Protaxymyia thuja Fitzgerald and Wood
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3857.1.4 |
|
publication LSID |
lsid:zoobank.org:pub:CFBE19A4-E8EC-494F-B8FA-72B4C1E2D93B |
|
DOI |
https://doi.org/10.5281/zenodo.6132044 |
|
persistent identifier |
https://treatment.plazi.org/id/03C6D643-FFF5-A501-0CC3-F49DF83CF8A0 |
|
treatment provided by |
Plazi |
|
scientific name |
Protaxymyia thuja Fitzgerald and Wood |
| status |
sp. nov. |
Protaxymyia thuja Fitzgerald and Wood View in CoL , new species
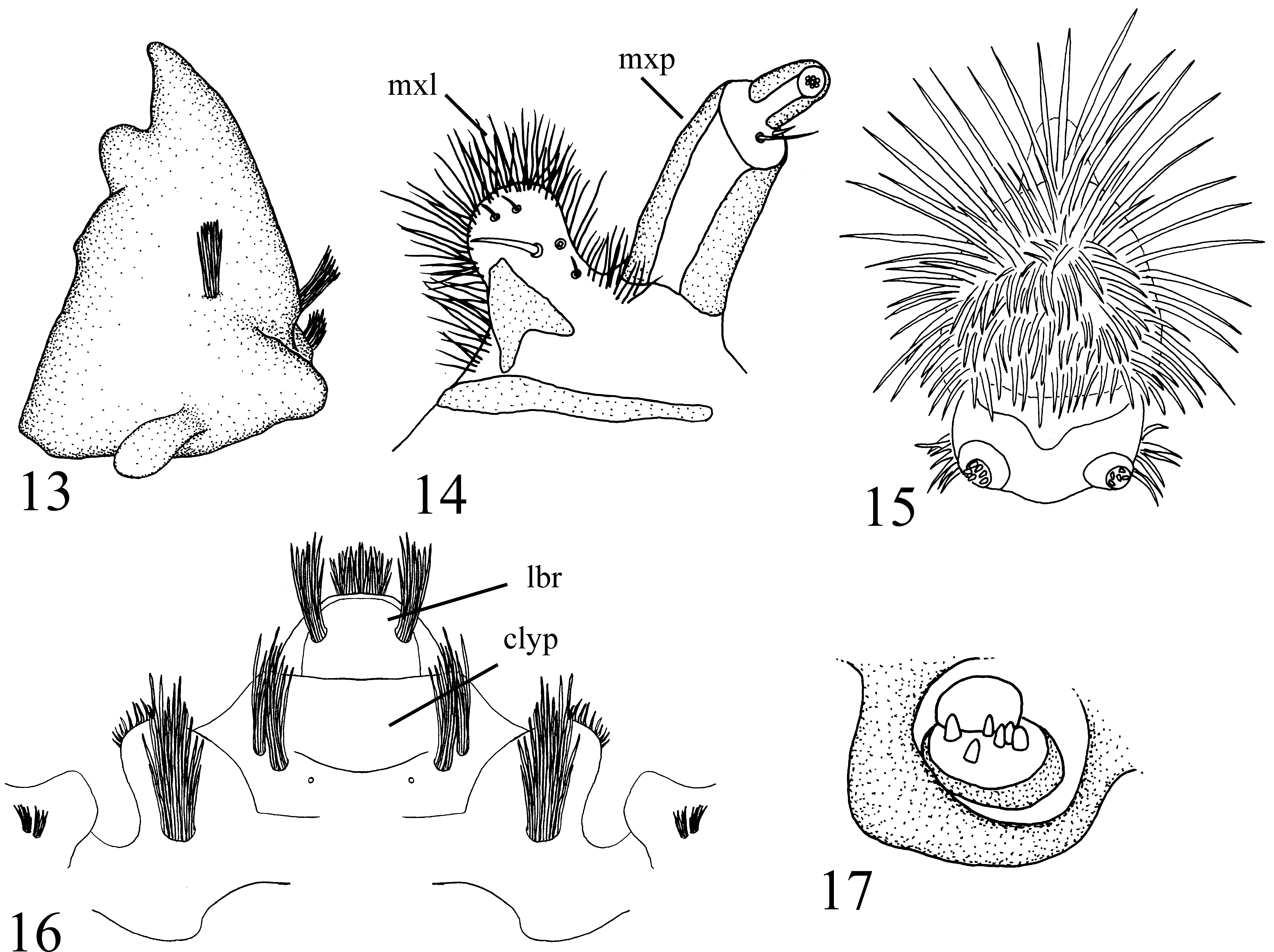
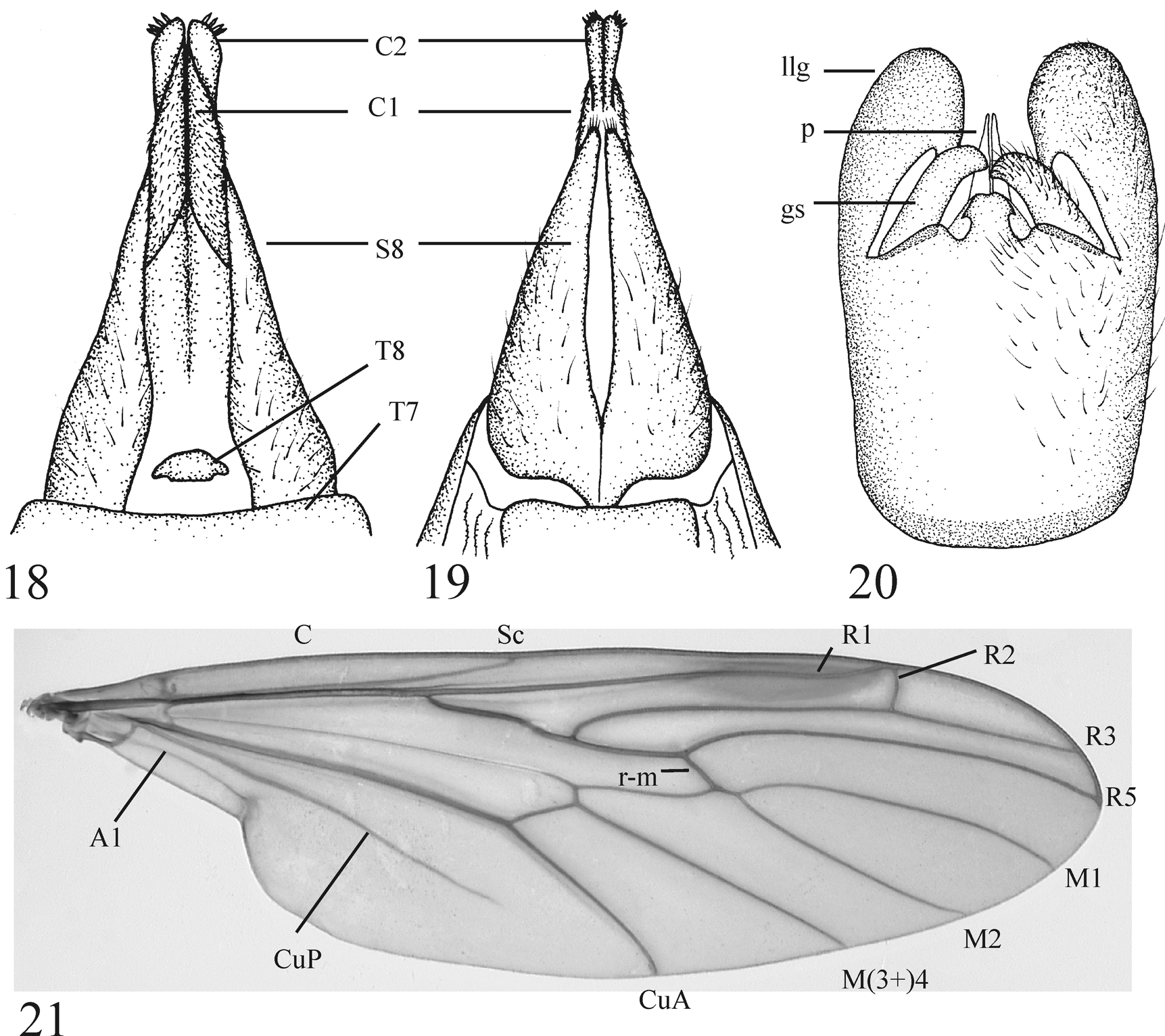
( Figs. 2–6 View FIGURES 1 – 4. 1 View FIGURES 5 – 8 , 9, 10 View FIGURES 9 – 12 , 13–21 View FIGURES 13 – 17. P View FIGURES 18 – 21. P )
Description. Male (n = 7): Head gray-black with light gray pruinescence. Holoptic, compound eye divided into dorsal region of larger facets and ventral region of smaller facets by distinctive longitudinal line level with dorsal edge of pedicel. Division line with smooth, glossy, unfaceted triangular region anteriorly. Dorsal portion of compound eye red (fading to dark brown with brick-red tinge in pinned specimens), ventral portion brown, both with minute, sparse, erect ommotrichia. Ocellar tubercle with short black setae; three ocelli present. Antennae divergent, about as long as head, black, with fourteen flagellomeres and short black setae. Flagellomeres gradually becoming smaller apically. Scape gray-black, pedicel light brown. Posterior margin of ventral division of compound eye with shiny, swollen tubercle similar to the stemmatic bulla of Simuliidae ( Mamaev 1968 describes this structure as a lateral ocellus). Face mostly bare. Clypeus gray-black, broader than long. Labrum shining, brown, short, triangular. Maxillary palpus four-segmented; black with short black setae at apex of each segment; apical segment with sensory pit. Occiput with moderately dense black setae. Thorax light brown in ground color with dark gray-brown pruinescence dorsally except along sutures. Dorsum with short, black setae anteromedially, laterally, and in two longitudinal rows on posteromedial portion of mesonotum and scutellum. Mesonotum strongly convex except posteromedial region slightly depressed and flattened. Antepronotal lobes light brown, without setae. Scutum with pair of shining, dark brown to black, oval spots anteriorly. Pleural sclerites light brown in ground color, anterior half of anepisternum and ventral half of katepisternum with dark gray-brown pruinescence. Coxae and trochanters light brown. Legs light brown basally becoming gradually black apically; femora light brown, tibiae brown-black, tarsi black. Legs with dense, appressed, black setae. Hind femur slightly tapered basally. Hind tibia essentially parallel-sided, approximately 2.5 times as long as basitarsus. Halter light brown except knob and basal spot on stem gray-brown, elongate; extending 2/3 length of abdominal tergite three. Stem of halter with dorsal brush of setae on basal 1/4. Wing 11.0– 11.5 mm, light brownish gray fumose. Pterostigma brown. Rs trifurcate; R2 perpendicular to slightly oblique to R2+3, R2 ending in R1 or ending in C just distal to R1/C intersection. Costa ending at R4+5. Costa and dorsal surface of R1 and Rs with minute setae. Wing with microtrichia. Alula absent. Tegula black with black setae. Abdomen dark gray, becoming darker posteriorly, with sparse appressed hairs. Tergite eight greatly reduced. Abdominal spiracle eight absent. Epandrium medially divided forming pair of posteriorly rounded lobes. Lateral edges of epandrium narrowly fused with gonocoxites + hypandrium. Proctiger largely membranous, cerci reduced to minute, lightly sclerotized nipples. Paramere apically laterally flattened into pair of median, fin-like lobes; in ventral view visible as pair of fins anterior to gonostyli ( Fig. 20 View FIGURES 18 – 21. P , p). Aedeagus between lobes of paramere; tubular posteriorly with posterolateral edges drawn out into heavily sclerotized, slender, anteriorly directed, apically free rods (apices not fused). Ejaculatory apodeme short, rod-like. In ventral view, posterior margin of gonocoxites + hypandrium with median mushroom-shaped lobe ( Fig. 20 View FIGURES 18 – 21. P ). In ventral view gonostylus robust, digitate, evenly curved, dorsomedially directed, apically broadly rounded to semi-truncate ( Fig. 20 View FIGURES 18 – 21. P ). In ventral and lateral views, lateral lobe of gonocoxite strongly developed, broadly rounded apically ( Fig. 20 View FIGURES 18 – 21. P ).
Female (n = 4): Habitus ( Fig. 2 View FIGURES 1 – 4. 1 ). As in male with following additions/exceptions: Dichoptic ( Fig. 2 View FIGURES 1 – 4. 1 ). Compound eye divided into dorsal and ventral regions; regions with similar sized facets. Frons black, opaque, medially depressed. Maxillary palpus five-segmented; apical segment with sensory pit. Wing 13.0–14.0 mm. Tergite 7 large and unmodified, tergite 8 minute (interpreted as tergite 9 by Iwata & Nagatomi (1981) with note saying that it may be tergite 8; we have taken the latter interpretation here), tergite 9 absent ( Fig. 18 View FIGURES 18 – 21. P ). Cercus twosegmented; basal segment more dorsal in position, elongate, weakly sclerotized, with fine setae, distal segment more ventrolateral in position, more heavily sclerotized, without fine setae, apically bulbous with cluster of rod or spine-like setae ( Figs 18–19 View FIGURES 18 – 21. P ). Sternite 7 narrow, much smaller than tergite 7. Sternite 8 large, heavily sclerotized, medially folded, laterally compressed, posterodorsally curved, broad anteriorly, tapering posteriorly ( Fig. 19 View FIGURES 18 – 21. P ). Posterior margin of sternite 8 medially cleft (on fold) 3/4 its length, though cleft represented by pale suture entire length of sternite ( Fig. 19 View FIGURES 18 – 21. P ). Apex of sternite 8 with stiff setae. Genital fork present as broad, dorsoventrally compressed plate with perpendicular, dorsally situated, median apodeme.
Larva (n = 5): Habitus ( Figs 4–5 View FIGURES 1 – 4. 1 View FIGURES 5 – 8 ). Plump, white with light brown head capsule and light brown sclerotization at apical tip of posterior siphon (modified segment 8). Body with distinct constrictions delineating each segment. Posterior siphon elongate, much longer than body (including head), minutely ribbed ( Figs 4–5 View FIGURES 1 – 4. 1 View FIGURES 5 – 8 ). Apex of posterior siphon without minute ribs, crowned with 5 lobes surrounding pair of posterior spiracles. Lobes with short stout spines, dorsal lobe with patch of short setae on each lateral edge. Two unbranched anal papillae present, much longer than body ( Figs 4–5 View FIGURES 1 – 4. 1 View FIGURES 5 – 8 ). Papillae sausage-link-like with lobes elongate, ovate. Respiratory system amphipneustic, anterior spiracle dark brown, on posterolateral edge of prothoracic segment. Dorsal and ventral surfaces of segments 1–5 with median transverse strip of indistinct minute spines. Head capsule large, brown, heavily sclerotized, non-retractable, posterior portion of cranium partly covered by invagination of thoracic cuticle. Cranium ventrally closed, hypostomal bridge with pair of rounded tubercles medially ( Fig. 10 View FIGURES 9 – 12 ). Positions of setal brushes on cranium as in Figures 9 and 10 View FIGURES 9 – 12 . Anterodorsal margin of cranium heavily sclerotized, with lateral and medial epicondyle for articulation with dorsal epicondyle of mandible. Lateral epicondyle dorsoventrally flattened, anteromedially directed, apically rounded, with two minute bundles of setae basally ( Figs 9 View FIGURES 9 – 12 , 16 View FIGURES 13 – 17. P ). Medial epicondyle larger than lateral, positioned posterolateral to clypeus, lobate, apically rounded, with large bundle of setae dorsally ( Fig. 16 View FIGURES 13 – 17. P ). Clypeus between medial epicondyles, apically truncate, distinct from labrum. Clypeus dorsally with two pairs of mediolaterally placed setal bundles ( Fig. 16 View FIGURES 13 – 17. P ). Labrum small, apically broadly rounded, with anterolateral bundle of setae on each side. Labrum ventrally with dense setae. Torma and premandible present. Mentum apparently absent. Sclerotization of labiohypopharynx horseshoe-shaped, medially membranous, apically broadly rounded, with dense hairs ( Fig. 15 View FIGURES 13 – 17. P ). Labial palpus cylindrical, longer than broad, two segmented. Basal segment barrel-shaped, apically with minute sensory rods ( Fig. 15 View FIGURES 13 – 17. P ). Maxilla well-developed, posteroventral to mandible. Cardo present, but thin, very lightly sclerotized, transparent and easily overlooked. Lacinia primarily membranous, densely haired, apically broadly rounded, approximately 1/2 length of and medial to maxillary palpus ( Fig. 14 View FIGURES 13 – 17. P ). Lacinia with sclerotized region along inner (proximal) basal edge and with a strong ventromedial seta. Maxillary palpus two-segmented; basal segment (palpifer) elongate cylindrical, apical segment (palpus) minute apically tapered ( Fig. 14 View FIGURES 13 – 17. P ). Single small seta located ventrally in membrane between basal and distal segments. Mandible large, stout, heavily sclerotized with one molar tooth posterior to apical tooth. Dorsally, mandible with two setal brushes posterolaterally and one posteromedially ( Fig. 13 View FIGURES 13 – 17. P ). Dorsal epicondyle of mandible lobate, dorsoventrally flattened, apically rounded ( Fig. 13 View FIGURES 13 – 17. P ).
Pupa (n = 5): Habitus ( Figs 3 View FIGURES 1 – 4. 1 , 6 View FIGURES 5 – 8 ). Approximately 12.0– 15.5 mm in length (including prothoracic respiratory organ). Dorsum of head and prothorax dark brown, heavily sclerotized, flattened with strong ridges and spines particularly around edge of flattened area ( Fig. 6 View FIGURES 5 – 8 ). Prothoracic respiratory organ elongate, anterolaterally curved ( Figs 3 View FIGURES 1 – 4. 1 , 6 View FIGURES 5 – 8 ), shoe-horn-shaped; basally circular in cross section with narrow wavy margined furrow becoming gradually broader distally; apex broadly rounded, slightly scoop-shaped. Antennal, wing, and leg sheaths free, but closely appressed. In position of adult stemmatic bulla is pair of setae each on slightly raised tubercle. Remainder of pupa light brown translucent to dark brown. Pair of strong setae in single alveolus just posterior to adult tegula. Position of base of adult halter with pair of strong setae in single alveolus. Additional smaller setae present on dorsum of thorax, scutellum, and abdomen. Posterior margin of tergites 2–4 with numerous spines; spines of tergites 2 and 3 smaller and posteriorly directed, those of tergite 4 larger with median spines anteriorly directed and lateral spines posteriorly directed ( Fig. 6 View FIGURES 5 – 8 ). Posterior margin of tergites 5–6 with only a few small spines. Fleshy remnant of larval posterior siphon arising between tergites 7 and 8; extending at most slightly beyond tip of abdomen. Anterior and posterior margins of sternites 4–6 with numerous posteriorly directed spines. Sternite 7 with posteriorly directed spines primarily on anterior margin.
Egg: (description of eggs based on those dissected from female abdomen and unclear whether form or surface of egg is further modified at time of oviposition). Plump, ovoid, watermelon-shaped, slightly tapered at ends, cream to amber-colored, approximately 1.0 mm long and 0.5 mm wide at middle. Chorion without distinct striations or pattern; surface smooth with grainy appearance.
Diagnosis. Protaxymyia thuja is most similar to P. japonica but is easily distinguished geographically, the two species being separated by the North Pacific. However, the following three character states will also distinguish the two species: medial fusion of gonocoxal apodemes obtuse (broadly U-shaped) in P. thuja versus acute (V-shaped) in P. japonica ; lateral, anteriorly directed extensions of aedeagus apically free in P. t hu j a versus apically fused in P. japonica ; gonocoxal lobes, in lateral view, broadly rounded and without a distinct apex in P. thuja versus narrower, apically rounded, but with a distinct apex in P. japonica . Protaxymyia thuja is also much larger than P. japonica or the eastern North American Axymyia furcata ; the wing is 11.0–14.0 mm long versus less than 8.0 mm in A. furcata ( Wihlm & Courtney 2011) and P. japonica . Characters for separating known life stages of the three Nearctic species, Protaxmyia thuja , Axymyia furcata , and Plesioaxmyia vespertina, are provided in the key given below.
Material examined. HOLOTYPE male (with pupal exuvia on same pin): USA: OREGON: Lane Co., Willamette National Forest, H.J. Andrews Experimental Forest, creek; Rd. 1506 (Lookout Crk. Rd.), 8.9 miles E. 1506/Rd. 15 jct., ex. wet Red Cedar, 44°10’N. 122°15’W., pupa collected 17 Oct. 1999, emerged 22 Nov. 1999, S. Fitzgerald, T. Sohns ( OSAC #0000770501).
PARATYPES (pinned): USA: OREGON: Lane Co., Andrews Experimental Forest, 11 miles NE Blue River, W.S. 10., 8 Nov. 1976, B. Gilmour, 1 ♂ ( OSAC #0000529781); same except, J.D. Lattin, 1 ♂ ( OSAC #0000529780); Benton Co., Mary’s Peak, N Fk Rock Creek jct Rd 2005, pupa ex. wet Red Cedar, 44°31.663’N 123°32.743’W, 8 Nov. 1999, S. Fitzgerald, 2 ♂ with pupal exuviae (1 SFC, 1 CNCI); same except, pupa collected 8 Nov. 1999, emerged 9 Dec. 1999, S. Fitzgerald, 1 ♂ with pupal exuviae ( OSAC #0000770503); Benton Co., Mary’s Peak, N Fk Rock Creek jct. Rd. 2005, pupa ex. wet Red Cedar, 44°31.663’N 123°32.743’W, 15 Nov., S. Fitzgerald, D.M. Wood, 1 ♀ with pupal exuviae ( CNCI); Benton Co., Mary’s Peak, N Fk Rock Creek jct. Rd. 2005, pupa ex. wet wood, 18 Nov 2013, emerged 30 Nov 2013, 44°31.663’N 123°32.743’W, S. Fitzgerald, 1 ♀ with pupal exuviae ( SFC). WASHINGTON: Mount Rainier National Park, EAL#21-2, Westside Road, 0.2 mi. (0.3 km.) NE of Entrance Road, 6.5 mi. (10.44 km.) E Ashford, UTM: 10Z, 584380E, 5177080N, Elev., 2170 ft. (661 m.), 19 Nov. 1995, E.A. Lisowski, 1 ♀ ( CASC).
Additional specimens: In addition to the type specimens listed above the following specimens (all in alcohol) of P. t h uj a were examined: USA: OREGON: Lane Co.: Willamette National Forest, H.J. Andrews Experimental Forest, creek; Rd. 1506 (Lookout Crk. Rd.), 8.9 miles E. 1506/Rd. 15 jct., ex. wet Red Cedar, 44°10’N. 122°15’W., 17 Oct. 1999, S. Fitzgerald, T. Sohns, 8 larvae, 1 pupa ( OSAC); H.J. Andrews Expt. Forest, Shorter Crk., June 1978, T. Dudley, 4 larvae ( OSAC); same except 14 Nov. 1978, lab reared, 1 larva ( OSAC), 1 ♀ with pupal exuviae ( CNCI); H.J. Andrews Exp. Forest, W2O Rip-up, 19 July 1978, 2 larvae ( OSAC); Benton Co., Mary’s Peak, N Fk Rock Creek jct. Rd. 2005, ex. wet Red Cedar, 44°31.663’N 123°32.743’W, 21 Oct. 1999, S. Fitzgerald, 4 larvae, 1 pupa ( SFC); same except 7 Nov. 2000, 2 larvae, 1 pupa ( CNCI). WASHINGTON: Pierce Co.: #504-01, Mt. Baker-Snoqualmie National Forest, Poch Creek at Carbon River Road, 9.3 mi. (15.0 km.) SSE Wilkeson, UTM:10Z, 579480mE, 5203840mN, Elev. 1800 ft. (548m.), 1 Jan. 1996, E.A. Lisowski, 1 ♀ ( CASC).
Etymology. The specific epithet is derived from the Latin Thuja plicata Donn. (Western Red Cedar) for the larval habitat of this species.
Biology. The biology of the eastern Nearctic A. furcata has been nicely documented by Wihlm & Courtney (2011) and Krogstad (1959). While very little has been reported about the biology of P. t hu j a, the aforementioned studies provide a foundation from which one can predict habits and habitats of other axymyiid species and much of the information provided by these authors fits the general pattern of observations made on the larvae, pupae, and adults of P. t hu j a during the course of this study. Nonetheless, some specific observations about P. thuja biology are provided below.
Both Oregon and Washington (Edward Lisowski per. comm.) collection sites are characterized as mature temperate rainforest with highly saturated soils belonging to the forest community type Western Hemlock/Devil’s Club Association which is characterized as mature, high volume stands of Douglas-fir ( Pseudotsuga menziesii (Mirbel)) , Western Red Cedar ( Thuja plicata), and Western Hemlock ( Tsuga heterophylla ) ( Franklin et al. 1988) ( Fig. 1 View FIGURES 1 – 4. 1 ). Larvae of P. thuja are primarily known from decaying, water-permeated logs of Western Red Cedar which are free from bark and moss and are lying (at least in part) in cold, running water of first order streams ( Fig. 1 View FIGURES 1 – 4. 1 ). Although the logs from which larvae have been collected were in a state of decay, Western Red Cedar is very resistant to breakdown and thus the wood is still quite hard and had to be split with a knife or screwdriver. Because Red Cedar is so resistant to rot one could argue that these flies are restricted to very mature (“old growth”) forests where fallen cedar logs have had adequate time to begin decaying. The authors have collected larvae and pupae from Western Red Cedar and possibly Douglas-fir (difficult to determine due to state of decay of log). The species has also been reported from Douglas fir (as “ Protaxymyia sp.”) by Wihlm & Courtney (2011) and Dudley & Anderson (1982) report finding larval exuvia of Axymyiidae in a “soft alder log” in the Oregon Coast Range. Until further collections are made with close attention to tree species and forest type, as well as the possibility of additional unknown species of axymyiids being present, the habitat restrictions of this species are still speculative.
Larval burrows were found in sections of the log which are either continually wet from splashing water or saturated with water because part of the log is submerged; larvae were never found in submerged portions of the log, but typically just above the water line. Burrows meet the surface of the log as a minute hole (original entrance point of the larva) and while some authors have noted piles of frass on “axymyiid logs” around burrow openings, this was not observed in most cases with P. t hu j a probably due to the fact that most of the collecting for this species was done during the rainy season when frass would be continually washed away by rainwater. Larvae are generally situated facing head down with the apex of the respiratory siphon meeting the minute entrance of the cavity at the surface of the log. Several instars are often present in one log at one collection time suggesting a multiple-year life cycle. Pereira et al. (1982) found woody particulate in the gut of P. t h uj a, but the exact larval diet of axymyiids remains unclear. Larvae of A. furcata , however, have regularly been collected in firm wood that has an unusual odor, suggesting the presence of a particular fungus (personal observations of DMW). As with A. furcata ( Wihlm & Courtney 2011) , larval P. thuja were sometimes found in logs with other xylophilic larvae of Syrphidae and Tipulidae . Prior to pupation, larvae turn 180 degrees before chewing their way to the surface of the log, as all pupae were collected with the anterior end facing out. Unlike the situation described for A. furcata by Wood (1981), the exit hole is pre-enlarged rather than left as a paper thin wall which is broken by the anterior spines of the pupa at emergence. The longest time interval between collection of pupae in the field and adult emergence was thirty-two days.
The paper-thin wall over the pupal chamber of A. furcata reported by Wood (1981) was observed in sections of wood that had been taken into the laboratory and kept in dishes of shallow water. These thin walls may be a response to environmental conditions (such as drought or predation pressure) rather than a character state restricted to A. furcata . The fact that thin walls were not observed in the pupal chambers of lab-reared or wild P. t hu j a may indicate a different set of environmental pressures for the winter-emerging P. thuja than in the spring-emerging A. furcata .
Each larva of A. furcata and P. thuja usually occupies its own tunnel, even in the first instar; though Wihlm & Courtney (2011) also note finding more complex galleries in some A. furcata . The published English translation of Mamayev & Krivoshena (1966) states “Retreating deep into the passage, which is often filled with a turbid mass of young larvae, the larva extends its respiratory tubule, the other end of which is always at the outer opening of the passage.” This statement was used by Wood (1981) to contrast the situation in A. furcata in which even the smallest larva each occupies its own tunnel. This statement of “a turbid mass of young larvae” could have been a mistranslation, because Krivosheina (2000) later stated “ Wood (1981) incorrectly cited the work by Mamaev and Krivosheina (1966), describing many small larvae living together in one cavity.”
Adults emerge in November and December and are presumably short lived as the adult mouthparts are vestigial. Although it is unknown if adults of P. t h uj a form a mating swarm, both males and females do fly as both sexes have been taken in a Malaise trap. One female dissected had 120 eggs in the abdomen. At higher elevations, at least one adult specimen has been collected off snow (Edward Lisowski per. comm.).
Discussion. There are two previous studies that have examined different aspects of P. thuja . Pereira et al. (1982) found woody particulate in the larval gut of P. t h uj a, but were unable to elucidate the exact larval diet. Fitzgerald (2004) included P. t h uj a in a phylogenetic analysis including numerous Bibionomorpha; Axymyiidae was unsurprisingly supported as a monophyletic group with the two axymyiid exemplars, P. t hu j a and A. furcata , forming a clade supported by numerous adult and larval characters though this clade was not supported as part of, or as the sister group to, Bibionomorpha. Additionally, Fitzgerald (2004) discussed the morphology of this species in the context of other Bibionomorpha and provided illustrations of some structures such as the male sperm pump which have not been reproduced here.
Distribution. Protaxymyia thuja is currently known from the Coast Range of Oregon and the Cascade Mountains of Oregon and Washington. Additionally, larvae of presumably the same species have been collected in the Siskiyou mountains of southwestern Oregon and burrows characteristic of axymyiid larvae have also been observed in Thuja plicata along the north fork of the Skokomish River in the coastal Olympic Mountains of Olympic National Park, Washington (Fitzgerald, pers. obs.).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.