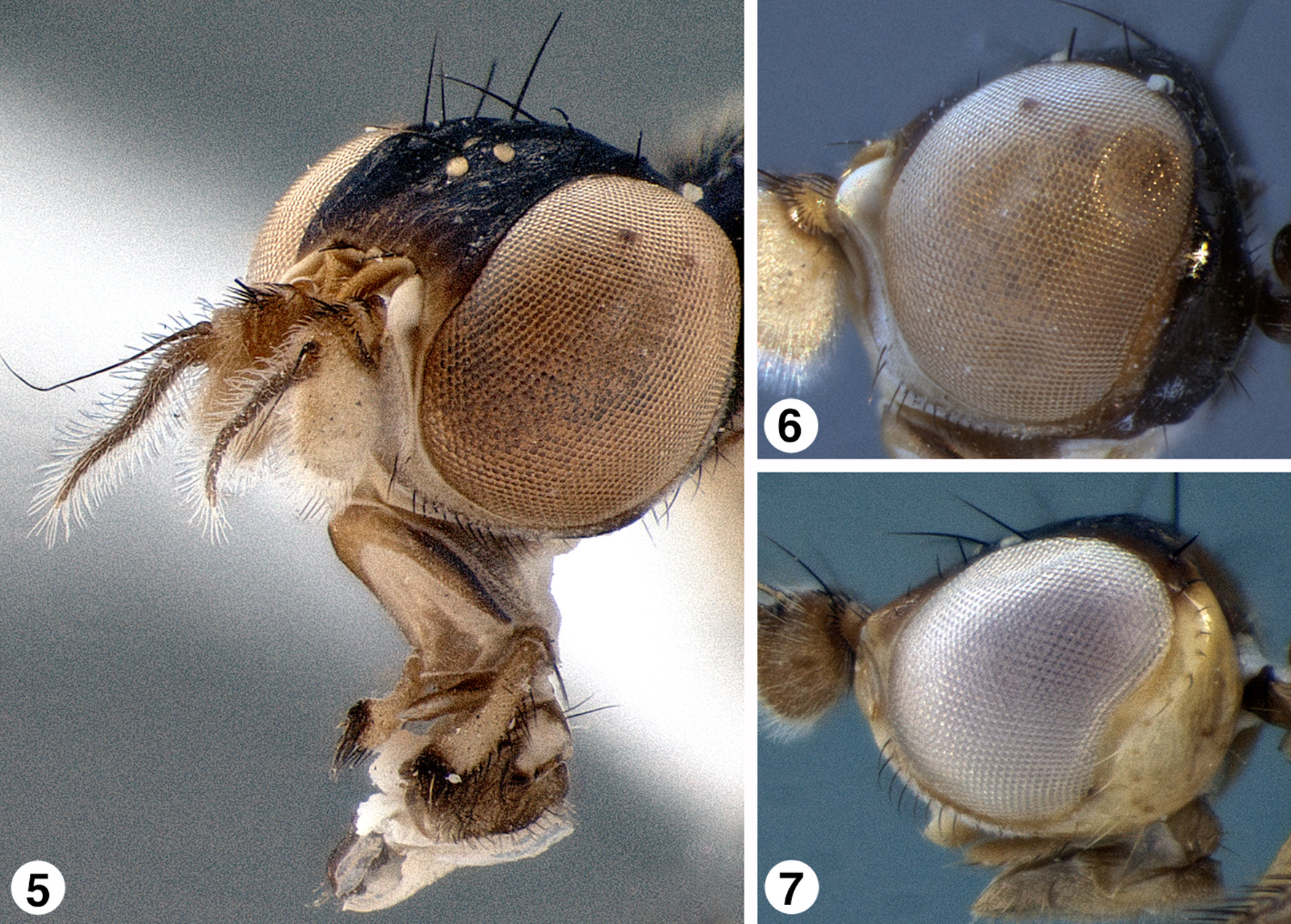
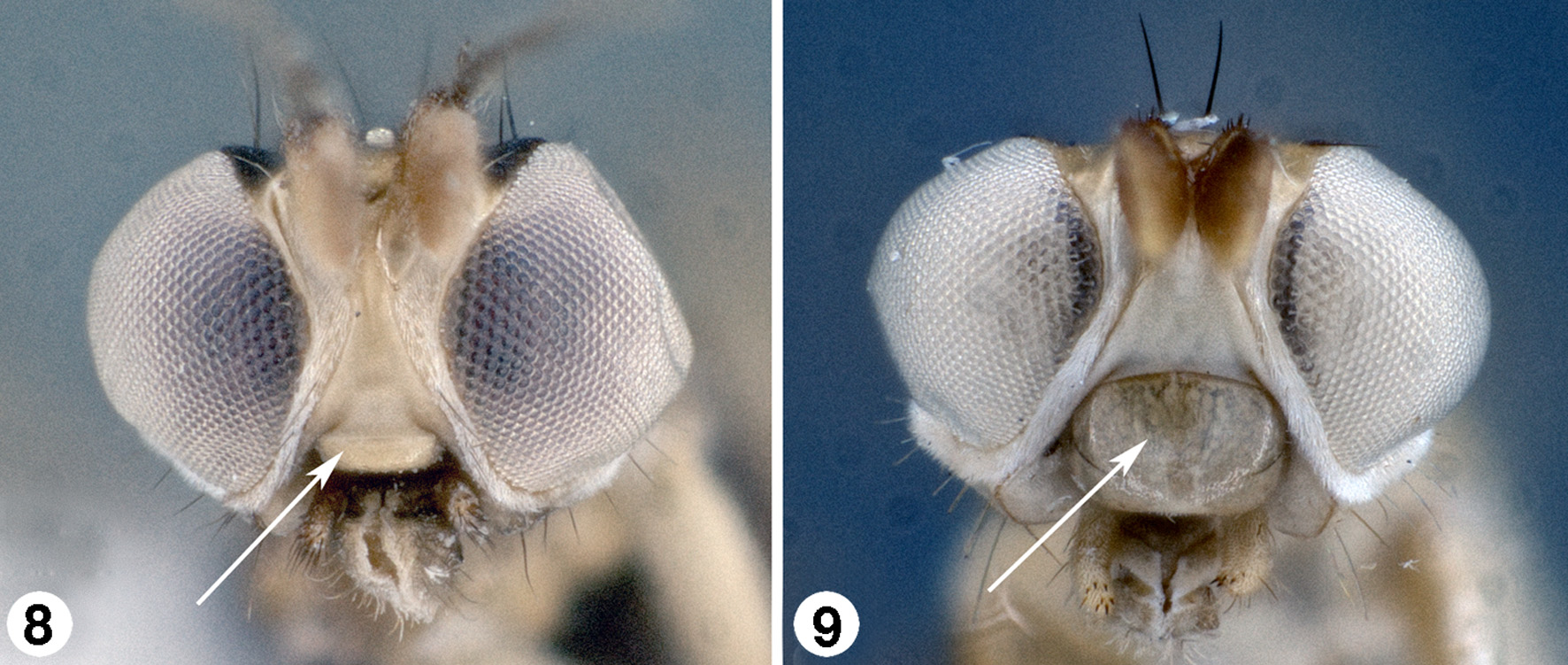
Head. Head in lateral view globose (e.g., Fig. 6 View FIGURES 5 – 7 ) or elongate (e.g., Fig. 7 View FIGURES 5 – 7 ), in dorsal view as wide or slightly wider than width of thorax; frons wide in both sexes with ocellar tubercle positioned anteriad of posterior margin of eye orbits (placed more posteriorly in S. sichuanica Evenhuis , n. sp. and S. gavryushini Galinskaya & Shatalkin, 2016 ); eye margin concave at postgena; inner eye margin converging below antennae to produce narrow face; face unsclerotized medially ( Figs. 8–9 View FIGURES 8 – 9 ); clypeus ( Fig. 8 View FIGURES 8 – 9 ) small, subquadrate, to very thin and band-like, or membranous and appearing absent; vibrissa absent; gena narrow, often pollinose, with short, fine or strong, yellow to black hairs; palpus white, yellowish brown, brown, or black, bacilliform to ovate in normal shape, but sometimes lengthened with dilated apex with flattened processes, glove-shaped ( S. caestus , n. sp.), racquet-shaped ( S. federeri , n. sp.), or disc-shaped ( S. palpalis Papp ), apex usually darker than remainder and/or with fine, thickened, or scale-like setae or spines on apical and/or ventral portions (cf. Figs. 10–17 View FIGURES 10 – 17 , 84–85 View FIGURES 82 – 86 ). Chaetotaxy as follows: 3–5 frontal-orbitals (very fine and hard to discern), 2–[rarely] 3 frontal-orbitals (distinct as easily seen), 1 ocellar, 1 postvertical, 2 verticals; vibrissae absent. [NB: The chaetotaxy of the head and thorax of Strongylophthalmyia is fairly consistent for the genus but has been found to be highly variable within species and thus not useful in characterizing species. It is therefore not added to the species descriptions in this work.]
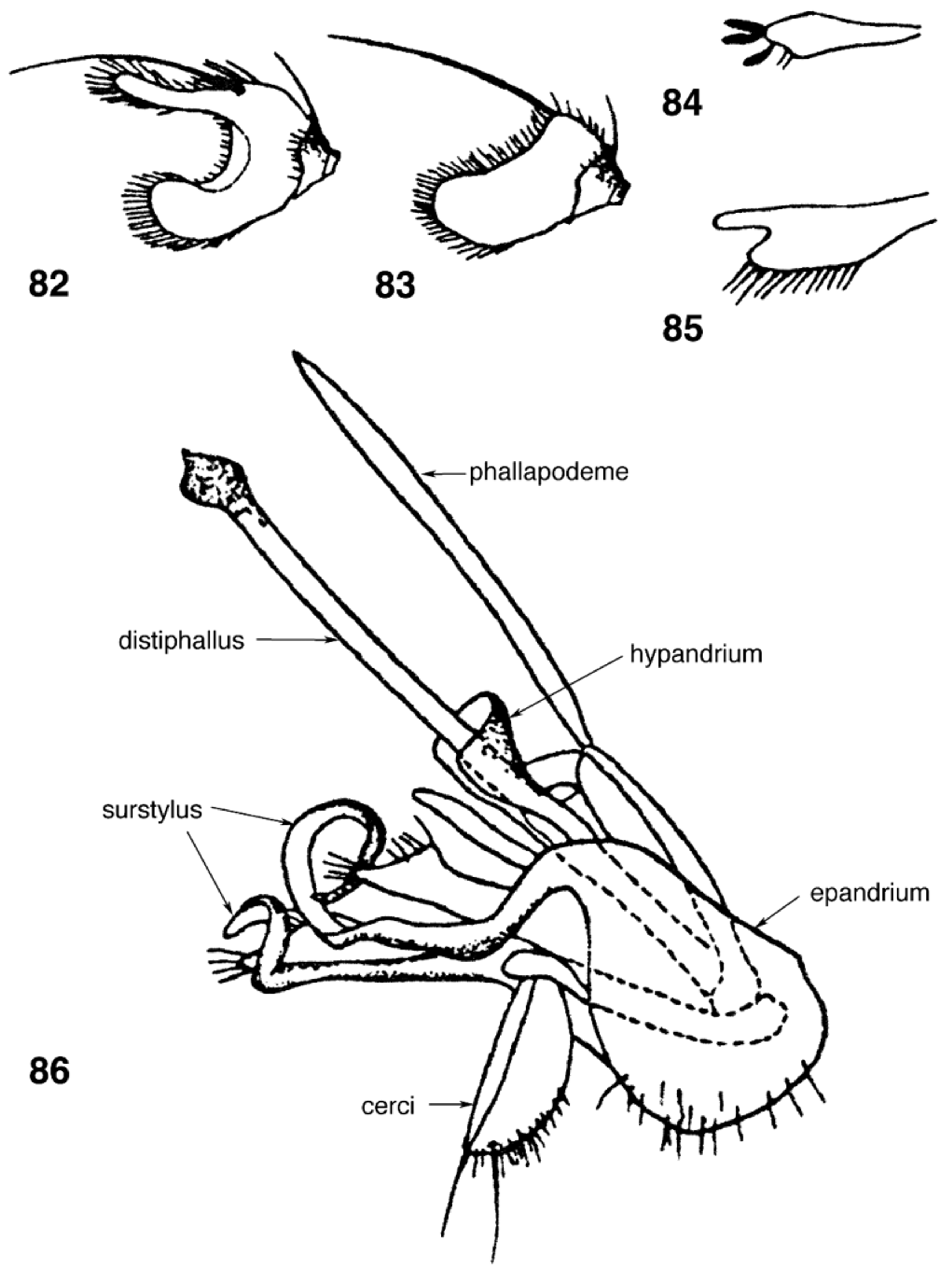
Antenna. Scape subcylindrical with ring of short stiff setae dorsoapically; pedicel flared apically and extending mesally onto medial portion of flagellomere, with dorsoapical ring of stiff setae, often with single strong apical seta that can be mistaken for a short antennal arista when the arista has been broken off; flagellomere ovate (normal) ( Fig. 30 View FIGURES 26 – 33 ) or extremely large and rounded ( S. macrocera Papp ), bifid ( S. raricornis Shatalkin ; Fig. 82 View FIGURES 82 – 86 ), or modified as axe-shaped, bean-shaped, or subrhomboid ( S. punctata group); flagellomere sometimes ( S. punctata group) with a dorsal process of varying lengths and shapes ( Figs. 26–41 View FIGURES 26 – 33 View FIGURES 34 – 37 View FIGURES 38 – 41 ); arista two-segmented, inserted subbasally on the dorsal surface, micro-pubescent (e.g., S. ustulata group) or bare, length varying from short, stump-like, one-half length of flagellomere to two times length of flagellomere.
Thorax. Micropezid or tanypezid-shaped in lateral view ( Figs. 38–45 View FIGURES 38 – 41 View FIGURES 42 – 45 ), twice as long as wide, with anterior portion extremely narrowed toward cervical area, with anepisternum and katepisternum expanded resulting in fore coxae being placed far from mid and hind coxae, which are in close approximation; propleuron, anepisternum and katepisternum appearing fused, without distinct sutures; mesonotum flattened and generally shining but some species dull (e.g., S. ustulata group), in dorsal view with distinct transverse suture; scutellum small, hemispherical to squarish; metanotum enlarged, extending posterior to scutellum; laterotergite with minute vestiture of varying colors; halter stalk with or without basal setulae. Non-chaetotaxic vestiture variable, mesonotum often with sparse or dense setulae or hairs, scattered or in rows, of varying lengths; ranging from decumbent, thick, and rather dense ( S. ustulata group; Fig. 97 View FIGURES 97 – 102 ) to erect, fine and sparse (e.g., Figs. 38–41 View FIGURES 38 – 41 ). Thoracic setae strong, black; with 0 proepisternals, 1 anepisternal seta, 0 katepisternals, 1–2 notopleurals, 0 acrostichals (although fine setulae occur in some species along the acrostichal row), 1–[2–3] [rarely 4–7] dorsocentrals, 1 supraalar, 1 postalar, 1 scutellar (lateral scutellars weak or absent in the family) [see remarks above under “Head” for the comments on the utility of chaetotaxy in distinguishing species in Strongylophthalmyia ]; anepisternum with hairs scattered, or clustered posteriorly, or along notopleural suture, tuft of curved hairs medioventrally on anepisternum in some species of the S. punctata subgroup (e.g., Figs. 38, 41 View FIGURES 38 – 41 , 42, 44 View FIGURES 42 – 45 ); katepisternum often with hairs ventrolaterally near mid coxa; laterotergite often with fine vestiture (concolorous with cuticle making it difficult to see).
Legs. Generally long and thin, with or without male secondary sexual character modifications including processes and setal patterns; fore coxae usually with strong hairs anteroapically; fore femur with short thorn-like spicules dorsally ( S. punctata subgroup) or without; other setal modifications of fore femur can include possessing extremely long, stiff lateral setae basally (e.g., S. darlingi , n. sp.), one or two clusters of hairs in close proximity ventrally that sometimes make thorn-like process(es) subbasally (e.g., S. palpalis Papp ) ( Fig. 72 View FIGURES 70 – 77 ), or a single strong black thorn-like seta ( S. microstyla Shatalkin ) ( Fig. 70 View FIGURES 70 – 77 ). Mid and hind legs up to two times longer than fore legs; mid legs normally unmodified, mid basitarsus modified in S. pengellyi [see Barber 2006; Figs. 16, 17 View FIGURES 10 – 17 )]; hind legs with dense, thin translucent or opaque ( Fig. 80 View FIGURES 78 – 81 ) scale-like setae on mesal surface of basitarsus in males and females [often difficult to see until light reflects off of them] (e.g., Fig. 78, 79 View FIGURES 78 – 81 ); hind legs otherwise mostly unmodified, some species with trochanter and/or base of femur possessing one, two, or three minute papillate protuberances of varying shapes (e.g., Fig. 81 View FIGURES 78 – 81 ), some bearing setae, others bare. Hind basitarsus normally subequal in length to second tarsal segment, but much longer in some species.
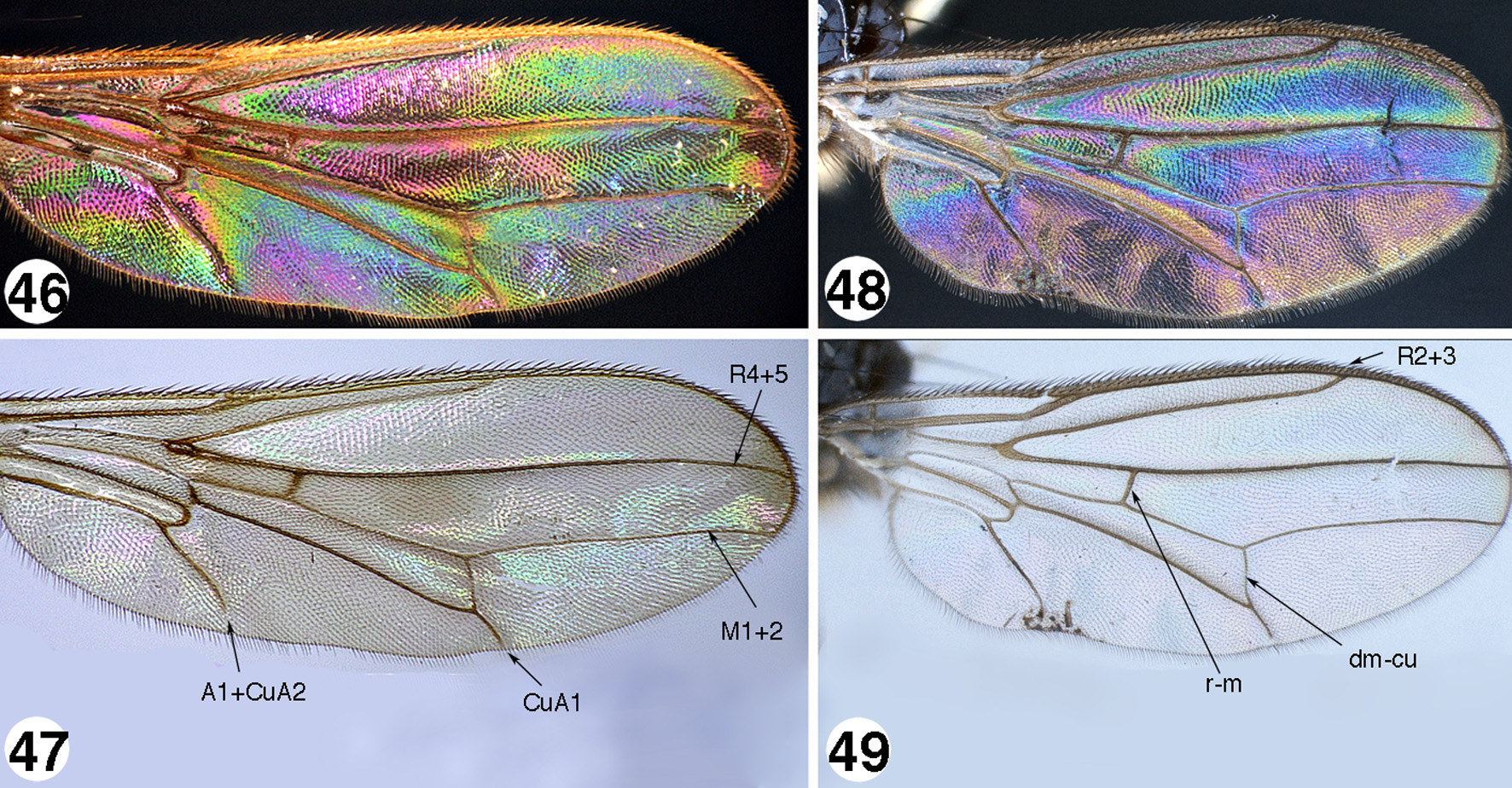
Wing ( Figs. 46–53 View FIGURES 46 – 49 View FIGURES 50 – 53 ). Hyaline (most species), fumose ( S. gigantica Iwasa & Evenhuis ), with a tinge of brown medially and apically (e.g., S. borneensis , n. sp.), infuscated with dark spot apically (e.g., S. fasciata Walker , S. ustulata group), or with transverse banding ( S. fascipennis group); single costal break just before junction with R1; costal vein ending at M1+2; Sc incomplete, effaced just before costal break; cell bm ends at or before level of R1 apex; R2+3 of variable length, ending before, at, or beyond level of crossvein dm-cu; distalmost portion of R4+5 and M1+2 parallel or slightly convergent at wing margin, never strongly convergent; cell dm long, length over five times width; crossvein r-m at or before halfway point of cell dm; crossvein dm-cu sloping toward ( Fig. 49 View FIGURES 46 – 49 ) or perpendicular to ( Fig. 50 View FIGURES 50 – 53 ) CuA1; distalmost section of CuA1 to wing margin much shorter than length of dm-cu (most species of S. ustulata group), subequal in length to, or longer than crossvein dm-cu; A1+CuA2 long, but not reaching wing margin; anal lobe present and well developed or slightly narrowed, but never extremely reduced; alula small; calypter rounded with fringe of long stiff hairs.
WIP ( Figs. 54–61 View FIGURES 54 – 61 , 96 View FIGURES 91 – 96 ). Wing Interference Patterns (WIP) were examined for all species not preserved in fluid, but could only be illustrated for a few species in this study due to distortion, folding, or inaccessibility to photography because of obstructions of body features. All species of Strongylophthalmyia possess WIP, although the number of individuals in this study that had wings suitable for study was minimal, so results shown here and described should be considered preliminary. These color patterns were recently discovered ( Shevtsova et al. 2011 et al.) as occurring in a number of flying insects. They may be used in sexual selection and/or mate recognition ( Katayama et al. 2014). The utility of this character in species identification bears further scrutiny. Some males and females in this study were found to exhibit similar patterns and further research may show this to be a character that can be used to associate females with males. There are clear species differences but there do not seem as yet to be clusters of pattern-types that can be used to distinguish species-groups. Most species exhibit a golden or brassy color with combinations of magenta, blue, and green in various parts of the wing. Strongylophthalmyia punctata Hennig ( Fig. 48 View FIGURES 46 – 49 ), S. sumatrana , n. sp. ( Fig. 59 View FIGURES 54 – 61 ), and S. sichuani c a, n. sp. ( Fig. 96 View FIGURES 91 – 96 ) differ from most species in this subgroup by having a predominant magenta or blue color in the distal medial field of the wing, this area being predominantly brassy to blue-green colored in other species. The anal lobe appears in most species to have a basal to subbasal spot of color lighter than the surrounding color.
Abdomen. Generally long and slender, dorsum generally flattened, shining brown to black with sparse erect pale hairs, longer setae posteriorly and laterally; tufts of stiff hairs may be present laterally on tergite V ( S. trifasciata Hennig ); anteriormost tergites (I, II and sometimes III) often paler in color than remainder of tergites; sternites normally sclerotized but weakly so medially, some species with sternites II and III with concavity at interstice separating the two segments, or sternites I, II and sometimes III membranous and appearing absent ( S. albisternum , n. sp.).
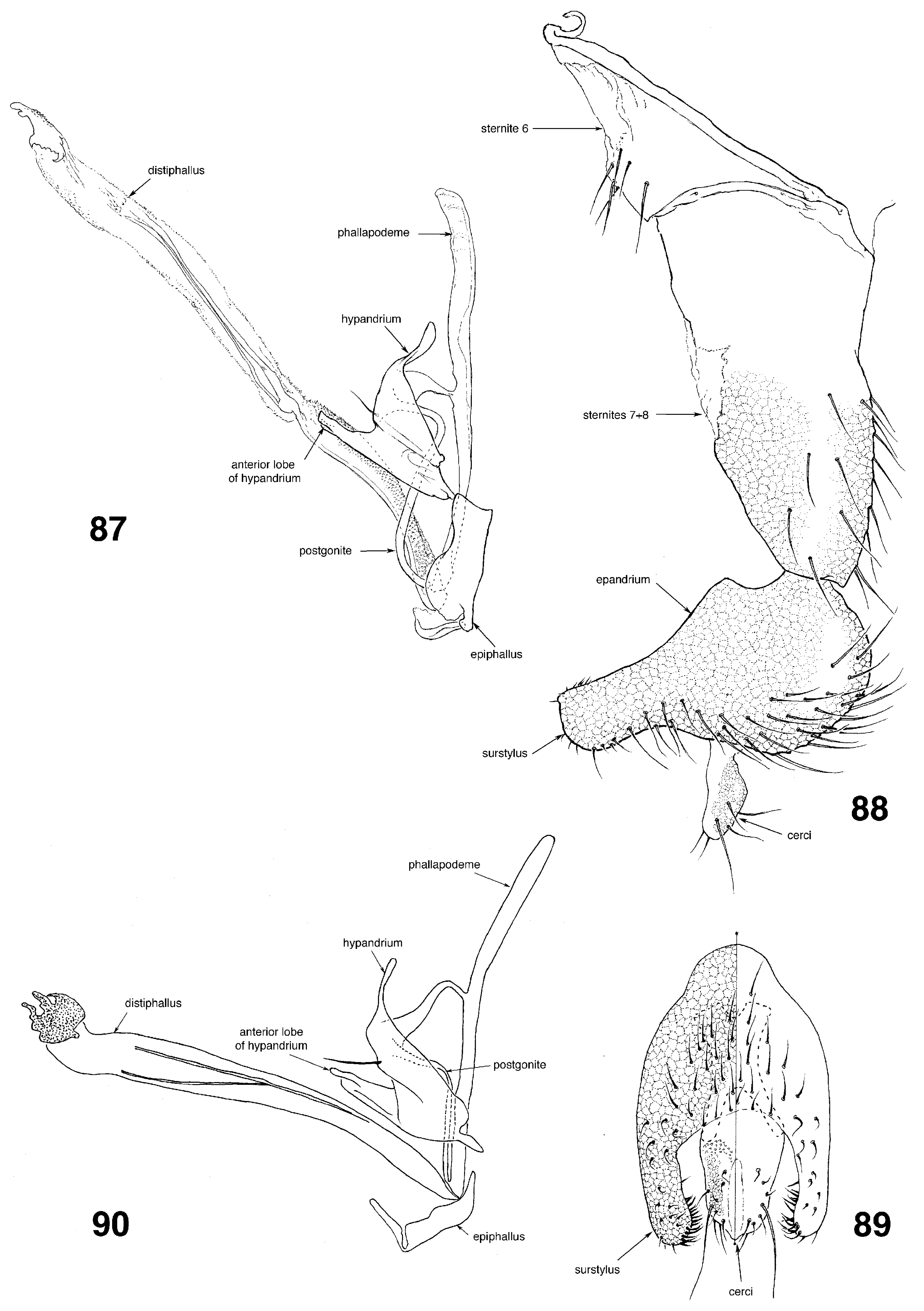
Genitalia (cf. Figs. 86–90 View FIGURES 82 – 86 View FIGURES 87 – 90 ). Male sternite VI subtriangular; sternites VII and III fused to form a syntergite 7+8; epandrium subovate in lateral view, fused to narrow surstylus making epandrium+surstylus appear pear-shaped [ Galinskaya & Shatalkin (2016) considered the surstylus to be absent]; surstylus rounded with patch of minute spicules mesoapically ( Fig. 89 View FIGURES 87 – 90 ) or surstylus expanded apically, long, curled ribbon-like ( S. crinita group) ( Fig. 86 View FIGURES 82 – 86 ); cerci fused for most their length, subequal to or longer than length of surstylus, narrow basally, flared and/or rounded apically, with long setae and hairs apically; hypandrium fused dorsally by thin bridge, with paired bifid anterior lobes, hypandrium attached to epiphallus via paired thin long, postgonites; epiphallus plate-like, consisting of two articulating segments; distiphallus long or short, straight, unsegmented ( Fig. 86 View FIGURES 82 – 86 , 90 View FIGURES 87 – 90 ), or appearing twosegmented ( S. ustulata group; Fig. 87 View FIGURES 87 – 90 ), up to two times length of hypandrial complex, with sclerotized apex of varying shapes; phallapodeme short or long, thin, straight, in some species length subequal in length to distiphallus, fused to hypandrium via pair of ventromedial processes
Female. As in male except for following: clypeus large, bulbous ( Fig. 9 View FIGURES 8 – 9 ); palpus linear, subtrapezoidal, or bacilliform in shape, without modifications, with or without ventral hairs, darker than in male, normally black; antennal flagellomere ovate, sometimes enlarged, but without modifications in shape found in males; arista up to two times length of flagellomere; pleuron as in male except those of S. punctata subgroup lack anepisternal tuft; legs normal, without modifications except scale-like setae on hind basitarsus; abdomen with segments VI–X lengthened and modified into a long tapering tubular ovipositor, posterior margins of ovipositor segments with long setae; one ( S. ustulata group) or two sclerotized spherical spermathecae.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Strongylophthalmyia Heller
| Evenhuis, Neal L. 2016 |
Strongylophthalmus
| Hendel 1902: 179 |
Strongylophthalmyia
| Heller 1902: 226 |