Lamprolonchaea brouniana ( Bezzi 1919 )
|
publication ID |
https://doi.org/ 10.5281/zenodo.199123 |
|
DOI |
https://doi.org/10.5281/zenodo.5698279 |
|
persistent identifier |
https://treatment.plazi.org/id/03C6F713-FFB9-0A4D-FF71-FF4FFDD3FC97 |
|
treatment provided by |
Plazi |
|
scientific name |
Lamprolonchaea brouniana ( Bezzi 1919 ) |
| status |
|
Lamprolonchaea brouniana ( Bezzi 1919) View in CoL
Lonchaea splendida Broun 1904: 307 View in CoL . (Homonymous with Lonchaea splendida Loew 1873 View in CoL ) Lonchaea brouniana Bezzi, 1919: 246 View in CoL .
Lamprolonchaea rugosifrons Bezzi, 1923: 183 View in CoL . (Synonymised by Pitkin, 1996: 476)
Material examined. Type specimens: ɗ, AUSTRALIA; Sydney, “ K48703 View Materials ”, 26 Dec 1920, Lamprolonchaea rugosifrons n.sp. mf “, “TYPE’, specimen on minuten pin, here designated lectotype (AMS). Ψ, Sydney 26 Dec 1920, SPHTM Coll, here designated paralectotype (AMS). Ψ, Sydney, 30.x.1921, Health Dept, “ Lonchaea rugosifrons Bezzi , here designated paralectotype (MVM).
Type (not previously designated) location Sydney, Broun (1904). Broun (1905) is a duplicate species description that includes a photographic plate; Broun (1905) notes that while being sent away for the preparation of this plate his specimens were damaged in transit. Bezzi (1919) was a nomenclatural note (see below) that did not add any associated type material for L. brouniana .
Other (adult) specimens: AUSTRALIA; New South Wales: Ψ, Bilpin nr Kurrajong, 27 Oct 1980, NW Rodd, in AM; ɗ, Wahroonga, Sydney, 20 Nov 1926, in AM; ɗ, Yass, 14 Dec 1931, in AM; ɗ, Northmead, 26.Jan.1963, DK McAlpine, in AM; ɗ, same loc., 4 Feb 1963, N. Gregg, in AM; ɗ, Ψ,Bronte, near Sydney, 13 Nov 1960, DK McAlpine, in AM; Ψ, Katoomba, 13.Nov 1958, “ Lamprolonchaea brouniana Bezzi det. by JF McAlpine (AMS). 2Ψ, Murray R 80 km W of Wentworth, 22.xi.1967, A. Neboiss (MVM). Ψ, Brewarrina WWF, “ Lamprolonchaea brouniana JF McAlpine 1960, in MV. 3ɗ, 3Ψ, NSW, Feb.1966, bred from tomato ( VAIC).
Northern Territory: 3ɗ,Ψ, Darwin, 22.ii.1984, S. Collins, ex peaches from Pickering Brook WA; 2ɗ, 4Ψ, Anula Supermarket, 11.ii. 1981, J. Freeman, ex nectarines; ɗ, 3Ψ, Curtain Springs Roadhouse, 1.vi.1993, M. Barton, reared from Solanum melongena ; 13ɗ, 4Ψ, Arid Gold Farm, Ti Tree, 29.i.2001, collected from CUE trap during an outbreak and eradication; ɗ, Tennant Creek, 20.ii.1997, Mrs Hopf, ex Citrus limon ; ɗ, Alice Springs, 4.vii.1977, J. Bobb, bred from orange; 5ɗ, 9Ψ, Alice Springs, 5.xii.1978, F. McEllister, ex tomato; 4ɗ, 15 km N of Alice Springs, 18.iv.1982, emerged 24.v.1982, ex Solanum sp. ( NTDR).
Queensland: 2ɗ, Dalby, 20 Feb 1935, bred from tomatoes, “ Lamprolonchaea brouniana Bezzi det. by JF McAlpine 1960. 2ɗ, 2Ψ, Esdivold, “24”, Bancroft, bred from tomatoes, SPHTM Coll (AMS).
Victoria: ɗ, 2Ψ, Warburton, F.E. Wilson, 13 Jan 1924; SPHTM Coll (AMS). ɗ, 3Ψ, Preston; ɗ, Eltham, 8.xii.1918, CE Cole; Ψ, Croydon, 13.ii.08; ɗ, Melbourne, 15.i.28, GF Hill; 3Ψ, Nunawading, 9.i.1968, Neboiss, “ Lamprolonchaea sp det. DK McAlpine 1979” ɗ, Mt Albert, 15.iii.47, R.T.; 3ɗ, 5Ψ, 1 puparium, Windsor, out of tomato, T.K., 02.08; ɗ, Ψ, Melton, 27.i.1957, A. Neboiss; ɗ, Kerang, 11.v.1946, RE Trebilock, ɗ, same except, 26.v.1946, 3Ψ, same except, 24.xi.1946; ɗ, 1(headless), Ardmona, 1.xi.1928, GF Hill, “ Lamprolonchaea brouniana det. J.F. McAlpine 1960 ”; Ψ, Grampians, 12.94; ɗ, Ψ, Little Desert, 12.xi.1958, FE Wilson; Ψ, 17 km SE Merrijig Howqua River, 1.xii.1971, Neboiss; ɗ,Ψ, 20 km NNE Horsham, 29.x.1982, KL Walker, on Eucalyptus; 2Ψ, 40 km NW Donald, 29.x.1982, KLWalker, on Eucalyptus; ɗ, Ψ, 38 km N Birchip, 29.x.1982, KLWalker, on Eucalytpus; ɗ, Ψ, 46 km N Ouyen, 30.x.1982, KLWalker; 2Ψ, Cape Otway, 29.xi.1966, A. Neboiss; 2Ψ, Glenelg R, 6 km NNE Nelson, 25.xi.1966, A. Neboiss (MVM).3 Ψ, Victoria, oranges, reared Dunedin, “X no. 6”, 20.i.1922; ɗ, same locality, reared Wellington, 22.i.1922; 2ɗ, same locality, reared Dunedin, i.1922, one labelled “ Lonchaea splendida ; all Coll. Miller ( NZAC). Ψ, Tatura, 14 15.iii.1991, on Japanese millet, M.Malipatil and K.L. Dunn; ɗ, 1 missing head, Kyabram, in tomatoes, emerged 10.iv.1907; 2ɗ, 2Ψ, 4 pupae, Ararat, H.W.Davey; ɗ, 2 pupae, Geelong, H.W.Davey; 2ɗ, 4Ψ, Port Melbourne, Feb.1950, V.Sloane; 2Ψ, Werribee, xi.1940, HA. Barnham; 3ɗ, 3Ψ, several larvae, several pupae, Yarrawonga, 13.ii.2009, S. Lewis, ex domestic tomatoes; ɗ, 6Ψ, 7 pupae, several larvae, Thornbury, 20.ii.2002, C.Pollard, ex tomato fruit; Ψ, 1pupa, Cobram, Dec 1997, ex Citrus sinensis fruit; ɗ, Cobram East, 23.i.2001, M.Malipatil, on peach leaves; ɗ, Knoxfield, Dec.2002, B.Henderson, in glasshouse; Ψ, also larvae and puparia, Barham, 18 Dec 2010, A. Anderson, ex avocado fruit reared 4 Jan 2010; 3ɗ, also larvae and puparia, Flemington, 6 Jan 2010, D. Mansell, ex tomato fruit reared 22 Jan 2010; ɗ, also larvae, Echuca, 12 Mar 2004, K. Ockerby, ex eggplant fruit reared; 5ɗ, 1 Ψ, also larvae and puparia, Heywood, 15 Jan 2010, W. Stevens, ex tomato fruit reared 31 Jan 2010; 2Ψ, also larvae and puparia, Ascot Vale, 8 Jan 2010, N. Gerad, ex tomato fruit reared 30 Jan 2010; 2ɗ, Ψ, also larvae and puparia, Sale, 18 Jan 2010, J. McBay, ex tomato fruit reared 9 Feb 2010 ( VAIC).
Western Australia: ɗ,Ψ, Bunbury, 17.ii.1954, A.Neboiss (MVM). 2ɗ, Bunbury, 1 20 Oct 1955, A. Snell; ɗ, Cape district, 28 km S of Bunbury, 7 Jan 1957, A. Snell; ɗ, 18 km E of Wicherina, 25.ix.1964, G.L. Bush; Ψ, 18 km W of Eucla, 13.ix.1964, G.L. Bush; all “ Lamprolonchaea brouniana Bezzi det. by JF McAlpine (AMS). ɗ, Ψ, Fremantle, 11.i.1954, KR Norris, “ Lamprolonchaea brouniana det. JF McAlpine 1960 ”; Ψ, Capel, 7.i.1957, Snell; ɗ, Beelerup, 25.ii.1958, Snell (MVM). 4ɗ, 2Ψ, Carnarvon, -. x.1955, ex capsicums; 2ɗ, Dalkeith, Perth, ex tomato fruit reared 16.ii.1989, J. Bradshaw; 2Ψ, Geraldton, 14 Oct 1947, F. Ryan; 2ɗ, 2Ψ, Kununurra, ex overripe rockmelon collected maggots 7.vi.1990, flies emerged 19.vi.1990, G.R. Strickland; 30ɗ, 4Ψ, Lake Bryde, 16.xii.1974, K.T. Richards; 2m 7Ψ, Middle Swan, 15.vi.1972, ex Sodam Apple, D.L. Hardey; 2ɗ 2Ψ, Narrogin, March 1979, ex Tomatoes; Ψ, Nedlands, 15.4.46 FE, reared in lab from Apple of Sodom; Ψ, North Gingin, 17.ix.1969, K.T. Richards; ɗ, Perth, bred in insectory from Walnuts, 22-4-55, E. Elkington; 4ɗ, 4Ψ, Swan River, March, L.J. Newman; ɗ, Wanneroo, May 1955, bred from tomatoes in lab, Mrs Edwards; 4ɗ 4Ψ, Wanneroo, ex cowdung, 15.iii.1997, D.F. Cook, “ Lamprolonchaea brouniana Bezzi det. D.K. MacAlpine”( DAFWA).
Diagnosis. Adult fly moderately large (relative to other Lamprolonchaea ), approximately 3.5–4.5 mm body length, bright metallic golden-green in colour, with black head, a shiny-black frons entirely covered with large irregular coarse pits (rugose), dusky grey antennal postpedicellus, white calypters, clear iridescent wings, legs with femora ventrally fringed with long black setae and yellowish basal tarsal segments
Description. Adults ( Figs. 1–19 View FIGURES 1 – 6. L View FIGURES 7 – 9. L View FIGURES 10 – 16. L View FIGURES 17 – 19. L )
Males. General body colour bright metallic golden-green except head which is black ( Figs. 1, 3, 5 View FIGURES 1 – 6. L ).
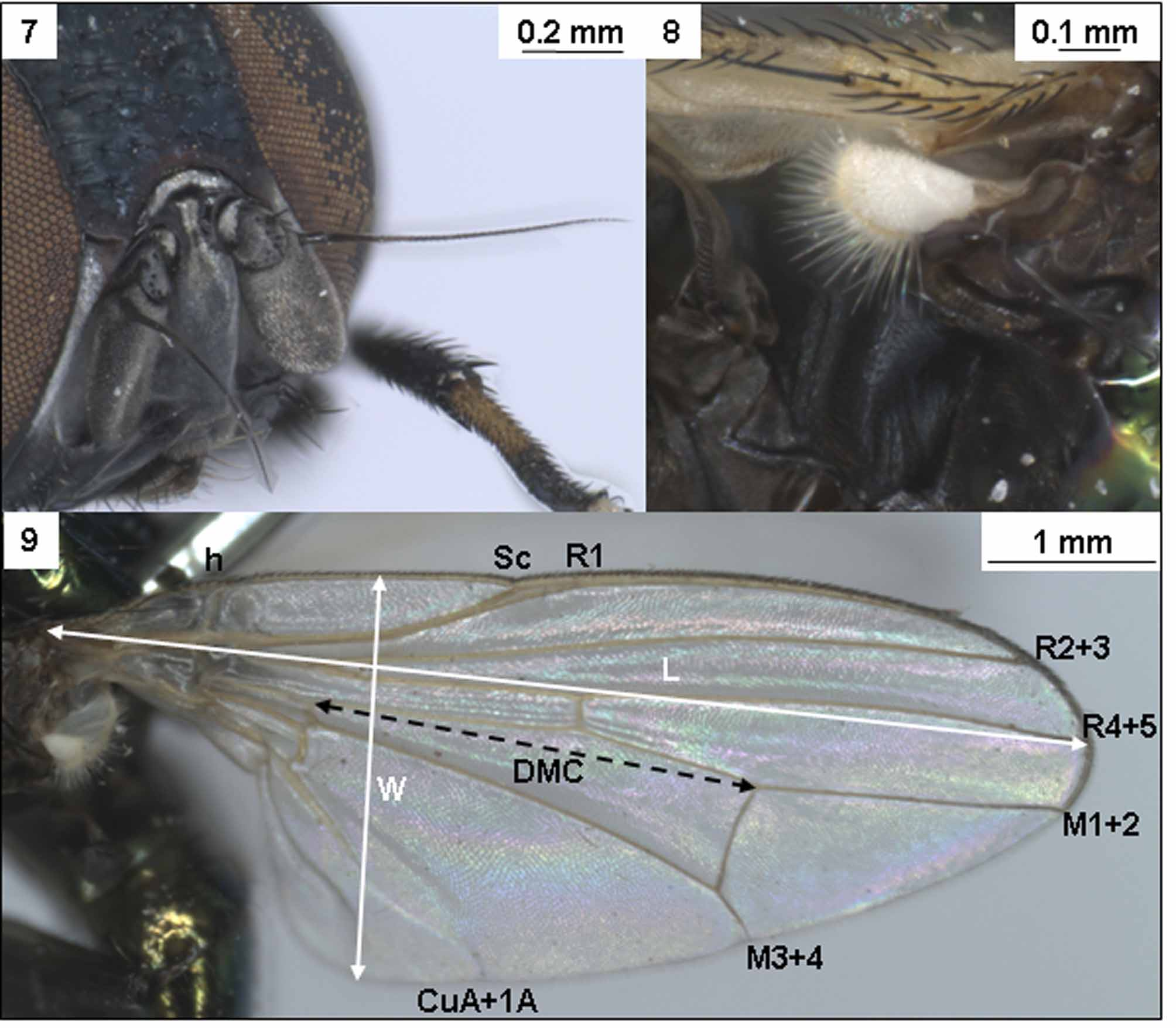
Head: Eyes chocolate-brown coloured, large rounded externally and oval in shape ( Fig. 3, 5 View FIGURES 1 – 6. L ). In live specimens the eyes are reddish-brown. Frons narrow, widening at top ( Fig. 5 View FIGURES 1 – 6. L ), lower frons width 0.5 of one eye, upper frons width 0.6 of one eye, shiny black with greenish or bluish reflections, covered with large irregular and indistinct pits on almost entire surface giving a rugose appearance. Ocelli small, forming three points of a triangle. Strong setae present along margin of genal plate, fine subequal setula on frons up to orbital setae. Head setae previously illustrated in Colless & McAlpine (1991); outer vertical setae 2/3 length of inner vertical setae, ocellar setae proclinate, subequal to inner vertical setae, orbital setae subequal to outer vertical setae. Postocular setae ½ (inner) to ¼ (outer) length of outer vertical setae. Lunule bare, black or reddish-brown and silver dusted, as is face and parafacials. The antennal postpedicellus dusky grey and three times as long as wide, arista often pale at base black at tip, entirely bare. Short robust oral setulae along subgenal margin. ( Figs. 1, 3, 5 View FIGURES 1 – 6. L ).
Thorax: Thorax dorsally only slightly longer than broad, narrowed at rear with a strong groove between the mesonotum and scutellum, entirely shining, and devoid of dust ( Figs. 1, 3 View FIGURES 1 – 6. L ). Scutellum, on margin, with 2, one apical and one subbasal, pairs of long setae (lateral setular) and 1–4 short lateral scutellar setulae (often broken off). Thoracic sclerites shining metallic-green with no dust, and numerous fine setula and long robust setae: single stigmatal seta, two posterior and two or more anterodorsal mesoplural setae, single humeral seta and single anterior and posterior notoplural seta, four strong postsupra-alar setae.
Legs black, basal tarsomeres yellow except at apices, other tarsomeres black and cordiform, femora ventrally fringed with long, dense black setae. Pretarsus and claws black, ariolum white with paired lobes that are often extended ( Figs. 3, 5 View FIGURES 1 – 6. L ).
Wings ~ 3.5 mm long, clear and iridescent, with a distinct whitish tint, anterior margin of costa covered with dense dark spinules which gradually become shorter and thinner from base to apex of wing, all veins pale yellowish. The pattern of veins was previously illustrated in Colless & McAlpine (1991). Calypters whitish and white fringed, halteres black, transversely knobbed at the tips, their stalks rather long and slender ( Figs. 3 View FIGURES 1 – 6. L , 8 View FIGURES 7 – 9. L ).
Abdomen: Abdominal tergites glistening and coloured like mesonotum, with golden and coppery reflections, 5 visible tergites, with strong closely placed, almost divided into two tufts, black setulae at end of tergite 5, the latter about as long as combined length of tergites 3 and 4 ( Fig. 3 View FIGURES 1 – 6. L ).
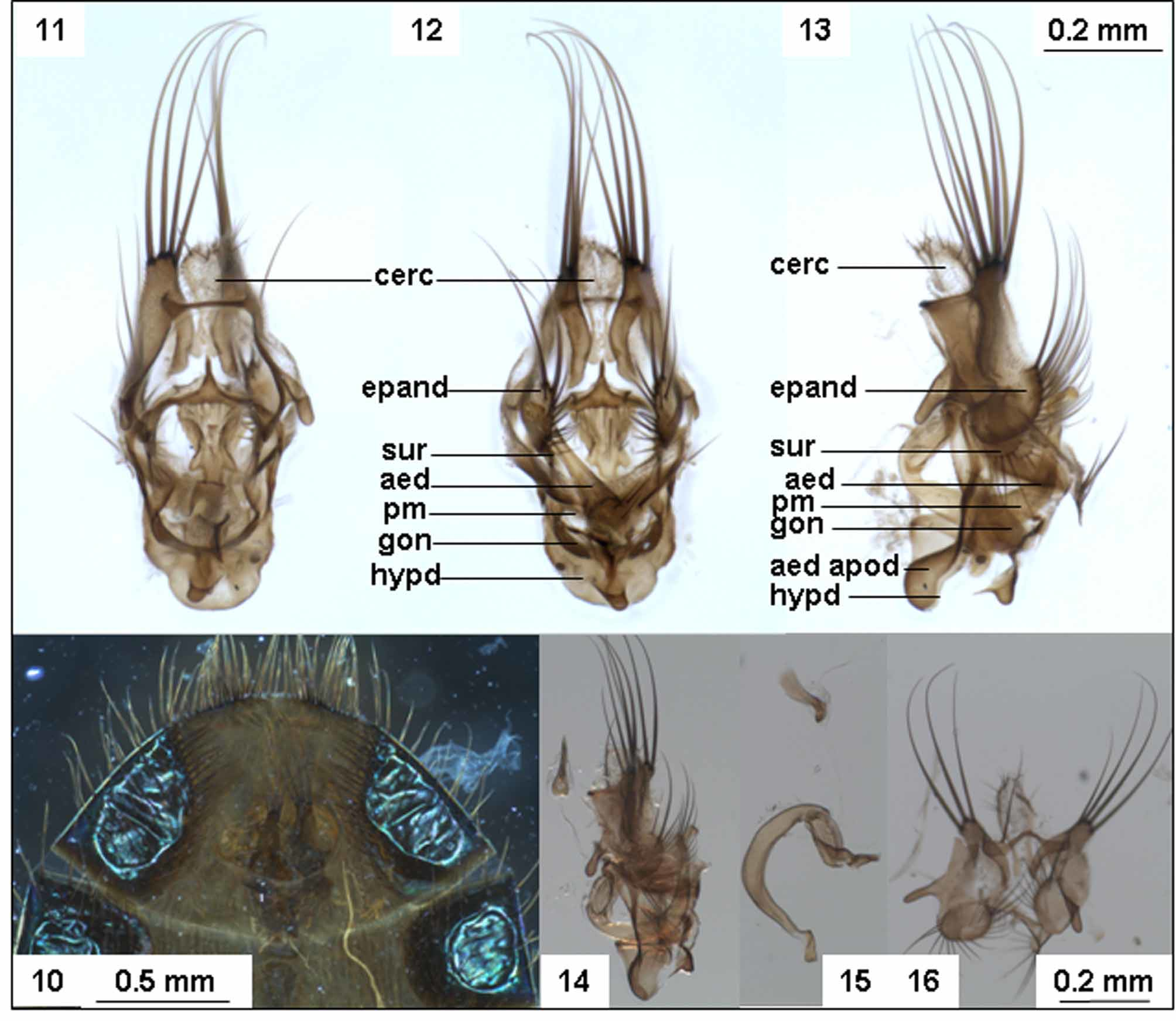
Terminalia: Short, projecting forward internally to about half length of abdomen, tips of setae on epandrium extending posteriorly to tip of abdomen ( Fig. 10 View FIGURES 10 – 16. L ). In dissected terminalia ( Fig. 11–16 View FIGURES 10 – 16. L ), cerci soft and flaplike, covered with fine setulae and spinules particularly on apical margin; epandrium large, posteriorly produced to a semi-circular, lobe with several strong setulae on posterior margin, apically produced to a small lobe with rounded margin and armed with 3 or 4 strong setae 1 ½ x longer than epandrium itself, and 2/3 length of remainder of terminalia, antero-laterally produced to a narrow pointed process. Surstyli smaller than epandrium, semi-circular and bearing a marginal fringe of strong setae. Phallus short and simple, in lateral view C-shaped with a characteristically angled and slightly thickened apex ( Fig. 15 View FIGURES 10 – 16. L ). Ejaculatory apodeme narrow platelike, slightly broadened towards apex ( Figs. 14–15 View FIGURES 10 – 16. L )
Females. Generally as in male ( Figs. 2, 4, 6 View FIGURES 1 – 6. L ) except: slightly shorter body, abdomen, eye, and scutellar setae ( Table 1 View TABLE 1 ); frons much wider and more parallel sided ( Fig. 6 View FIGURES 1 – 6. L ), lower frons width 0.8 of one eye, upper frons width 0.8; of one eye; femoral fringe slightly less prominent and the tarsomeres are paler in colour, with both the basal plus the second tarsomere often yellow; abdomen with 6 visible tergites, sixth tergite about as long as fifth ( Fig. 4 View FIGURES 1 – 6. L ), with no paired tuft of setulae at apex.
Aculeus relatively short (~1.0 mm), broad (~ 0.2 mm), and black, shaft slender ( Figs. 17–19 View FIGURES 17 – 19. L ); basal segment has one row of submarginal sparse short setulae on both dorsal and ventral surfaces, central membrane spiculose in middle 1/3 area (as in Fig. 52 View FIGURES 52 of McAlpine & Steyskal 1982), gradually widening slightly from base to apex; apical segment ends in bluntly rounded apex, about 1 ½ as long as broad, subbasal dorsal setae (one pair) about ½ as long as apical segment, and as long as apical pair, two longest preapical ventral setulae about as long as the segment, Note, apical setulae number variable and often rubbed off ( Figs. 17–19 View FIGURES 17 – 19. L ).
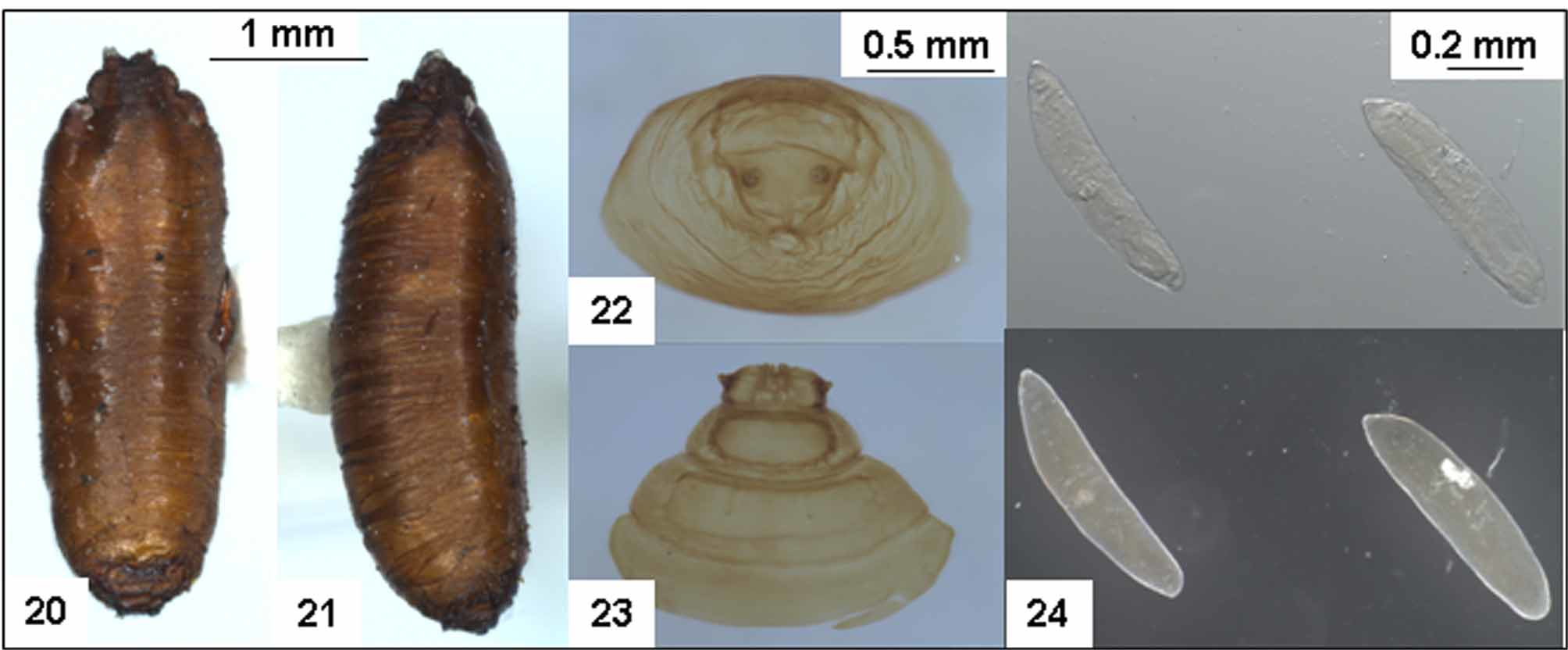
Pupae ( Figs. 20–23 View FIGURES 20 – 24. L )
Material examined: AUSTRALIA; Victoria: Ararat, 4 specimens; Ascot Vale, 2 specimens; Cobram, 1 specimen; Flemington, 3 specimens; Geelong, 2 specimens; Heywood, 6 specimens; Knoxfield, 1 specimen; Sale, 3 specimens; Thornbury, 8 specimens; Yarrawonga, 15 specimens ( VAIC).
Approximately 4 mm long and 1 mm wide ( Fig. 20–21 View FIGURES 20 – 24. L ) barrel-shaped, with former larval segments marked by slight indentations, surface covered in transverse wrinkles, anterior end slightly compressed dorsolaterally, with a short lateral ridge on either side, and anterior spiracles represented by two small knobs on either side of “head” area ( Fig. 23 View FIGURES 20 – 24. L ). The posterior spiracles represented by a pair of knobs, each with 3 narrow slits similar to in larvae ( Fig. 22 View FIGURES 20 – 24. L ). In the laboratory at Knoxfield DPI, pupal stadium lasted for 11 to 14 days at 25o C (mean =12 days, n =5 clutches, total 17 pupae).
Larvae ( Figs. 25 View FIGURES 25 – 32. L –44)
Material examined: AUSTRALIA; New South Wales: Avocado: Barham, 18 Dec 2009; Capsicum: Barooga , 17 Mar 2008; Tocumwal, 6 Mar 2007; Citrus: Tocumwal , 17 Feb 2009; 27 Nov 2008; 1 Dec 2008; 1 Nov 2008; 2 Dec 2008; Stone Fruit: Tocumwal, 17 Feb 2009; 4 Feb 2009; 3 Feb 2009; 28 Feb 2007; Tomato: 32 km from Swan Hill, 18 Jan 2006; Barooga, 10 Apr 2008; Tocumwal, 2 Feb 2009; 14 Feb 2008; 18 Dec 2002; 14 Mar 2007; unknown host: Rock Valley, 28 Dec 1950.
Victoria: Apricot: Wangaratta, 20 Jan 2009; Avocado: Nichols Point, 10 Feb 2005; Capsicum: Chiltern , 30 Mar 2007; Rutherglen, 20 Feb 2007; Wahgunyah, 28 Mar 2007; Citrus: Moonee Ponds , 10 Nov 2008; Eggplant: Ascot Vale, 8 Apr 2008; Echuca, 12 Mar 2004; Eldorado, 28 Apr 2008; Myrrhee, 17 Mar 2008; FIGURES 33–44. L. brouniana larvae, Porepunkah, Vic, 36–44) after clearing in KOH – 33) 1st, 2nd and 3rd instars; 34) 1st instar posterior, lateral view; 35) 2nd instar posterior, lateral view; 36) 1st instar anterior, lateral view; 37) 1st instar, lateral view; 38) 1st instar posterior, lateral view; 39) 2nd instar anterior, lateral view; 40) 2nd instar, lateral view; 41) 2nd instar posterior, lateral view; 42) 3rd instar anterior, lateral view; 43) 3rd instar, lateral view; 44) 3rd instar posterior, lateral view.
Rutherglen, 29 Mar 2007; Waaia, 4 May 2005; Grapefruit: Euroa, 15 Apr 2008; Rutherglen, 18 Dec 2007; Yarrawonga, 13 Jan 2009; Lemon: Moonee Ponds, 18 Nov 2008; Wangaratta, 21 Jan 2009; Mandarin: Springhurst, 3 Dec 2008; Nectarine: Wangaratta, 11 Feb 2009; Orange: Boorhaman, 3 Dec 2008; Wangaratta, 17 Feb 2008; Peach: Eldorado, 18 Jan 2006; Kyneton, Dec 1999; Mildura, 31 Mar 2009; Narre Warren, 15 Jan 2001; Wangaratta, 8 Feb 2008; Warracknabeal, 22 Feb 2008; Peach or Tomato: Bendigo, 5 Jan 2001; Stone Fruit: Baddaginnie, 31 Mar 2008; Benalla, Feb 2002; Shepparton, 2 Mar 2006; Waaia, 4 May 2005; Tomato: Airport West, 20 Mar 2008; Ascot Vale, 16 Apr 2008; 5 Mar 2009; Barwidgee, 4 Feb 2009; Beechworth, 2 Mar 2009; 27 Feb 2009; 2 Apr 2008; Benalla, 2 Dec 2008; 19 Feb 2007; 3 Apr 2007; 4 Apr 2007; 4 Apr 2007; 4 Apr 2007; 5 Apr 2007; Bendigo, 9 Feb 2009; Boho, 11 Mar 2009; Bunbartha, 1 Apr 2004; Bundalong, 20 Mar 2009; Castlemaine, 30 Jan 2001; Chiltern, 30 Mar 2007; 30 Mar 2007; Dereel, 3 Mar 2001; Devenish, 20 Mar 2008; Echuca, 21 Jan 2009; 14 Feb 2005; Eltham, 28 Mar 2008; 31 Jan 2000; 12 Feb 1999; Essendon, 27 Mar 2008; Euroa, 11 Apr 2008; 10 Apr 2008; 15 Apr 2008; Flemington, 9 Feb 2009; Footscray, 11 Mar 2008; Gapsted, 21 Feb 1940; Glenrowan, 10 Feb 2009; Kensington, 16 Feb 2008; Kerang, Jan 1999; Koonoomoo, 16 Feb 2007; Kotupna, 13 Feb 2006; Lower Templestowe, 21 Feb 2008; Lurg Upper Greta, 6 Mar 2009; Maffra, 3 Feb 2004; Melbourne, Jan 2001; Mildura, 30 Nov 1999; Moama, 8 Mar 2009; Moonee Ponds, 25 Mar 2008; Moorabbin, 22 Jan 2001; Myrtleford, 14 Mar 2008; Narre Warren, 5 Feb 2009; Oxley, 18 Apr 2008; Oxley, 21 Apr 2008; Parkville, 25 Jan 2001; Paynesville, 6 Feb 2008; Pyramid Hill, 21 Jan 2005; Raywood, 7 Mar 2006; Rushworth, 20 Mar 2008; Rutherglen, 20 Feb 2007; 29 Mar 2007; 5 Apr 2007; 29 Mar 2007; 29 Mar 2007; 20 Feb 2007; 29 Mar 2007; 27 Mar 2007; 18 Feb 2005; Sale, 2 Feb 2005; Scoresby, 2 Feb 2005; Shepparton, Jan 2000; South Yarra, 5 Feb 2008; Springhurst, 1 May 2008; Swan Hill, Mar 2002; Tatura, Jan 2001; 28 Mar 2006; 30 Mar 2006; Thornbury, 20 Feb 2002; Toolamba, 7 Jan 2004; Wahgunyah, 27 Mar 2007; 27 Mar 2007; Wandin East, 2 Mar 2004; Wangandary, 13 Jan 2009; Wangaratta, 23 Jun 2004; 13 Apr 2007; 12 Apr 2007; 12 Apr 2007; 12 Apr 2007; 19 Apr 2007; 18 Feb 2008; 18 Feb 2008; 18 Feb 2008; 22 Jan 2009; 29 Feb 2009; 21 Dec 2009; 20 Jan 2009; Yackandandah, 17 Mar 2008; Yarrawonga, 13 Feb 2009; 17 Feb 2009; Jan 2009; 20 Feb 2009; Vegetable: Plenty, 29 Jan 2003. Unkown host: Bayswater , 12 Feb 2009; Kerang, 21 Jan 2004; Kyabram, 10 Apr 1907; ( VAIC).
Third instar. Body: Body strongly tapering anteriorly, average length 7.1 mm ± 0.3 (SD), average width 1.0 mm ± 0.2 (SD) ( Figs. 25 View FIGURES 25 – 32. L –33, 43). Fresh specimens mostly white or creamy white in colour, except posterior spiracles and mouth hooks and associated internal structures contrastingly dark; often darken once preserved in ethanol.
Head: Cephlopharangeal skeleton as in Fig. 27 View FIGURES 25 – 32. L , with a pair of symmetrical stout sickle shaped mouthhooks without preapical teeth, but with a pair of distinct dental sclerites, labial and hypopharyngeal sclerites present, parastomal bars very narrow, posterolateral apodeme generally large, dorsal arch and cornu well developed and sclerotized, ventral cornu weakly sclerotized, fine ventral pharyngeal ridges present along lower margin of ventral cornu ( Figs. 27 View FIGURES 25 – 32. L , 42).
Thoracic and abdominal segments: Ventral locomotory welts of spines indistinct on thoracic segments, but distinctly present on abdominal segments, welts approximately 1/3 width of the body (laterally) becoming slightly more conspicuous from anterior to posterior segments ( Figs. 25 View FIGURES 25 – 32. L , 43). Each abdominal “proleg” elliptical in outline with gradually rounded ends, margined with a continuous row of very fine spicules ( Figs. 32 View FIGURES 25 – 32. L , 44). Each abdominal proleg, particularly the posterior ones, with five transverse parallel rows of locomotory spicules ( Figs. 32 View FIGURES 25 – 32. L , 44)—the most anterior row with relatively large spicules forming a broken line, followed by two continuous lines of fine spicules, the fourth line containing the largest spicules arranged in raised linear-clumps of 3–6 broad teeth, followed by a shorter fifth continuous row of fine spicules. Anterior spiracles: hand-like, with five to seven lobular papillae projecting from a stalk ( Figs. 25–26, 28 View FIGURES 25 – 32. L ). Posterior spiracles: mounted above centre of the depressed posterior spiracular disk ( Figs. 25, 30 View FIGURES 25 – 32. L ), characteristically projected on very short tubular horn-like projections ( Figs. 30–31 View FIGURES 25 – 32. L ), in lateral view appears as a narrow pigmented band, sometimes with a clear layer above ( Fig. 30 View FIGURES 25 – 32. L ), with three spiracle-slits roughly at right-angles to each other, in older specimens there are often sclerotized tubercules present laterally adjacent to the posterior spiracles ( Fig. 30 View FIGURES 25 – 32. L ). Anal area: spines present around anal plate, with teeth on posterior of the last locomotory welt and behind anal lobes more strongly developed ( Figs. 30, 32 View FIGURES 25 – 32. L , 44). When extended anal lobese can be raised from the body ( Fig. 30 View FIGURES 25 – 32. L ). Anal lobes large and heart-shaped ( Fig. 32 View FIGURES 25 – 32. L ).
Second instar. Average body length 5.5 mm ± 0.4 (SD), width 0.8 mm ± 0.1 (SD). Generally as in third instar, except: ventral locomotory welts indistinct; anal lobes less developed; anterior spiracle with longer lobular papillae; posterior spiracles higher on posterior with the pigmentation around them reduced or entirely absent, spiracular slits more conspicuous, and horn projections reduced (Figs. 33, 35, 39–41).
First instar. Average body length 3.9 mm ± 0.4 (SD), width 0.5 mm ± 0.2 (SD). Generally as in second instar, posterior horn projections reduced further and posterior spiracles higher on posterior (Figs. 33–34, 36– 38).
Eggs ( Fig. 24 View FIGURES 20 – 24. L ). Generally, Ferrar (1987) reports that lance flies have typical muscine eggs, white in colour with the anterior end being relatively sharply pointed. Eggs dissected from the abdomen of a specimen collected from Bunbury WA (from the MVM collection) agree with this description, and were approximately 0.75 x 0.15 mm in size ( Fig. 24 View FIGURES 20 – 24. L ).
Comments. Synonymies of L. brouniana . Broun (1904) originally described this species of lance fly from NSW material as Lonchaea splendida . However, this name was a homonym of Lonchaea splendida Loew [now regarded as a junior synonym of the cosmopolitan Lamprolonchaea smaragdi (Walker) ]. Hence, Bezzi (1919) proposed the new name Lonchaea brouniana (in honour of Broun) as a replacement name. Subsequently, Bezzi (1923) described L. rugosifrons as a new species, noting that L. brouniana was likely to be synonymous with L. aurea Macquart , and followed by Malloch (1928) who did not include L. brouniana in his key to Australian species. However, Bezzi (1923) erroneously noted that L. brouniana differed from L. rugosifrons in not (1) possessing a pitted frons and (2) being much smaller in size. In Broun (1904, 1905) the body length of L. brouniana is given as 1 ¾ lines (= 3.7 mm) and the frons is noted to be “rugosely punctured”, both of which could equally apply to L. rugosifrons . Pitkin (1996) formally synonymised L. rugosifrons Bezzi with L. brouniana (Bezzi) , which is supported by evidence from our study (see below).
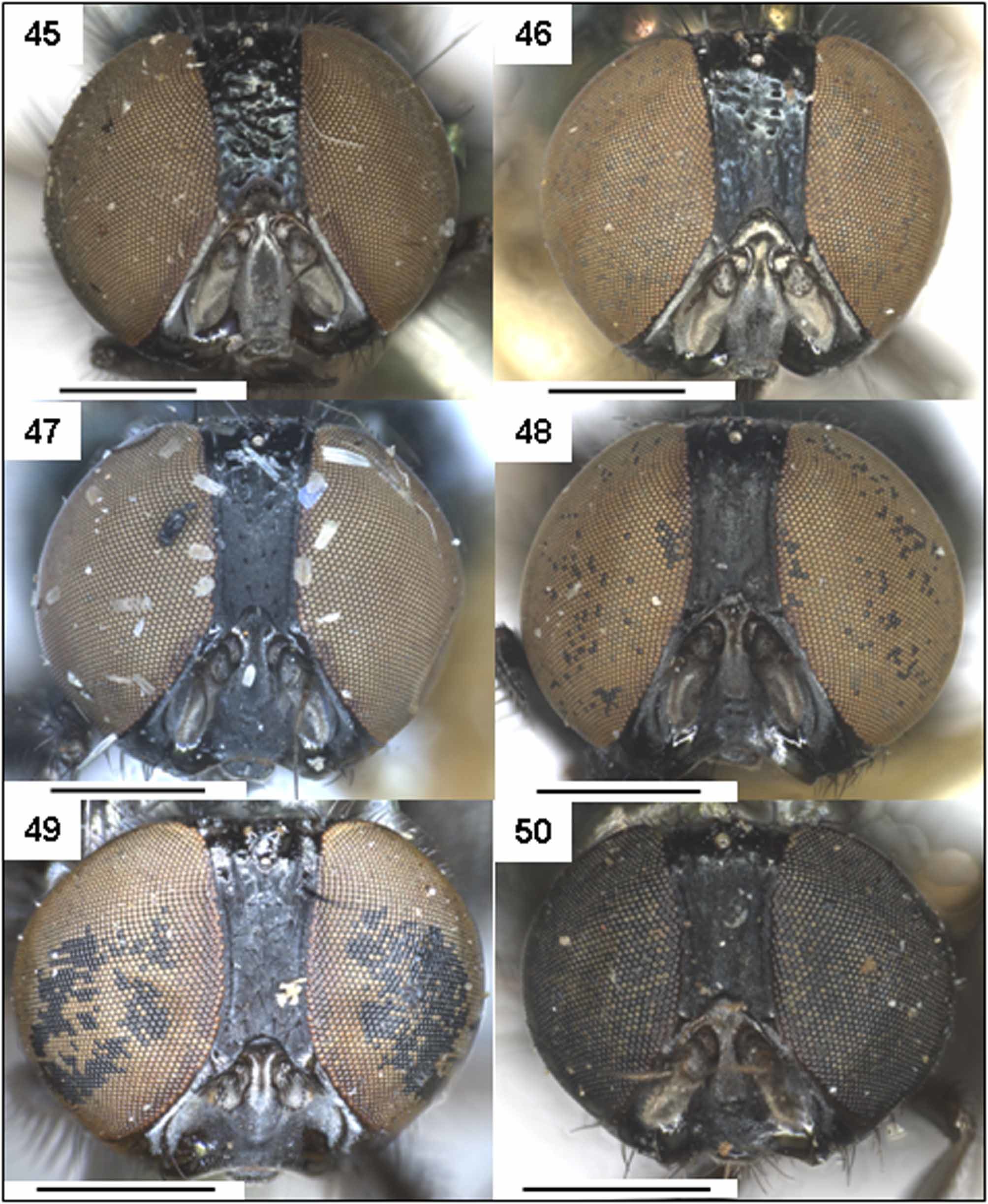
Morphological variation within L. brouniana adults. All adult specimens examined here exhibited pitting of the frons, however there was variation in the degree of pitting and colour of specimens from different localities which may represent currently unrecognised taxonomic diversity: (1) The two female specimens from Glenelg River Victoria are slightly coppery in body colour. In one female specimen from Glenelg River basal segment of tarsi except apices are less conspicuously yellow; (2) The two male adult specimens from Northmead (NSW) exhibit variation in punctation of frons – one specimen has frons evenly pitted and rugose, the other has frons with only a few widely spaced distinct punctures below ocelli ( Fig. 46 View FIGURES 45 – 50 ); (3) Two specimens from Bunbury were particularly small (e.g. body length of male = 3.4 mm, female =3.0 mm), outside the size range of other specimens examined ( Table 1 View TABLE 1 ), the female exhibited slight variation in the morphology of the ovipositor, being stockier and more parallel sided at the tip ( Fig. 19 View FIGURES 17 – 19. L ); (4) Also, a number of specimens from the Pilbara and Kimberley regions of WA examined in the current study had paler tarsi and weakly pitted frons, further work is required to clarify their taxonomic status, hence these specimens have not been included in the above description or on the distribution map above; (5) A series of specimens collected from Wanneroo WA that were raised from larvae exhibited blue rather than green body colour, otherwise morphologically they match L. brouniana (including male and female genitalia); and (6) Among the specimens examined there were minor variations in number of setae and colour of calypters, wings, antennae and the lunules.
Molecular variation. The six DNA sequences obtained from the (COI) DNA barcoding region (with the primer sequences removed) were 658 b.p. in length, with 1–3 single base differences (0.2–0.5 %) in pairwise sequence comparisons. The predicted amino acid sequence contained no substitutions (0% variation) and no putative stop codons. These sequences have been submitted to GenBank ( HQ 261232 View Materials – HQ 261237 View Materials ) and the Bar Code of Life (BOLD Project: AAPL) databases.
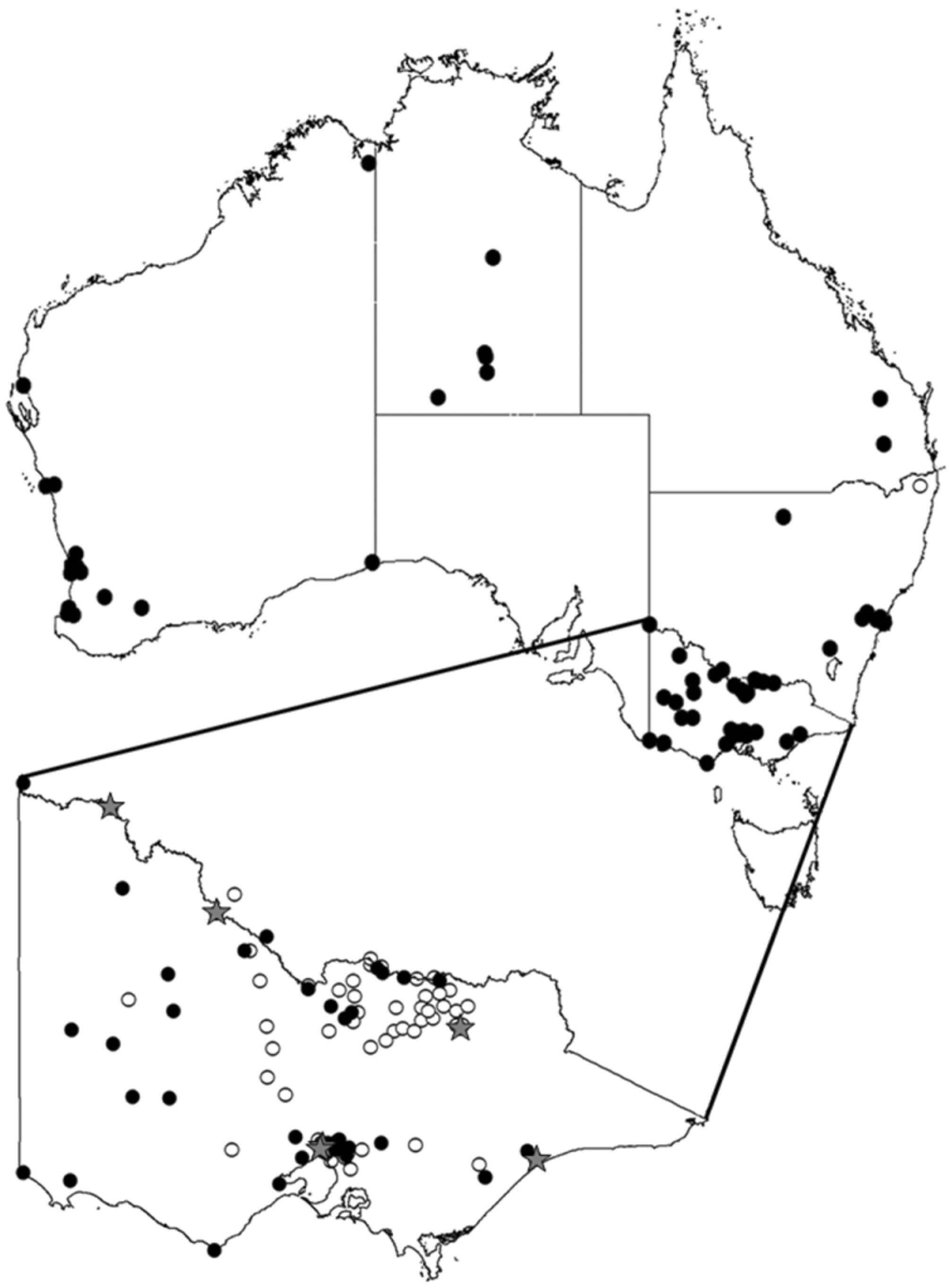
Distribution ( Fig. 51 View FIGURE 51 ). This species appears to be restricted to Australia, and has been most commonly collected from the temperate south. It was previously recorded from south-eastern Australia (Sydney and Como, NSW; Mt Gambier, South Australia; Linga, north-western Victoria. ( Broun 1904, 1905; Bezzi 1923; Malloch 1928). In the current study adult specimens have been examined from south-eastern Queensland, the Northern Territory and Western Australia, and additional localities from New South Wales and Victoria. Figure 51 View FIGURE 51 shows the geographic distribution of L. brouniana specimens examined in the present study. The specimens examined in the present study were almost all collected from southerly non-tropical areas (with the exception of some adults reared from larvae at Kununurra WA, DAFWA collection), Fig. 51 View FIGURE 51 ; a number of additional L. brouniana specimens from non-locally grown stone fruit were intercepted in Darwin (from the NTDR collection listed above) and are not included on Fig. 51 View FIGURE 51 . All of the northerly records may be introductions associated with horticulture.
References to L. brouniana being collected from Fiji (e.g. Bezzi 1919) is an historical error that has apparently occurred through confusion with a portion of the species description of Dacus xanthodes Broun, 1905 (from Suva, Fiji) which precedes the description of L. splendida (= L. brouniana ) on the same page in Broun (1905), as previously noted by McAlpine (1960). An additional historical error that has occurred is that this species has become established in New Zealand. Broun (1904, 1905) notes that L. brouniana was bred from imported tomatoes in New Zealand, but does not state that the species was established there. Subsequent references to L. brouniana being present in New Zealand by Froggatt (cited in French 1911) and Tillyard (1926) are apparently mistakenly based on Broun (1904, 1905). No species of Lonchaeidae are recorded from New Zealand ( Ferrar 1987, T. Crosby, pers comm. Jan. 2010).
Host plants ( Fig. 52 View FIGURES 52 ). Generally L. brouniana View in CoL appears to be capable of breeding in a wide variety of organic matter, but they appear to have a particular preference for Lycopersicon and Solanum View in CoL plants ( Solanaceae View in CoL ). Previously, Broun (1904, 1905), French (1911), and Tillyard (1926) recorded that Metallicgreen tomato flies have been reported as major pests of tomato plants in NSW and Victoria (and mistakenly in New Zealand, see notes under Distribution above) and were also known from potato, eggplant, and other Solanaceae View in CoL . Ferrar (1987) recorded them from tomato, wild tobacco, rotting potato stalks, cowdung, and even dead grasshoppers. They have also been noted to commonly breed in cow dung in southern NSW ( Ferrar 1979), and Hughes & Woolcock (1976) include them in with other dung breeding flies. A series of adult specimens examined in the current study were raised from larvae collected from cow dung at Wanneroo (WA), while other individuals were raised from rockmelon and walnut fruit. However, in south-eastern Australia, larvae have been most commonly collected from tomato fruit (>70% of records, Fig. 52 View FIGURES 52 ), they also regularly occur in other Solanaceae View in CoL ( capsicum View in CoL and eggplant), Rosaceae View in CoL (apricot, nectarine, peach), Rutaceae View in CoL (grapefruit, lemon, mandarin, orange), and Lauraceae View in CoL (avocado), during the warm summer (Dec–Feb) and autumn (Mar–May) months ( Fig. 52 View FIGURES 52 ).
TABLE 1. Specimen measurements of L. brouniana. Details of each measurement are described in the text. T-test probability values for differences between males and females of P <0.10 are included. Abbreviations: L, Length; W, Width; DMC, Discal Medial Cell; SD, Standard Deviation; n / s, non-significant.
| Lectotype Male | Paralectotype Female | Male Average (± SD) | Male Range | Female Average (± SD) | Female Range | T-test | |
|---|---|---|---|---|---|---|---|
| Body L (lateral) | 4.17 | 3.63 | 4.07 ± 0.23 | 3.68–4.31 | 3.74 ± 0.25 | 3.44–4.06 | <0.04 |
| Head L (lateral) | 1.27 | 1.06 | 1.22 ± 0.10 | 1.02–1.30 | 1.14 ± 0.07 | 1.06–1.25 | n / s |
| Head W (lateral) | 0.69 | 0.56 | 0.66 ± 0.08 | 0.59–0.77 | 0.63 ± 0.04 | 0.56–0.67 | n / s |
| Head W (dorsal) | 1.51 | 1.37 | 1.45 ± 0.09 | 1.28–1.57 | 1.42 ± 0.09 | 1.30–1.55 | n / s |
| Eye L (lateral) | 1.14 | 0.95 | 1.10 ± 0.09 | 0.93–1.18 | 1.00 ± 0.06 | 0.94–1.09 | <0.05 |
| Eye W (lateral) | 0.66 | 0.51 | 0.61 ± 0.10 | 0.47–0.75 | 0.58 ± 0.04 | 0.51–0.64 | n / s |
| Frons L | 0.48 | 0.43 | 0.51 ± 0.06 | 0.42–0.59 | 0.45 ± 0.04 | 0.41–0.50 | <0.07 |
| Frons W Lower | 0.31 | 0.45 | 0.30 ± 0.02 | 0.27–0.33 | 0.45 ± 0.03 | 0.40–0.48 | <0.01 |
| Frons W Upper | 0.38 | 0.47 | 0.38 ± 0.02 | 0.34–0.40 | 0.48 ± 0.04 | 0.42–0.53 | <0.01 |
| Antenna L (postpedicellus) | 0.31 | 0.33 | 0.32 ± 0.02 | 0.28–0.34 | 0.33 ± 0.02 | 0.30 – 0.37 | n / s |
| Antenna W (postpedicellus) | 0.13 | 0.11 | 0.11 ± 0.01 | 0.10–0.12 | 0.12 ± 0.01 | 0.11–0.13 | n / s |
| Arista L | 0.68 | 0.59 | 0.59 ± 0.05 | 0.51–0.65 | 0.59 ± 0.04 | 0.53–0.65 | n / s |
| Thorax L (dorsal) | 1.32 | 1.13 | 1.24 ± 0.14 | 0.99–1.36 | 1.18 ± 0.06 | 1.10–1.26 | n / s |
| Thorax W (dorsal) | 1.32 | 1.11 | 1.24 ± 0.13 | 1.02–1.40 | 1.22 ± 0.10 | 1.11–1.34 | n / s |
| Scutellum L | 0.47 | 0.41 | 0.45 ± 0.02 | 0.42–0.48 | 0.43 ± 0.04 | 0.36–0.47 | n / s |
| Scutellum W | 0.67 | 0.59 | 0.67 ± 0.07 | 0.60–0.79 | 0.62 ± 0.06 | 0.54–0.69 | n / s |
| Scutellar seta L | 0.60 | 0.62 | 0.64 ± 0.07 | 0.56–0.75 | 0.56 ± 0.04 | 0.50–0.62 | <0.07 |
| Wing L | 3.61 | 3.19 | 3.50 ± 0.22 | 3.16–3.76 | 3.42 ± 0.33 | 3.04–3.81 | n / s |
| Wing W | 1.33 | 1.24 | 1.38 ± 0.11 | 1.20–1.52 | 1.29 ± 0.13 | 1.17–1.53 | n / s |
| Wing DMC L | 1.54 | 1.44 | 1.51 ± 0.12 | 1.30–1.64 | 1.52 ± 0.10 | 1.40–1.66 | n / s |
| Abdomen L (lateral) | 2.03 | 1.63 | 1.85 ± 0.16 | 1.69–2.12 | 1.67 ± 0.13 | 1.48–1.84 | <0.06 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Lamprolonchaea brouniana ( Bezzi 1919 )
| Blacket, Mark J. & Malipatil, Mallik B. 2010 |
Lamprolonchaea rugosifrons
| Pitkin 1996: 476 |
| Bezzi 1923: 183 |
Lonchaea splendida
| Bezzi 1919: 246 |
| Broun 1904: 307 |