Troglocubazomus inexpectatus, Teruel & Rodríguez-Cabrera, 2019
|
publication ID |
https://doi.org/ 10.37828/em.2019.20.4 |
|
publication LSID |
urn:lsid:zoobank.org:pub:DA579664-F49E-4F15-B604-C1C3FCB4B86A |
|
DOI |
https://doi.org/10.5281/zenodo.8028410 |
|
persistent identifier |
https://treatment.plazi.org/id/5730987B-0E1D-400E-8E1A-0265C117EFD7 |
|
taxon LSID |
lsid:zoobank.org:act:5730987B-0E1D-400E-8E1A-0265C117EFD7 |
|
treatment provided by |
Felipe |
|
scientific name |
Troglocubazomus inexpectatus |
| status |
sp. nov. |
Troglocubazomus inexpectatus View in CoL sp. n.
Figs. 5 View Figures 5-6. 5 , 7 View Figures 7-8. 7 , 10 View Figures 9-11. 9 , 13 View Figures 12-13. 12 . Table I View Table I
Type data. CUBA: GRANMA PROVINCE: Niquero Municipality: Cabo Cruz: El Guafe : Cueva Funeraria # 2 (19°51'11"N - 77°42'52"W), 8 m a.s.l.; 24/October/2018; T GoogleMaps . M. Rodríguez; under rock, dark zone; 1♂ homomorphic holotype ( RTO). 28/October/2018; T . M. Rodríguez; under rocks, dark zone; 3 juveniles ( RTO) .
Diagnosis. Adult size large for the genus (male 5.8 mm). Coloration immaculate, pale olivaceous yellow, chelicerae and pedipalps light reddish, abdominal segments X–XII and flagellum reddish brown. Propeltidium with 2–3 pairs of dorsal setae. Heteromorphic male: unknown. Homomorphic male: Abdominal segment XII large, with the dorsal surface almost flat and with only three pairs of modified macrosetae (dorsolateral pair absent), which are all very large and very strongly curved. Flagellum large, very strongly sculptured (edges more raised and depressions deeper) and with microsetae patch strongly developed (microsetae much larger than usual); bulb in dorsal view broadly oval (1.21 times longer than wide), in lateral view conspicuously depressed (2.80 times wider than deep). Female: adult unknown (only a single immature specimen available).
Description (homomorphic male holotype). Coloration (fig. 5): immaculate, pale olivaceous yellow. Chelicerae and pedipalps light reddish. Abdominal segments X–XII and flagellum reddish brown. In general, the entire animal has a very pale olivaceous shade all over, which quickly fades to pale yellowish after preservation.
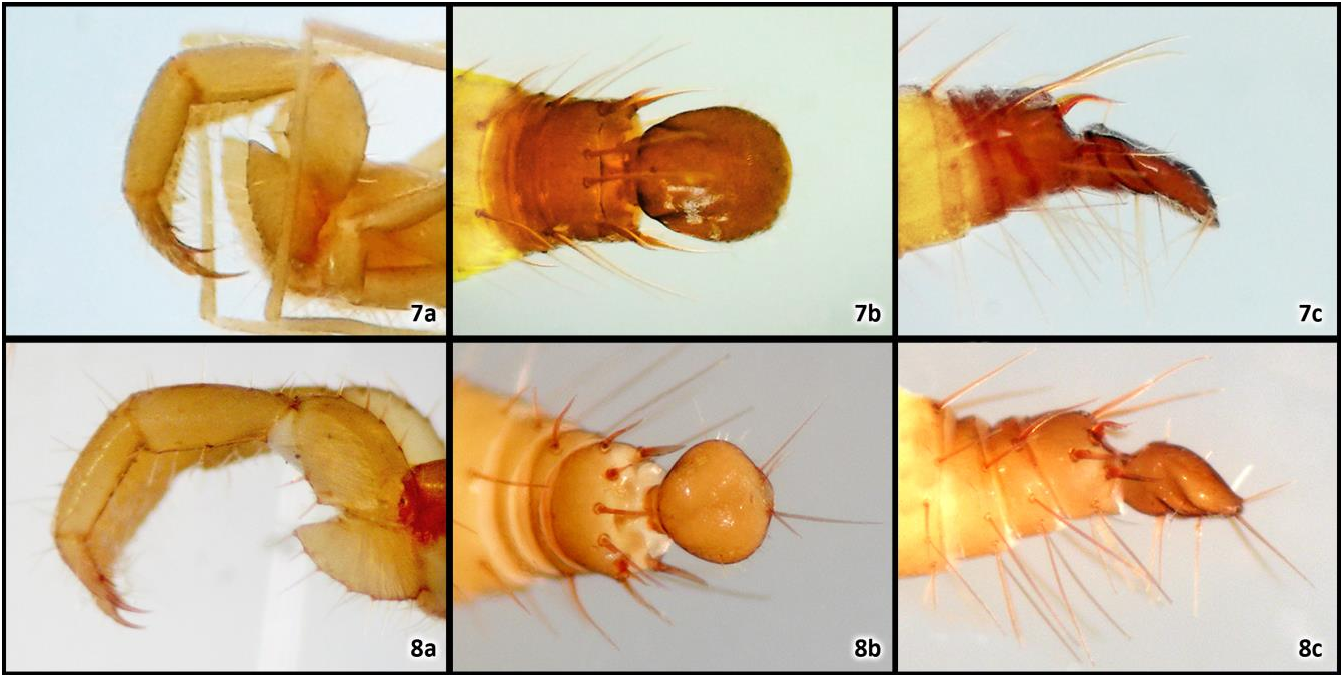
Pedipalp (figs. 7a): short and robust (1.45 times shorter than body). Trochanter lanceolate (2.16 times longer than deep), compressed, straight, and apically produced into a large, flat, narrowly paraboloid projection; dorsal margin evenly concave and bare; ventral margin shallowly convex, with 10–12 spiniform macrosetae; inner surface with three spiniform setae arranged into a curved row, essentially parallel to ventral margin, internal spur vestigial (very small, translucent and located near the dorsal margin). Femur rhomboidal, stout (1.89 times longer than deep), straight and very slightly bent basally; dorsal margin strongly convex, with two irregular rows of spiniform macrosetae (five dorsoexternals, three dorsointernals); ventral margin strongly angulose (at about 130°), with two irregularly parallel rows of spiniform macrosetae (three ventrointernals and three ventroexternals). Patella club-shaped, stout (2.56 times longer than deep) and straight basally; dorsal margin smooth, with 14–16 variously sized, rigid macrosetae; ventral margin very shallowly convex, with four pairs of large, subequal macrosetae irregularly arranged into two rows (the ventrointernals sinuose and plumose, the ventroexternals rigid and spiniform). Tibia subcylindrical, stout (3.08 times longer than deep), and moderately bent basally; dorsal margin with 16–18 variously sized setae, most of them rigid (some spiniform); ventral margin with three irregular rows of long, rigid setae all along: the ventrointernal row with six setae (sinuose and plumose), the closely-set ventromedian row with six setae (sinuose and plumose), and the ventroexternal row with six setae (rigid and spiniform), inner surface with many macrosetae scattered all over, obscuring the row pattern. Tarsus conical, elongate (3.13 times longer than deep), straight and densely covered with variously sized, sedose setae; apical spurs very large, thick and asymmetric (outer larger). Claw very large, thick, sharp, and shallowly curved.
Propeltidium (figs. 5a–b): with 1 + 1 apical and two pairs of dorsal setae (large and rigid), plus a single smaller seta in between on the left side (incomplete median pair). Eyespots absent.
Mesopeltidia (figs. 5a–b): very short and wide, subtriangular and widely separated.
Metapeltidium (figs. 5a–b): entire (without any traces of median suture or pale band), subrectangular and much wider than long.
Legs (figs. 5a–b): I very attenuate especially I. Leg IV femur elongate but robust (3.16 times longer than deep), slightly curved downwards and with anterodorsal margin angled at about 75°.
Abdomen (figs. 5a–b, 7b–c): moderately attenuate distally. Tergite I with two pairs of anterior microsetae, II with three pairs. Tergites II–VII with setal formula standard: 2 / 2 / 2 / 2 / 2 / 2; setae very large, dark, rigid and erect. Segment X slightly modified: slightly more sclerotized than usual and darkened. Segment XI moderately modified: more sclerotized than usual, darkened, and armed with a dorsosubmedian pair of long, thickened, sinuose macrosetae. Segment XII highly modified: heavily sclerotized, swollen, darkened, dorsally with distal third concave and sloped down at about 90° (so the flagellar joint when viewed from behind is transversely semicircular, instead of round as in most schizomids), and armed with three pairs of modified macrosetae: a dorsomedian pair (very long, thick and distally curved downwards), a dorsosubmedian pair (long, thick and sickle-shaped), and a lateral pair (very long, thick and distally curved inwards); posterodorsal process absent. Sternites densely covered with long, rigid macrosetae, most of them with tip ramified (mostly trifid, a few bifid or with four points).
Flagellum (figs. 7b–c): large, with pedicel/bulb angled at about 180°. Pedicel very short and wide. Bulb in dorsal view broadly oval (1.21 times longer than wide), anterior and lateral margins undefined due to round outline; bulb in lateral view strongly depressed (3.40 times longer than deep, 2.80 times wider than deep), with dorsal and ventral surfaces parallel and largely flat, and with a very large and deep pair of oblique ventrolateral furrows; dorsal surface with a very wide and shallow depression all along, flanked basally by a coarse U–shaped carina; dm 1 seta located basally on bulb, dm 4 in subapical position; apex undefined in dorsal view due to round outline, and strongly depressed in lateral view.
Female. No adults available, only one early-instar juvenile without evident sexual dimorphism except for the multi-segmented flagellum (three flagellomeres and two annuli).
Variation. No other adults are available, but the three juveniles exhibit very interesting variation in some non-maturity related features. First, the internal spur of pedipalp trochanter is slightly more developed. Second, the number of dorsal setae of propeltidium is the same as in the holotype, but the unpaired smaller seta is located on the right side of one specimen; evidently, the third (median) pair is in process of being either gained or lost.
Comparisons (males only). T. inexpectatus sp. n. is very easy to distinguish from the only other congener as follows:
1. Size. Much larger, up to 5.8 mm. T. orghidani is much smaller, 4.1–4.8 mm.
2. Coloration. More vivid and sharply contrasting, with chelicerae and pedipalps light reddish and abdominal segments X–XII and flagellum reddish brown. T. orghidani is paler and duller, with chelicerae, pedipalps, abdominal segments X–XII and flagellum light orange.
3. Shape and setation of abdominal segment XII. Larger, with the dorsal surface almost flat and with only three pairs of modified macrosetae (dorsolateral pair absent), which are all much longer and less strongly curved. In T. orghidani it is smaller, with the dorsal surface inflate and with four pairs of modified macrosetae (dorsolateral pair present), all much shorter and strongly curved.
4. Shape and setation of flagellum. Larger, bulb with dorsal and ventral surfaces flattened, parallel and strongly sculptured and with the microsetae patch markedly more developed (microsetae longer and thicker). In T. orghidani it is smaller, the bulb has dorsal and ventral surfaces convex, bulky and weakly sculptured and the microsetae patch is less developed (microsetae shorter and thinner).
5. Shape of leg IV femur. More slender and with posterodorsal margin angled at about 75°. In T. orghidani it is more robust and with posterodorsal margin angled at slightly less than 90°.
All these differences can be readily seen in detail in the photographs included here for comparison (figs. 6, 8). These show a standard-sized, adult male topotype T. orghidani with the following collecting data: CUBA: SANTIAGO DE CUBA PROVINCE: Santiago de Cuba Municipality: Siboney: Cueva Atabex (19°57'42"N - 75°42'57"W, type-locality), 50 m a.s.l.; 12/December/2009; R. Teruel, C. Martínez; walking on the floor, dark zone; 1♂ homomorphic ( RTO) GoogleMaps .
Etymology. The selected epithet is a Latin adjective that literally means "unexpected". It alludes to the surprising discovery of a second member of this remarkable genus, plus an even more shocking fact: that it occurred in a locality sampled long and well by arachnologists and other skilled collectors.
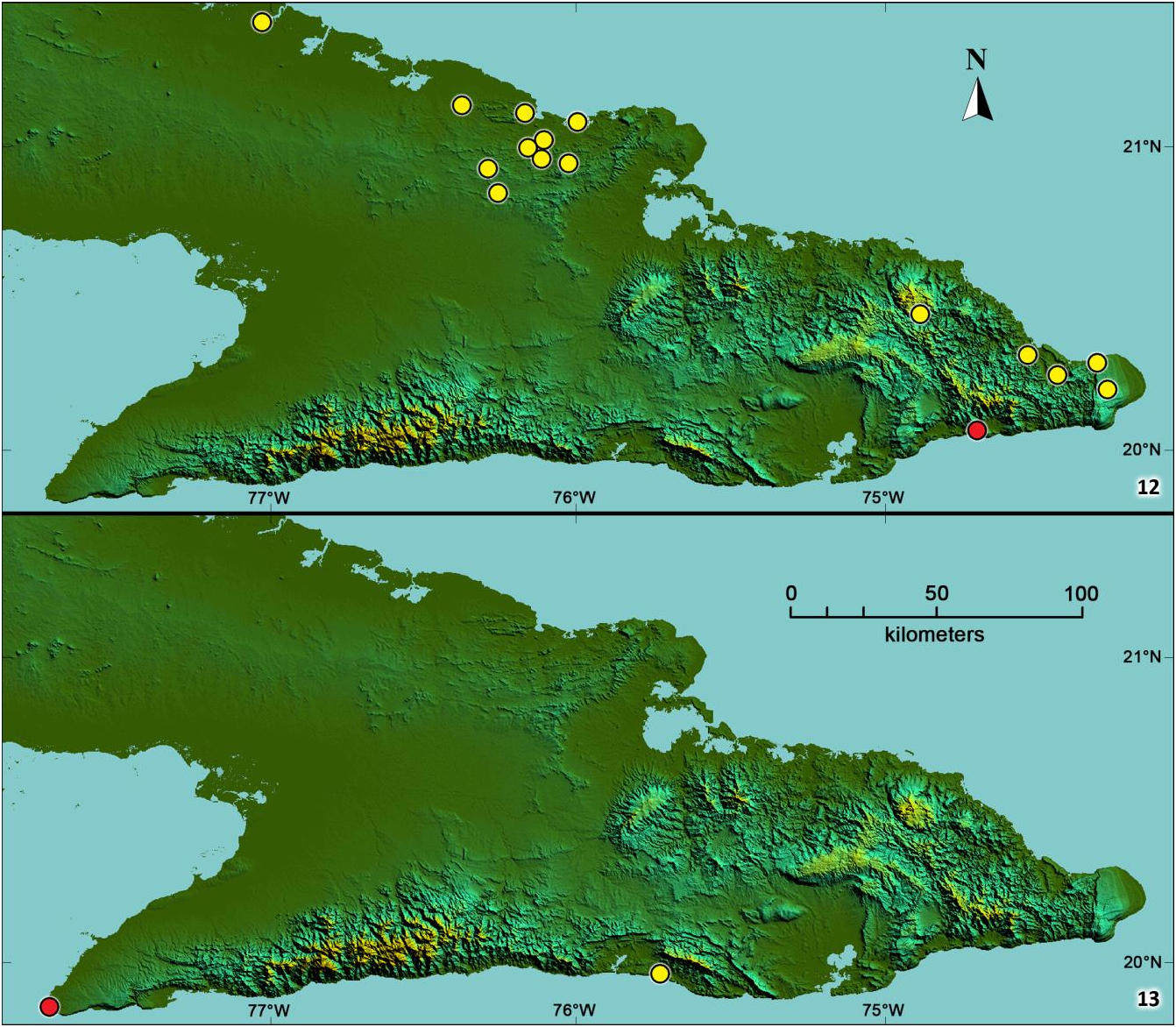
Distribution (fig. 13). Known only from the type-locality, a limestone cave in the extreme western tip of the Sierra Maestra Mountains.
Ecological notes (fig. 10). All available specimens of T. inexpectatus sp. n. were found inside a phreatic cave, under rocks deeply buried in the damp clay soil, very near some dripping pools and water-filled burrows of the land crab Cardisoma guanhumi Latreille, 1825 . The adult male was found in a small chamber more than 100 m away from the nearest entrance, but the three juveniles were all found in the semidarkness zone, less than 15 m deep from the cave main entrance. The male and two juveniles were located directly on the soil under the rocks, whereas the other juvenile was hanging to the underside of the rock. Air humidity and temperature along the cave were both relatively high, approaching 100% and 30°C in most chambers and galleries.
It lives syntopically with Rowlandius digitiger (Dumitresco, 1977) , which is by far more abundant and widespread all along the cave. At least five different bat species use this cave as their diurnal roosting site, forming colonies up to several hundred individuals that cause the subsequent accumulation of guano on the soil. R. digitiger is particularly common in and around the bat guano (maybe due to the observed higher abundance of potential prey, such as mites, collembolans and micro-moth larvae), but T. inexpectatus sp. n. was found only on bare soil, devoid of bat guano.
Cabo Cruz is a limestone region composed of different levels of staggered marine terraces with abundant caves (fig. 10a–b). Therefore, it will not be surprising that T. inexpectatus sp. n. may be more widely distributed through the interconnected subterranean systems of the area. Moreover, since 1985 the region is under the protection of the Desembarco del Granma National Park, thus, the long-term conservation of this species seems legally warranted.
Remarks. The anterodorsal margin of leg IV femur angled at about 75° represents a striking attribute in T. inexpectatus sp. n., because such angle is only slightly less than 90° in the only other congener T. orghidani and such intrageneric variation was unprecedented for Schizomida . In other words, the anterodorsal angling of the leg IV femur has been so far a 100% stable and reliable genus-level taxonomic character in the entire family, without any significant variation found to date in any genus despite how heterogeneous and speciose it could be, such as Rowlandius , Stenochrus and Surazomus Reddell & Cokendolpher, 1995 , for example. With the material currently at hand, nothing else supports separating this species in a different genus; on the contrary, it matches T. orghidani in all other odd characters that are currently diagnostic for Troglocubazomus . Anyway, such generic assignment will be confirmed only when adult females become available for study, especially their spermathecae.
General Remarks
With the present additions, the continuously growing diversity of the Cuban schizomid fauna reaches 59 species in 13 genera. Both additions are highly remarkable in different ways, as explained as follows.
On one hand, A. eremita sp. n. represents the first Cuban member of Antillostenochrus that is found in a desertic habitat. Almost all species of this genus are forest-dwellers restricted to mesic to very humid conditions, which range from seasonally dry, coastal semicaducifolious forests through montane rainforests and even elfin forests. The single previous exception was the southern Hispaniolan endemic Antillostenochrus subcerdoso (Armas & Abud, 1990) , which also lives in a semidesert environment. Thereafter, such kind of arid habitats cannot be considered anymore as exceptional for the genus and additional occurrences (and even undescribed taxa) become expected.
On the other hand, the discovery of a second member of Troglocubazomus is very important, because it allows the redefinition the generic diagnosis and for the first time to produce independent species-level diagnoses for its members. By the way, the redescription of T. orghidani will follow very soon (R. Teruel, in preparation).
| T |
Tavera, Department of Geology and Geophysics |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Hubbardiinae |
|
Genus |